Article Contents
| Clin Exp Pediatr > Volume 66(12); 2023 |
|
Abstract
The prevalence of food allergy and food-induced anaphylaxis in children is increasing worldwide. Cow’s milk, hen’s eggs, and wheat allergies in young children have a more favorable prognosis with a relatively early outgrow, while allergies to peanuts, tree nuts, and seafood are more likely to be persistent. Although our understanding of the mechanism underlying the resolution of food allergy is incomplete, the roles of dendritic cells, regulatory T cells, and regulatory B cells are important. Many past studies on the natural course of food allergy were retrospective analyses of specific study groups, but large-scale population-based prospective studies are now being published. This review summarizes recent studies of the natural course of cow’s milk, hen’s eggs, wheat, peanuts, tree nuts, soy, sesame, and seafood allergies. The potential factors affecting the natural course of food allergy include symptom severity on ingestion, age at diagnosis, allergic comorbidities, skin prick test reaction size or serum food-specific immunoglobulin (Ig) E levels, changes in sensitization degree, IgE epitope specificity, ratio of food-specific IgE to IgG4, food-specific IgA levels, component-resolved diagnostic profile, diet, gut microbiome, and interventions such as immunotherapy. Since food allergy places a significant burden on patients and their caregivers in daily life, clinicians should be able to provide relevant knowledge on the natural course of food allergy, appropriately evaluate its resolution, and offer therapeutic options whenever possible.
The prevalence of immunoglobulin (Ig) E–mediated food allergy in children has increased remarkably worldwide in recent decades, placing a substantial burden on patients, their caregivers, and health care systems. The results of food allergy epidemiologic studies vary by study method, such as whether the diagnosis is based on an oral food challenge (OFC), a clinician’s history taking, or self-reported questionnaires, but it is apparent that the prevalence of IgE-mediated food allergy in children is increasing despite variations in research designs [1]. Large-scale population-based studies in Australia reported that the prevalence of food allergy exceeded 10% in infants and was 4%–5% in older children [2,3]. According to a cross-sectional population-based survey in the United States, the prevalence of IgE-mediated food allergy in children was 7.6%.4) In a Korean birth cohort study, the prevalence of immediate-type food allergy was 5.3% among infants [5]. Potentially life-threatening food-induced anaphylaxis is of greater importance, especially in younger individuals, in whom a sharp increase has been observed compared to older individuals. The annual admission rates due to foodinduced anaphylaxis in Australia increased 9-fold between 1998–1999 and 2018–2019, with the highest rates in those younger than 1 year of age [6]. According to a national big data analysis in Korea, the prevalence of all-cause anaphylaxis increased 1.7-fold from 2010 to 2014 and was greatest in the 0–2 years age group, in which food-induced anaphylaxis was most frequent [7].
Allergies to peanuts, tree nuts, and seafood tend to persist, whereas those to cow’s milk, hen’s eggs, wheat, and soy are typically outgrown during the preschool years. However, some studies suggested that the resolution timing of these food allergies may now be delayed compared with past decades [8,9]. Food allergy patients and/or their caregivers often experience a significant decrease in quality of life, especially if they experience severe symptoms, have multiple food allergies, or have allergies to staple foods that are more difficult to avoid, such as cow’s milk, hen’s eggs, or wheat [10,11]. Therefore, it is important for clinicians to equip themselves with current knowledge about the natural course of food allergy to guide elimination diets as well as to evaluate food allergy resolution at appropriate timing during follow-up. This review discusses the natural course of major IgE-mediated food allergies in children.
Our understanding remains poor about why some food allergies outgrow earlier versus persist longer. Tolerance to food allergens is driven mainly by antigen-presenting cells within the gut lamina propria by the promotion of T-cell differentiation. CX3CR1+ dendritic cells directly uptake antigens from the intestinal lumen and have greater inflammatory potential, whereas CD103+CX3CR1− dendritic cells have tolerogenic properties [12]. Goblet cell-associated antigen passages deliver antigens to CD103+CX3CR1− dendritic cells and, therefore, are associated with oral tolerance induction [13]. Migratory CD103+ dendritic cells from the lamina propria in the mesenteric lymph nodes can promote the development of gut-homing regulatory T cells through multiple mechanisms involving transforming growth factor-β (TGF-β), retinoic acid, transmembrane proteins, and enzymes involved in tryptophan catabolism [14-16].
Regulatory T cells, especially Foxp3+ regulatory T cells characterized by CD25 expression, play an essential role in oral tolerance. Foxp3 knockout mice developed multi-organ allergic inflammatory responses, whereas adoptive transfer of regulatory T cells could suppress anaphylaxis in a food allergy animal model [17,18]. Higher numbers of milk-specific CD4+CD25+ T regulatory cells were associated with the development of oral tolerance to cow’s milk [19,20]. Regulatory T cells can also produce several inhibitory cytokines, such as interleukin (IL)–10 and TGF-β. Interleukin (IL)-10 suppresses Th2 immune reactions and allergic inflammation by reducing IgE production and promoting allergen-specific IgG4 responses. Children who developed natural tolerance to hen’s eggs or peanuts had considerably increased IL-10 levels expressed by CD4+ T regulatory, CD25+ CD127lo, and Foxp3+ cells [21,22].
Immunosuppressive regulatory B cells regulate the immune responses by suppressing effector T cells via the production of suppressor cytokines such as IL-10 and TGF-β [23]. Also, IL-10-secreting regulatory B cells produce IgG4, a non-inflammatory isotype that prevents IgE-mediated mast cell and basophil degranulation [24].
Numerous studies have shown that IgE-mediated allergies to cow’s milk, hen’s eggs, wheat, and soy are more likely to resolve in childhood, while allergies to peanuts, tree nuts, and seafood tend to persist [25-30]. However, the majority of studies on the natural course of food allergy were inherently limited by their design because population-based prospective studies using OFC at predetermined intervals are almost impossible to perform. Many past studies were retrospective analyses that included both OFC and medical history for assessing tolerance, whereas recent studies in Australia released longitudinal population-level challenge-based results of the natural course of food allergy in children [3,26,29]. The factors believed to affect the tolerance development or persistence of food allergy include symptom severity on ingestion, age at diagnosis, comorbid allergic diseases, and various immune-related markers that will be described later [31]. As the current status and results of studies related to the natural course of food allergy vary widely by the food in question, large-scale recent studies are mainly described in the text, while other various research results including Korean data for each major food allergy are listed in Tables 1 and 2 for the reference for each major food allergen.
The EuroPrevall birth cohort study from 9 countries across Europe revealed that 56.5% of children with IgE-associated cow’s milk allergy passed double-blind placebo-controlled food challenges at re-evaluation 1 year after the initial diagnosis [32]. From a relatively large-scale retrospective analysis of children with cow’s milk allergy in the United States with a median follow-up duration of 54 months, the resolution rates of cow’s milk allergy were 19% by 4 years, 42% by 8 years, 64% by 12 years, and 79% by 16 years [25]. In a retrospective analysis of Korean children, half of the children with a cow’s milk allergy outgrew it at a median 8.7 years of age [33].
The resolution of challenge-proven hen’s egg allergy from a population-based prospective study in Australia was 89% by 6 years of age [34]. In Japan, where hen’s egg accounts for the highest frequency of all food allergies, the resolution rates were 30% by 3 years of age, 59% by 5 years of age, and 73% at 6 years of age in a retrospective analysis [35]. In a recent prospective report, a continuation of the previous study, the estimated acquired resolution rates were 14.6% by 7 years, 40.8% by 9 years, and 60.5% by 12 years [36]. In Korean children with immediate-type hen’s egg allergy, the median age at tolerance acquisition in 50% of patients was 5.6 years [33]. This result shows that the age of resolution of hen’s egg allergy is slightly younger than those reported in the United States and similar to that in Japan. A prospective larger-scale study is needed in Korea in the future.
In Thailand, where wheat allergy is particularly common and research is actively conducted, OFC was performed for evaluation of wheat allergy resolution if the patients’ specific IgE levels to wheat and ω-5 gliadin were ≤26 kUA/L and ≤1.06 kUA/L, respectively [37]. In this study, the proportion of children with wheat tolerance was 14.7% by 2 years, 27% by 4 years, 45.7% by 5 years, and 69% by 9 years. Another prospective analysis of 50 challenge-proven wheat-allergic children in Poland revealed that the resolution rates were 20% by 4 years, 52% by 8 years, 66% by 12 years, and 76% by 18 years [38].
As described above and summarized in Table 1, allergies to cow’s milk, hen’s eggs, and wheat are often outgrown before school age but not rarely persist into late childhood, so clinicians must pay attention to each individual patient when managing these allergies.
There are relatively few studies of the natural course of soy allergy compared to other food allergies. A retrospective analysis in a tertiary clinic in the United States reported that the resolution rates for soy allergy in children were 25% by age 4 years, 45% by age 6 years, and 69% by age 10 years [28]. Peanut allergy is a very active research field, especially in the West, and according to the latest Australian population-based prospective study, peanut allergy had resolved in 29% by 6 years of age [34]. As reported by the same research team, the rate of resolution of peanut allergy by the age of 4 years was reportedly 22% in 2015 [29]. A retrospective analysis conducted at 3 hospitals in Korea reported that the probabilities of peanut allergy resolution were 10.3% at age 6 years and 32.8% at age 10 years [39].
Tree nut allergy accounts for a considerable proportion of food allergies in children and frequently cause severe reactions, but the natural course of tree nut allergy compared to other major food allergies remains understudied [40,41]. A study published in 2005 that is still consistently cited when referring to the natural course of tree nuts reported a resolution rate of 8.9% among children with previous clinical reactivity and evidence of tree nut sensitization [30].
Most studies of sesame allergy have been published in Israel; according to a retrospective observation of 45 sesame allergy patients for 6 years, 20% achieved tolerance [42]. In a more recent retrospective analysis of 190 children with sesame allergy, 32.1 % had spontaneous resolution during the mean follow-up period of 3.86±4.43 years [43].
Among allergies to legumes, tree nuts, and seeds, only soy allergy seems to have good prognosis, but there are currently no Korean data of the natural course of soy allergy. Although walnuts are the most common cause of anaphylaxis in children aged 2–12 years in Korea, there is no study on the natural course of walnut allergy in Korean children, strongly suggesting the need for additional research in this field [44].
Studies of the natural course of seafood versus other allergies in children are relatively rare. A recently published prospective study examining the natural course of IgE-mediated fish allergy in Greek children reported a rate of 22% tolerance development to cod during the follow-up period [45]. Complete tolerance to fish increased with age from 3.4% in preschool children to 45% in adolescents in that study. According to a longitudinal cohort study of 49 children and 14 adults with seafood allergy in Canada, the resolution rate was 0.6% per person-year for fish and 0.8% per person-year for shellfish [46]. Reports on the natural course of shellfish allergy are rare, and no studies have been conducted in Asian children. In 11 adults with shrimp allergy, the levels of shrimp-specific IgE remained constant over a 24-month period suggesting the persistence of allergy [47].
Studies of the natural course of seafood allergy in Korean children have not been published to date. Contrary to the point that seafood allergy generally persists into adulthood, in actual clinical practice, it seems not uncommon for young children with fish allergy to improve within a few years after diagnosis, suggesting the need for future research into the natural course of seafood allergy in Korean children.
Evidence of markers predicting the resolution or persistency of food allergy remains insufficient today. Factors associated with the timing of food allergy resolution include age at diagnosis, comorbid allergic diseases and their severity, symptom severity on ingestion, skin prick test (SPT) reaction size, foodspecific IgE levels, rate of change of food-specific IgE levels or SPT reaction sizes, ratio of specific IgE to IgG4, diet, gut microbiome, and interventions such as oral immunotherapy (Fig. 1). The degree of sensitization to the specific food by serum-specific IgE levels or SPT is among few tools that can be repeatedly performed in the actual clinical setting to monitor the natural course of food allergy. Diagnostic cutoff levels for predicting the persistency of food allergy have been suggested by numerous studies, but the results varied widely by age, region, and study design bearing considerable limits when applied in clinical practice [48-51].
The extensive research results related to specific IgE for predicting OFC outcomes at specific time points are not covered in detail in this article, as they refer more to the diagnosis of food allergy. This review mainly examined and summarized the findings related to predicting persistent food allergy in studies that prospectively or retrospectively followed the natural course of food allergy over time (Table 3). Studies showed that the persistence of food allergy was considerably associated with the baseline, peak (highest at all ages), and/or time point food-specific IgE levels. The resolution rates of cow’s milk allergy by 10 years was 87% with a peak cow’s milk-specific IgE <2 kUA/L and 5% with a peak level of ≥50 kUA/L [25].
Component-resolved data in some foods may provide additional information to enable a more precise diagnosis as well as for persistence predictions of food allergy. High levels of ovomucoid- and casein-specific IgE were associated with persistent hen’s egg and cow’s milk allergies, respectively, but specific cutoff levels for ovomucoid- or casein-specific IgE for predicting persistent food allergy have not been reported to date [35,52]. High IgE levels to gliadins were correlated with persistent wheat allergy and the development of asthma in children [53]. Other components such as Ara h 2 and Ara h 6 from peanut, Jug r 1 from walnut, Cor a 9 and Cor a 14 from hazelnut, and Ana o 3 from cashew nut have been studied for their useful value in diagnosing food allergy, but the role of these components with regard to the natural course of food allergy should be further investigated [54-57].
Diet and the gut microbiome also influence the persistence of food allergy by influencing mucosal immune tolerance [58]. It was recently suggested that regular consumption of the food in the form of reduced allergenicity, such as baked products containing egg and/or milk, or at an amount that does not induce symptoms, may improve the prognosis of food allergy [59]. Certain strains such as Bifidobacterium longum and Bacteroides fragilis can induce intestinal regulatory T cells, possibly by pattern-recognition receptor activation on dendritic cells [60]. Metabolites such as short-chain fatty acids produced by bacteria after the digestion of dietary fibers play an important role in enhancing the regulatory activity of dendritic cells, leading to the induction of regulatory T cells and IL-10-secreting T cells [61].
It is imperative for clinicians to aptly guide the timing of food allergy resolution assessments using appropriate parameters. As the course of food allergy can vary substantially depending on food type, patient age, and test results, clinicians would have to integrate all available information to evaluate the patient’s food allergy resolution status. By incorporating the results of various studies (Table 3), Santos et al. recently suggested an approach to assessing food allergy resolution: for cow’s milk and hen’s egg, a baseline specific IgE <2 kUA/L, >50% decrease in specific IgE levels over >12 months, and specific IgE <3 kUA/L (cow’s milk) or <2 kUA/L (hen’s egg) at other time points were associated with likely resolution; for peanuts, a baseline specific IgE <2 kUA/L and other time point-specific IgE <2 kUA/L (if reaction) or <5 kUA/L (if no reaction) were associated with likely resolution; and for tree nuts, a baseline specific IgE <2 kUA/L was associated with likely resolution, whereas an SPT reaction size ≥13 mm and/or specific IgE ≥5 kUA/L at other time points were associated with likely persistence [62]. When considering OFC for the assessment of food allergy resolution, clinicians should consider multifaceted factors such as patent age, the importance of the food in the diet, previous history of reactions, potential for risk, comorbidities especially severe atopic dermatitis, uncontrolled asthma, or eosinophilic gastrointestinal diseases, and patient and family preferences (Fig. 2).
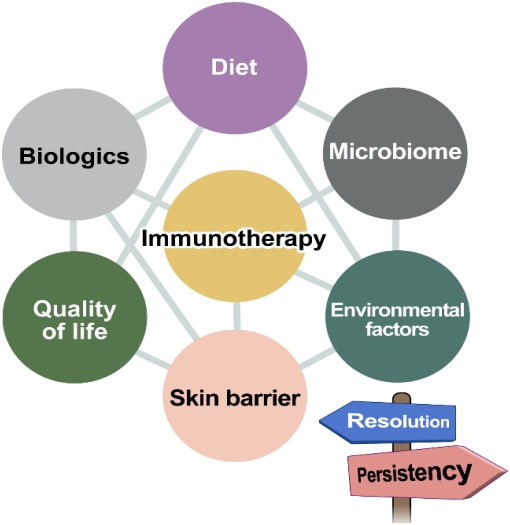
The current leading treatment for modifying the natural course of food allergy is oral immunotherapy, an allergen-specific approach based on progressive incremental ingestion of the food allergen doses until a daily maintenance dosage is reached to achieve desensitization. Although oral immunotherapy still requires some attention, such as adverse events occurring not rarely during the treatment and achievement of continued tolerance, its implementation is gradually expanding worldwide and studies proving its efficacy are abounding [63-65]. Epicutaneous immunotherapy has been actively studied, particularly for peanut allergy, and has shown safe and modest treatment responses, especially in younger children [66]. In cases of severe or multiple food allergies, studies of allergen-specific immunotherapy combined with biologics such as omalizumab have been reported, enabling faster dose escalation and lower rates of adverse reactions during immunotherapy [67,68]. The purpose of allergenspecific immunotherapy in food allergy may be sufficient tolerance induction in some patients or the increase of the tolerated dose to improve quality of life in others, which will not be discussed in detail here. Other than immunotherapy, aspects that may affect the natural course of food allergy are diet, gutmicrobial modulation, skin barrier management, psychological state, and other environmental factors, which are outlined in Fig. 3.
To provide the optimal treatment to patients with food allergy and help improve their quality of life, it is essential that clinicians understand the natural course of individual food allergies. At this point, it remains unclear why some children outgrow their food allergy and others do not. Past studies of the natural course of food allergy are heterogeneous and bear limitations in terms of study design, but lately, population-based prospective data are becoming available. Based on the various factors associated with food allergy persistence, health care practitioners should offer relevant information to patients and their caregivers and conduct relevant assessments to evaluate food allergy resolution at appropriate timing. Using therapeutic tools including allergen-specific immunotherapy, gut-microbial modulation, and other potential therapeutics to be developed, we may be able to alter the course of food allergy in the future.
Fig. 1.
Suggested elements associated with the natural course of food allergies. sIgE, specific immunoglobulin E; SPT, skin prick test.

Fig. 2.
Factors related to the natural course of major food allergies. sIgE, specific immunoglobulin E; SPT, skin prick test.
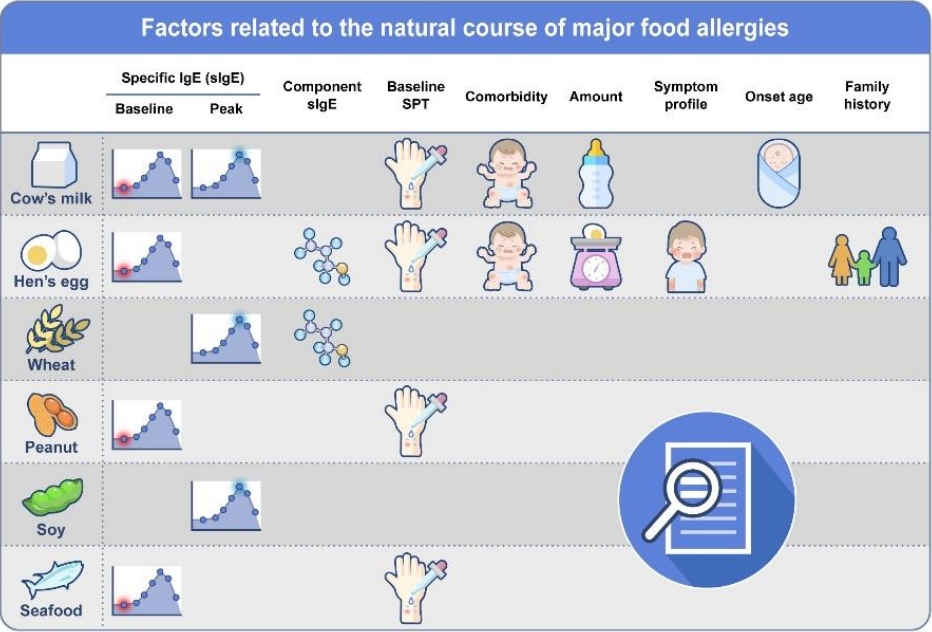
Table 1.
Studies of the natural course of cow’s milk, hen’s egg, and wheat allergy in children
| Food | Age or rates of resolution | Criteria for resolution | Study type, country | Year |
|---|---|---|---|---|
| Cow’s milk | 19% by 4 ears, 42% by 8 years, 64% by 12 years, and 79% by 16 years [25] | Passed OFC or sIgE <3 kU/L with no symptoms within 12 months | Retrospective, US | 2007 |
| 57.4% during 48- to 60-month follow-up [69] | Passed OFC or successful consumption history | Prospective, Israel | 2012 | |
| 52.6% at a median age of 63 months [9] | Passed OFC or ingestion of uncooked milk products in serving size quantities | Observational cohort, USA | 2013 | |
| 57% after 1 year from the diagnosis [32] | Passed OFC | Birth cohort, Europe | 2015 | |
| 25% by 43 months, 50% by 104.8 months [33] | Passed OFC or successful consumption history | Retrospective, Korea | 2020 | |
| 81.8% by 6 years [70] | Passed OFC or successful consumption history | Retrospective, Singapore | 2022 | |
| Hen’s egg | 11% by 4 years, 41% by 8years, 65% by 12 years, 82% by 16 years [8] | Passed OFC or sIgE < 2 kU/L with no symptoms within 12 months | Retrospective, USA | 2007 |
| 49.3% at a median age of 74 months [71] | Passed OFC or ingestion of whole, concentrated egg products in serving size quantities | Observational cohort, USA | 2014 | |
| 30% by 3 years, 59% by 5 years, and 73% by 6 years [35] | Passed OFC | Retrospective, Japan | 2016 | |
| 83.9% by 4 years [26] | Passed OFC | Longitudinal cohort, Australia | 2019 | |
| 25% by 46.7 months, 50% by 67.6 months [33] | Passed OFC or successful consumption history | Retrospective, Korea | 2020 | |
| 14.6% by 7 years, 40.8% by 9 years, 60.5% by 12 yearsa), [36] | Passed OFC | Prospective, Japan | 2022 | |
| 89% by 6 years [34] | Passed OFC or SPT < 3 mm or SPT 3–7 mm with successful consumption history | Longitudinal cohort, Australia | 2022 | |
| Wheat | 29% by 4 years, 56% by 8 years, 65% by 12 years [27] | Passed OFC or successful consumption history | Retrospective, USA | 2009 |
| 20% by 4 years, 52% by 8years, 66% by 12 years, 76% by 18 years [38] | Passed OFC | Prospective, Poland | 2014 | |
| 14.7% at 2 years, 27% at 4 years, 45.7% at 5 years 69% at 9 years [37] | Passed OFC | Prospective, Thailand | 2017 |
Table 2.
Studies of the natural course of legume, tree nut, seed, and seafood allergies in children
| Food | Age or rates of resolution | Criteria for resolution | Study type, country | Year |
|---|---|---|---|---|
| Peanut | 13.2% at 3 years, 21.4% at 5 years, 34.2% at 7 years [72] | Passed OFC | Longitudinal cohort, Australia | 2008 |
| 10% by 4 years, 18% by 6 years, 22% by 8 years, 26% by 10 years [73] | Passed OFC | Prospective, Canada | 2013 | |
| 22% by 4 years [29] | Passed OFC | Longitudinal cohort, Australia | 2015 | |
| 10.3% by 6 years, 32.8% by 10 years [39] | Passed OFC or successful consumption history | Retrospective, Korea | 2021 | |
| 29% by 6 years [34] | Passed OFC or SPT <3 mm or SPT 3–7 mm with successful consumption history | Longitudinal cohort, Australia | 2022 | |
| Tree nuts | 8.9% among children with previous clinical reactivity and evidence of tree nut sensitization [30] | Passed OFC | Retrospective, USA | 2005 |
| Soy | 25% by 4 years, 45% by 6 years, 69% by 10 years [28] | Passed OFC or successful consumption history | Retrospective, USA | 2010 |
| Sesame | 20% during 6.4-year follow-up [42] | Successful consumption history | Retrospective, Israel | 2007 |
| 32.1% during 3.86±4.43-year follow-up [43] | Passed OFC or successful consumption history | Retrospective, Israel | 2022 | |
| Seafood | 0.6% per person-year for fish, 0.8% per person-year for shellfisha), [46] | Successful consumption history or decision of resolution by a physician | Longitudinal cohort, Canada | 2019 |
| Complete fish tolerance 3.4% in preschool children, 45% in adolescents [45] | Passed OFC | Prospective, Greece | 2021 |
Table 3.
Factors associated with persistent food allergy
| Food | Factors associated with persistent food allergy | Study type, country |
|---|---|---|
| Cow’s milk | Baseline milk-sIgE level (>10 kU/L), SPT wheal size (>10 mm), moderate/severe AD [9] | Observational cohort, USA |
| Reaction to <10 mL on OFC, SPT wheal size (>6 mm), age of ≤30 days at first reaction [69] | Prospective, Israel | |
| Peak milk-sIgE within the first 24 months of birth (≥15 kU/L)a), [74] | Retrospective, Korea | |
| High milk-sIgE at the first reaction [33] | Retrospective, Korea | |
| Hen’s egg | Initial reaction profile (presentations other than isolated urticarial/angioedema), baseline egg white-sIgE levels (≥10 kU/L), baseline SPT wheal size (≥10 mm), moderate/severe AD, baseline egg-sIgG4 levels (<0.1 mg/L) [71] | Observational cohort, USA |
| High egg white-sIgE at the first reaction, positive family history of allergic diseases [33] | Retrospective, Korea | |
| Previous history of egg-induced anaphylaxis before 6 years of age, symptoms to small amounts of heated egg by 6 years of age, higher ovomucoid-sIgE levels at 6 years of age (≥12.5 kU/L) [36] | Prospective, Japan | |
| Ovomucoid sensitization at 12 months [26] | Longitudinal cohort, Australia | |
| High peak egg white-sIgE, presence of other atopic disease, presence of other food allergy [8] | Retrospective, USA | |
| Wheat | ω-5-gliadin-sIgE (≥0.35 kU/L) [37] | Prospective, Thailand |
| High peak wheat-sIgE (≥50 kU/L) [27] | Retrospective, USA | |
| Peanut | Peanut-sIgE at diagnosis (≥1 kU/L) [39] | Retrospective, Korea |
| SPT wheal size (≥6 mm), peanut-sIgE (≥3 kU/L) [72] | Longitudinal cohort, Australia | |
| Increasing SPT wheal size [29] | Longitudinal cohort, Australia | |
| Soy | High peak soy-sIgE (≥50 kU/L) [28] | Retrospective, USA |
| Seafood | Prechallenge cod-sIgE (>4.87 kU/L), SPT to sardine (>6.5 mm) [45] | Prospective, Greece |
References
1. Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep 2020;20:6.




2. Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year followup. J Allergy Clin Immunol 2017;140:145–53.e8.


3. Sasaki M, Koplin JJ, Dharmage SC, Field MJ, Sawyer SM, McWilliam V, et al. Prevalence of clinic-defined food allergy in early adolescence: the SchoolNuts study. J Allergy Clin Immunol 2018;141:391–8.e4.


4. Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 2018;142:e20181235.




5. Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol 2011;22:715–9.


6. Mullins RJ, Dear KBG, Tang MLK. Changes in Australian food anaphylaxis admission rates following introduction of updated allergy prevention guidelines. J Allergy Clin Immunol 2022;150:140–5.e1.


7. Jeong K, Lee JD, Kang DR, Lee S. A population-based epidemiological study of anaphylaxis using national big data in Korea: trends in age-specific prevalence and epinephrine use in 2010-2014. Allergy Asthma Clin Immunol 2018;14:31.




8. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol 2007;120:1413–7.


9. Wood RA, Sicherer SH, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol 2013;131:805–12.



10. Allen CW, Bidarkar MS, vanNunen SA, Campbell DE. Factors impacting parental burden in food-allergic children. J Paediatr Child Health 2015;51:696–8.



11. Ward CE, Greenhawt MJ. Treatment of allergic reactions and quality of life among caregivers of food-allergic children. Ann Allergy Asthma Immunol 2015;114:312–8.e2.


12. Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 2009;31:502–12.


13. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012;483:345–9.




14. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic aciddependent mechanism. J Exp Med 2007;204:1757–64.


15. Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, et al. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 2011;141:1813–20.



16. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010;59:595–604.


17. Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, et al. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol 2005;116:1106–15.


18. Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy 2012;67:201–9.


19. Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol 2009;123:43–52.e7.


20. Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+ CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med 2004;199:1679–88.




21. Fishbein AB, Qamar N, Erickson KA, Kwasny MJ, Cai M, Szychlinski C, et al. Cytokine responses to egg protein in previously allergic children who developed tolerance naturally. Ann Allergy Asthma Immunol 2014;113:667–70.e4.

22. Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ, et al. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy 2015;45:1663–72.



23. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015;42:607–12.


24. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 2013;131:1204–12.


25. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol 2007;120:1172–7.


26. Dang TD, Peters RL, Koplin JJ, Dharmage SC, Gurrin LC, Ponsonby AL, et al. Egg allergen specific IgE diversity predicts resolution of egg allergy in the population cohort HealthNuts. Allergy 2019;74:318–26.



27. Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol 2009;102:410–5.


28. Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol 2010;125:683–6.


29. Peters RL, Allen KJ, Dharmage SC, Koplin JJ, Dang T, Tilbrook KP, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population-based assessment. J Allergy Clin Immunol 2015;135:1257–66.e1-2.


30. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol 2005;116:1087–93.


31. Kattan J. The prevalence and natural history of food allergy. Curr Allergy Asthma Rep 2016;16:47.



32. Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy 2015;70:963–72.


33. Kim M, Lee JY, Yang HK, Won HJ, Kim K, Kim J, et al. The natural course of immediate-type cow’s milk and egg allergies in children. Int Arch Allergy Immunol 2020;181:103–10.



34. Peters RL, Guarnieri I, Tang MLK, Lowe AJ, Dharmage SC, Perrett KP, et al. The natural history of peanut and egg allergy in children up to age 6 years in the HealthNuts population-based longitudinal study. J Allergy Clin Immunol 2022;150:657–65.e13.


35. Ohtani K, Sato S, Syukuya A, Asaumi T, Ogura K, Koike Y, et al. Natural history of immediate-type hen's egg allergy in Japanese children. Allergol Int 2016;65:153–7.


36. Taniguchi H, Ogura K, Sato S, Ebisawa M, Yanagida N. Natural history of allergy to hen’s egg: a prospective study in children aged 6 to 12 years. Int Arch Allergy Immunol 2022;183:14–24.



37. Siripipattanamongkol N, Vichyanond P, Jirapongsananuruk O, Veskitkul J, Visitsunthorn N, Pacharn P. Age of resolution from IgE-mediated wheat allergy. Asian Pac J Allergy Immunol 2017;35:113–7.

38. Czaja-Bulsa G, Bulsa M. The natural history of IgE mediated wheat allergy in children with dominant gastrointestinal symptoms. Allergy Asthma Clin Immunol 2014;10:12.




39. Jung M, Jeong HI, Kyung Y, Kim SK, Lee JS, Choi M, et al. Natural course and prognostic factors of immediate-type peanut allergy in children. Int Arch Allergy Immunol 2021;182:1072–6.



40. Jeong K, Kim J, Ahn K, Lee SY, Min TK, Pyun BY, et al. Age-based causes and clinical characteristics of immediate-type food allergy in Korean children. Allergy Asthma Immunol Res 2017;9:423–30.




41. McWilliam VL, Perrett KP, Dang T, Peters RL. Prevalence and natural history of tree nut allergy. Ann Allergy Asthma Immunol 2020;124:466–72.


42. Cohen A, Goldberg M, Levy B, Leshno M, Katz Y. Sesame food allergy and sensitization in children: the natural history and long-term follow-up. Pediatr Allergy Immunol 2007;18:217–23.


43. Mahlab-Guri K, Guri A, Kadar L, Asher I, Sthoeger Z, Elbirt D, et al. Characteristics of patients with spontaneous resolution of sesame allergy. Ann Allergy Asthma Immunol 2022;128:206–12.


44. Jeong K, Ye YM, Kim SH, Kim KW, Kim JH, Kwon JW, et al. A multicenter anaphylaxis registry in Korea: clinical characteristics and acute treatment details from infants to older adults. World Allergy Organ J 2020;13:100449.



45. Xepapadaki P, Christopoulou G, Stavroulakis G, Freidl R, Linhart B, Zuidmeer L, et al. Natural history of IgE-mediated fish allergy in children. J Allergy Clin Immunol Pract 2021;9:3147–56.e5.


46. Zotova V, Clarke AE, Chan ES, Asai Y, Chin R, Van Lambalgen C, et al. Low resolution rates of seafood allergy. J Allergy Clin Immunol Pract 2019;7:690–2.


47. Daul CB, Morgan JE, Lehrer SB. The natural history of shrimp hypersensitivity. J Allergy Clin Immunol 1990;86:88–93.


48. Peters RL, Allen KJ, Dharmage SC, Tang ML, Koplin JJ, Ponsonby AL, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol 2013;132:874–80.


49. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001;107:891–6.


50. Roberts G, Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol 2005;115:1291–6.


51. Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy 2000;30:1540–6.



52. Boyano-Martinez T, Garcia-Ara C, Pedrosa M, Diaz-Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow's milk proteins. J Allergy Clin Immunol 2009;123:883–8.


53. Kotaniemi-Syrjänen A, Palosuo K, Jartti T, Kuitunen M, Pelkonen AS, Mäkelä MJ. The prognosis of wheat hypersensitivity in children. Pediatr Allergy Immunol 2010;21(2 Pt 2): e421–8.

54. Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy 2007;37:1221–8.


55. Lee J, Jeong K, Jeon SA, Lee S. Component resolved diagnosis of walnut allergy in young children: Jug r 1 as a major walnut allergen. Asian Pac J Allergy Immunol 2021;39:190–6.

56. Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol 2013;132:393–9.


57. Lange L, Lasota L, Finger A, Vlajnic D, Busing S, Meister J, et al. Ana o 3-specific IgE is a good predictor for clinically relevant cashew allergy in children. Allergy 2017;72:598–603.



58. Marrs T, Sim K. Demystifying dysbiosis: can the gut microbiome promote oral tolerance over IgE-mediated food allergy? Curr Pediatr Rev 2018;14:156–63.


59. Esmaeilzadeh H, Alyasin S, Haghighat M, Nabavizadeh H, Esmaeilzadeh E, Mosavat F. The effect of baked milk on accelerating unheated cow's milk tolerance: a control randomized clinical trial. Pediatr Allergy Immunol 2018;29:747–53.



60. Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy 2010;40:811–9.


61. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119.


62. Foong RX, Santos AF. Biomarkers of diagnosis and resolution of food allergy. Pediatr Allergy Immunol 2021;32:223–33.



63. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 2019;393:2222–32.


64. Miura Y, Nagakura KI, Nishino M, Takei M, Takahashi K, Asaumi T, et al. Long-term follow-up of fixed low-dose oral immunotherapy for children with severe cow's milk allergy. Pediatr Allergy Immunol 2021;32:734–41.

65. Palosuo K, Karisola P, Savinko T, Fyhrquist N, Alenius H, Makela MJ. A randomized, open-label trial of hen’s egg oral immunotherapy: efficacy and humoral immune responses in 50 children. J Allergy Clin Immunol Pract 2021;9:1892–901.e1.


66. Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 2017;139:1242–52.e9.



67. MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 2017;139:873–81.e8.



68. Andorf S, Purington N, Kumar D, Long A, O'Laughlin KL, Sicherer S, et al. A phase 2 randomized controlled multisite study using omalizumabfacilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. EClinicalMedicine 2019;7:27–38.



69. Elizur A, Rajuan N, Goldberg MR, Leshno M, Cohen A, Katz Y. Natural course and risk factors for persistence of IgE-mediated cow’s milk allergy. J Pediatr 2012;161:482–7.e1.


70. Chong KW, Goh SH, Saffari SE, Loh W, Sia I, Seah S, et al. IgE-mediated cow’s milk protein allergy in Singaporean children. Asian Pac J Allergy Immunol 2022;40:65–71.

71. Sicherer SH, Wood RA, Vickery BP, Jones SM, Liu AH, Fleischer DM, et al. The natural history of egg allergy in an observational cohort. J Allergy Clin Immunol 2014;133:492–9.



72. Ho MH, Wong WH, Heine RG, Hosking CS, Hill DJ, Allen KJ. Early clinical predictors of remission of peanut allergy in children. J Allergy Clin Immunol 2008;121:731–6.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


