Association between pre- and postnatal exposure to endocrine-disrupting chemicals and birth and neurodevelopmental outcomes: an extensive review
Article information
Abstract
Endocrine-disrupting chemicals (EDCs) are natural or synthetic chemicals that mimic, block, or interfere with the hormones in the body. The most common and well- studied EDCs are bisphenol A, phthalates, and persistent organic pollutants including polychlorinated biphenyls, polybrominated diphenyl ethers, per- and polyfluoroalkyl substances, other brominated flame retardants, organochlorine pesticides, dioxins, and furans. Starting in embryonic life, humans are constantly exposed to EDCs through air, diet, skin, and water. Fetuses and newborns undergo crucial developmental processes that allow adaptation to the environment throughout life. As developing organisms, they are extremely sensitive to low doses of EDCs. Many EDCs can cross the placental barrier and reach the developing fetal organs. In addition, newborns can be exposed to EDCs through breastfeeding or formula feeding. Pre- and postnatal exposure to EDCs may increase the risk of childhood diseases by disrupting the hormone-mediated processes critical for growth and development during gestation and infancy. This review discusses evidence of the relationship between pre- and postnatal exposure to several EDCs, childbirth, and neurodevelopmental outcomes. Available evidence suggests that pre- and postnatal exposure to certain EDCs causes fetal growth restriction, preterm birth, low birth weight, and neurodevelopmental problems through various mechanisms of action. Given the adverse effects of EDCs on child development, further studies are required to clarify the overall associations.
Key message
· Sensitivity to endocrine-disrupting chemical (EDC) exposure increases during critical developmental periods (in embryos, fetuses, and neonates).
· Pre- and postnatal exposure to EDCs is associated with fetal growth restriction, preterm birth, and low birth weight.
· Exposure to EDCs during fetal and early postnatal life can have lasting and lifelong neurodevelopmental outcomes, including autism spectrum, attention deficit hyperactivity, and other cognitive and behavioral disorders.
Graphical abstract. Association between pre- and postnatal exposure to endocrine-disrupting chemicals and birth and neurodevelopmental outcomes
Introduction
A functional endocrine system is required to coordinate the actions of the hormones that regulate the body’s physiological and behavioral activities [1]. Hormones produced by the endocrine glands are transported to target cells to regulate body development, growth, reproduction, metabolism, immunity, and behavior by binding to cellular receptors [2]. However, some environmental chemicals, termed endocrine-disrupting chemicals (EDCs) [3], directly interfere with the production, release, binding, transport, and elimination of hormones in the body, thereby altering their effects on target cells [4].
The most extensively studied EDCs include bisphenol A (BPA) and phthalate, which are plastics and plasticizers. Other common EDCs are persistent organic pollutants (POPs) [4]. BPA is an organic chemical used in the manufacture of epoxy resins, polycarbonates, and polyvinyl chloride plastics [5]. It is widely used in plastic bottles, feeding bottles, plastic kitchenware, electronic materials, paints, thermal papers, medical and dental materials, and the inner surface coatings of food and beverage cans [6]. Phthalates are chemicals used in medical and building materials, toys, personal care products, plastic kitchenware, and food packaging [7]. POPs, which are pesticides, industrial chemicals, and byproducts of industrial processes, are organic chemicals that can persist longer in the environment [8]. These include polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), per- and polyfluoroalkyl substances (PFAS), other brominated flame retardants, organochlorine pesticides (OCPs), dioxins, furans, and others [9]. EDCs can enter the human body through ingestion, inhalation, and dermal absorption via leakage into the environment, food, and consumer products [4]. The sources of exposure to common EDCs in humans are listed in Table 1.
The incidence rates of noncommunicable diseases, particularly birth defects, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), asthma, obesity, diabetes, and childhood cancers, have increased over the last 30 years. There is a growing concern that there is a strong association between childhood diseases and exposure to industrial chemicals and other environmental toxins acting as EDC [10]. Pre- and postnatal exposure to EDCs can have profound effects on health during infancy, childhood, and adulthood by causing irreversible changes in differentiated tissues [11]. Phthalates, phenols, perfluorinated compounds, flame retardants, PCBs, and OCPs can cross the placental barrier and reach the developing fetal organs [11,12]. In addition, newborns may be exposed to EDCs through breastfeeding and formula feeding as well as inhalation and dermal absorption [11]. The early life stages from fertilization to 2 years of age are critical developmental windows characterized by the maturation and epigenetic programming of neuronal, metabolic, and immune pathways as well as endocrine, reproductive, and other systems [4]. However, sensitivity to EDCs increases during such critical periods (embryo, fetus, and neonate) because of rapid cellular proliferation/differentiation, immature metabolism, and inadequate detoxification mechanisms [13]. Many EDCs accumulate in the adipose tissues because of their lipid solubility; therefore, the long-term effects of pre and postnatal exposure are observed in later years [14]. Thus, this review discusses the evidence linking pre and postnatal exposure to several EDCs to various childbirth and neurodevelopmental outcomes.
Methods
We searched for publications published between 2018 and 2023 in PubMed, Google Scholar, and Science Direct using some keyword combinations. The following terms were used to describe exposure: prenatal exposure, postnatal exposure, EDCs, infant, newborn, neonate, pregnancy, BPA, phthalates, POPs, PCBs, PBDEs, PFAS, OCPs, dioxins, and furans. These terms were matched with the following keywords describing birth and neurodevelopmental outcomes: birth outcomes, fetal growth, birth weight, birth length, birth size, neurodevelopment, neurotoxicity, neurobehavior, mental development, cognitive develop- ment, psychomotor development, language development, ASD, attention deficit, and hyperactivity disorder.
1. Birth outcomes
The placenta is considered both a filter for the passage of EDCs and an endocrine organ acting as a conduit between the mother and fetus to maintain fetal homeostasis. EDCs affect pregnancy as direct hormonal agonists/antagonists affecting endocrine functions as well as indirectly by disrupting maternal, placental, and fetal homeostasis [15,16]. Environmental exposure to EDCs impairs placental function [17,18]. The placental barrier is not completely impermeable to harmful substances; therefore, exposure to environmental triggers can permanently reprogram normal physiological responses that affect both intrauterine and postpartum life [17]. Prenatal exposure to EDCs can result in epigenetic changes that alter fetal programming and increase the risk of certain noncommunicable diseases in postnatal life as suggested by the Developmental Origins of Health and Disease hypothesis [19].
Prenatal exposure to EDCs leads to preterm birth, fetal intrauterine growth retardation, changes in birth weight and size, small-for-gestational-age status, large-for- gestational-age status, older gestational age, macrosomia, and congenital disorders, all of which may have negative consequences [20-23]. EDCs can interfere with the insulin- like growth factor (IGF) system that is a critical growth regulator in fetal development [24].
The IGF system represents a particularly critical growth regulator for fetal development, and EDCs can interfere with this system [25]. Correlations between in utero exposure to EDCs and birth outcomes have been reported in epidemiological studies, but results are conflicting [26,27]. Table 2 shows the effects of EDCs on birth outcomes.
1) Bisphenol A
BPA is found in human plasma, urine, amniotic fluid, follicular fluid, placental tissue, breast milk, umbilical cord blood, and adipose tissue [28-30]. In utero exposure to BPA can affect fetal growth through multiple hormone-mediated mechanisms by mimicking estrogen, inhibiting androgen production, altering thyroid signaling, and inducing oxidative stress [31-33]. A high amount of BPA in trophoblast cells during the first trimester of pregnancy reportedly inhibits cellular growth and affects deoxyribonucleic acid (DNA) methylation [34]. However, the effect of prenatal exposure to BPA on postnatal growth remains unclear [35].
BPA is transported through the placenta and affects placental growth by increasing beta human chorionic gonadotropin levels [36,37]. BPA can easily cross the placenta and is associated with preterm birth [38]. At the same time, prenatal exposure to BPA may cause disease onset in childhood and adulthood by changing fetal epigenetic programming [39]. Combined prenatal exposure to BPA from dietary and nondietary sources (especially when the first-half exposure occurs) may contribute to fetal growth restriction [40-42].
Although in utero exposure to BPA may adversely affect placental development and function and lead to inadequate fetal growth and adverse birth outcomes, some meta-analyses reported no association between fetal BPA exposure and birth weight, height, or head circumference [43-47]. No association between fetal exposure to BPA and gestational age at birth was also reported [46,47].
Due to concerns about the potential negative effects of BPA, it is increasingly being replaced by other bisphenols, such as bisphenol S (BPS) and bisphenol F (BPF). However, whether these bisphenols have fewer adverse health effects than BPA remains unclear [48]. Higher maternal BPS concentrations, particularly in the first trimester, are reportedly associated with greater fetal head circumference and weight, suggesting that BPS exposure enhances fetal growth. In utero BPS exposure affects maternal hormone levels, which may affect fetal growth differently depending on time of exposure [49-51]. Two studies investigating maternal urine BPF concentrations during pregnancy showed an inverse relationship with birth weight [32,52]. However, other studies reported no relationship between BPF exposure and birth weight [48,50,53,54]. Although fetal growth appears to be affected by BPF and BPS exposure, the data remain inconsistent.
2) Phthalates
Phthalates cross the placental barrier and impair placental growth and development [55]. Due to its immature metabolism, the fetus is more vulnerable to phthalate metabolite exposure during pregnancy. Higher phthalate levels may be present in pregnant women, especially through dietary changes occurring during pregnancy and the widespread use of body care products [56]. Exposure to phthalates during pregnancy reportedly increases the risk of adverse pregnancy outcomes and has deleterious effects on the offspring’s health, including during adulthood [57,58]. High phthalate concentrations in pregnant women may lead to adverse birth outcomes such as preterm birth, younger gestational age at birth, spontaneous abortion, a shorter anogenital distance, and a smaller birth size [59,60].
Various mechanisms have been proposed for the manner by which exposure to phthalates affects preterm birth [61]. One mechanism involves interference with placental function through trophoblast differentiation and placental steroidogenesis, which may increase the risk of preterm birth. This risk increases in individuals with certain genetic mutations through gene-environment interactions [62]. Evidence suggests that maternal exposure to phthalates can increase the levels of certain hormones. Another mechanism is that in utero exposure to phthalates causes epigenetic changes in the placenta, which may affect delivery time [63]. Phthalate exposure may lead to sex-specific differences by regulating peroxisome proliferator-activated receptor (PPAR) activity, which affects sex hormone metabolism and functions [64]. One proposed mechanism by which phthalates and bisphenols affect health is by affecting DNA methylation [65]. Studies examining the relationship between phthalate or bisphenol exposure during fetal life and DNA methylation in humans have reported inconsistent results. Candidate gene studies reported varying associations between fetal exposure to phthalates or BPA and DNA methylation of the IGF2 gene [66-70]. A systematic review and meta-analysis reported a positive association of prenatal phthalate exposure with preterm birth and a negative association with gestational age [71]. A recent meta-analysis of 59 studies suggested that maternal exposure to phthalates carries an increased risk of preterm birth [27,72,73].
Potential endocrine disruptors such as phthalates can interfere with hormone activity and affect birth outcomes [74,75]. Prenatal exposure to low-molecular-weight phthalate monoester metabolites is positively associated with gestational age and head circumference [76]. Exposure to mono-oxo-isonyl phthalate with high-molecular- weight phthalate monoesters reportedly reduces head circumference [77]. Maternal prenatal exposure to high- molecular-weight phthalates is associated with impaired fetal growth and birth size [78].
Exposure to phthalate metabolites such as bis (2-ethylhexyl) phthalate (DEHP), diethyl phthalate, dibutyl phthalate (DBP), butyl benzyl phthalate, di-isobutyl phthalate (DIBP), and di-isononyl phthalate may cause malfunction [61]. In a review, exposure to the most frequently studied phthalates, DEHP and its metabolites, was associated with reduced birth weight. Exposed pregnant women show a variety of changes reflecting a disruption in normal fetal growth with endocrine, placental, and epigenetic modifications and high oxidative stress, indicators of such deterioration [79]. The sex- and trimester- specific effects of DEHP exposure on fetal growth and birth outcomes have been demonstrated and confirmed in early childhood [80]. However, a recent systematic review and meta-analysis of 22 longitudinal and 17 cross- sectional studies showed that prenatal exposure to DEHP is associated with reduced body mass index (BMI) z scores in children [81].
The association of phthalates with birth weight appears to be metabolite-dependent. Especially in newborns with fetal growth retardation, mono (2-ethyl-5-hydroxyhexyl) phthalate and mono (2-ethyl-5-oxohexyl) phthalate urine concentrations are associated with IGF2 DNA methylation, the main regulator of placental and fetal growth [67,82].
3) Persistent organic pollutants
POPs can cross the placental barrier and enter the fetal bloodstream [83]. Some studies have reported that low- dose prenatal POP exposure can destroy the developing fetal endocrine and immune systems, eventually leading to irreversible birth defects such as intrauterine growth retardation [84-86]. Although their manufacture and use have long been banned, these substances are still widely distributed in the environment because of their persistence. Exposure to various POPs is associated with changes in gene methylation, including those of the IGF2 gene [82].
4) Polychlorinated biphenyls
PCBs can cross the placenta and adversely affect fetal development [87-89]. By disrupting hormonal balance, they can cause changes in the secondary sex ratio, increase the risk of preterm birth, cause major malformations, and change fetal growth [26]. In one study, different effects were observed according to the degree of chlorination, with low-chlorinated PCBs reportedly associated with lower luteinizing hormone and testosterone levels, lower gestational age, and smaller head circumference [90]. In addition, prenatal exposure to PCBs is associated with an increased risk of low birth weight [91,92], small-for-gestational-age status [93,94], and prolonged pregnancy [95,96]. High maternal blood PCB concentrations at the end of pregnancy are associated with reduced anogenital distances in male neonates [97].
5) Polybrominated diphenyl ethers
Chronic exposure to PBDEs in pregnant women can have potential adverse effects on the developing fetus. The presence of PBDEs in maternal and umbilical cord blood suggests that they are transported to the fetus via the placental interface [98-100].
The chemical structure of PBDEs is very similar to that of thyroid hormones; therefore, they are thought to act as thyroid disruptors and influence birth outcomes by affecting thyroid homeostasis. By mimicking thyroid hormones, PBDEs can disrupt the necessary roles of these hormones in fetal growth and development [101-103]. The thyroid hormones triiodothyronine (T3) and thyroxine (T4) play important roles in fetal growth and development during pregnancy; therefore, PBDE-induced thyroid disruption may have downstream effects on birth outcomes [101,104]. PBDE exposure is associated with placental epigenetic dysregulation, altered messenger ribonucleic acid expression, and metabolomic disruptions [105-107]. Birth outcomes are extremely important indicators of future adult health; therefore, adverse birth outcomes are associated with several adult diseases, including obesity, hypertension, heart disease, diabetes, and stroke [108-110].
Animal and human studies have demonstrated an association between prenatal PBDE exposure and adverse birth outcomes. PBDEs are generally associated with decreased birth weight, birth length, gestational age, birth weight z scores and head circumference [105,111-113].
6) Per- and polyfluoroalkyl substances
Higher PFAS concentrations are associated with an increased risk of low birth weight and preterm birth [114-116]. According to a recent meta-analysis, epidemiological evidence indicates that PFAS exposure during pregnancy is associated with adverse conditions such as preterm birth and small-for-gestational-age status. However, some researchers have reported that such exposure has none or an inverse relationship. These associations vary with outcomes and the specific PFAS studied. Because of the diversity of PFAS sources and pathways, PFAS exposure in pregnancy occurs globally but differs between countries [117,118].
The biological mechanisms by which PFAS may affect birth outcomes are largely unknown, but research has focused on potential mechanisms such as endocrine disruption [119], systemic inflammation [120], metabolic dysfunction [121], placental function [122], and epigenetic changes [123].
7) Organochlorine pesticides
Pesticide exposure is a risk factor for growth disorders in children living in agricultural areas [124]. Prenatal exposure to pesticides is associated with increased prematurity and preterm birth. Birth weight may be related to height and head circumference [125-127]. Conversely, the overall frequency of household pesticide exposure reportedly had no effect on body weight or height. However, significant associations exist between the use of fumigation insecticides and reduced body weight and between exposure to pyrethroid pesticides and the suppression of neonatal height growth, but this finding requires validation in other studies [128].
A recent systematic review found no consistent association between prenatal pesticide exposure and birth weight or height for any pesticide class. Prenatal exposure to organochlorine is reportedly associated with birth weight; however, the direction of this relationship remains unclear, with studies showing both positive and negative relationships. Additionally, there is no consistent evidence of an association between prenatal pesticide exposure, low birth weight, and preterm birth [129]. A meta-analysis reported that prenatal exposure to organophosphate pesticides was weakly associated with birth head circumference but not with birth weight or length [130]. Exposure to chlordecone, an OCP, was reportedly not associated with changes in birth weight [131].
In utero exposure to OCPs (dichlorodiphenyltrichloroeth ane [DDT], dichlorodiphenyldichloroethylene [DDE], and hexachlorobenzene [HCB]) may be associated with rapid weight gain in infancy [132,133] and later in childhood evidenced by a higher BMI [134,135]. Positive longitudinal associations have been reported between prenatal exposure to DDT and DDE in children and other obesity-related outcomes [136]. Prenatal DDE and DDT levels were significantly associated with increased newborn birth weight for both sexes. DDE exposure is positively associated with overweight status or a high BMI at 6, 12, or 14 months of age [132,133,137]. HCB exposure is significantly associated with increased newborn birth weight, especially in girls [138].
2. Neurodevelopmental outcomes
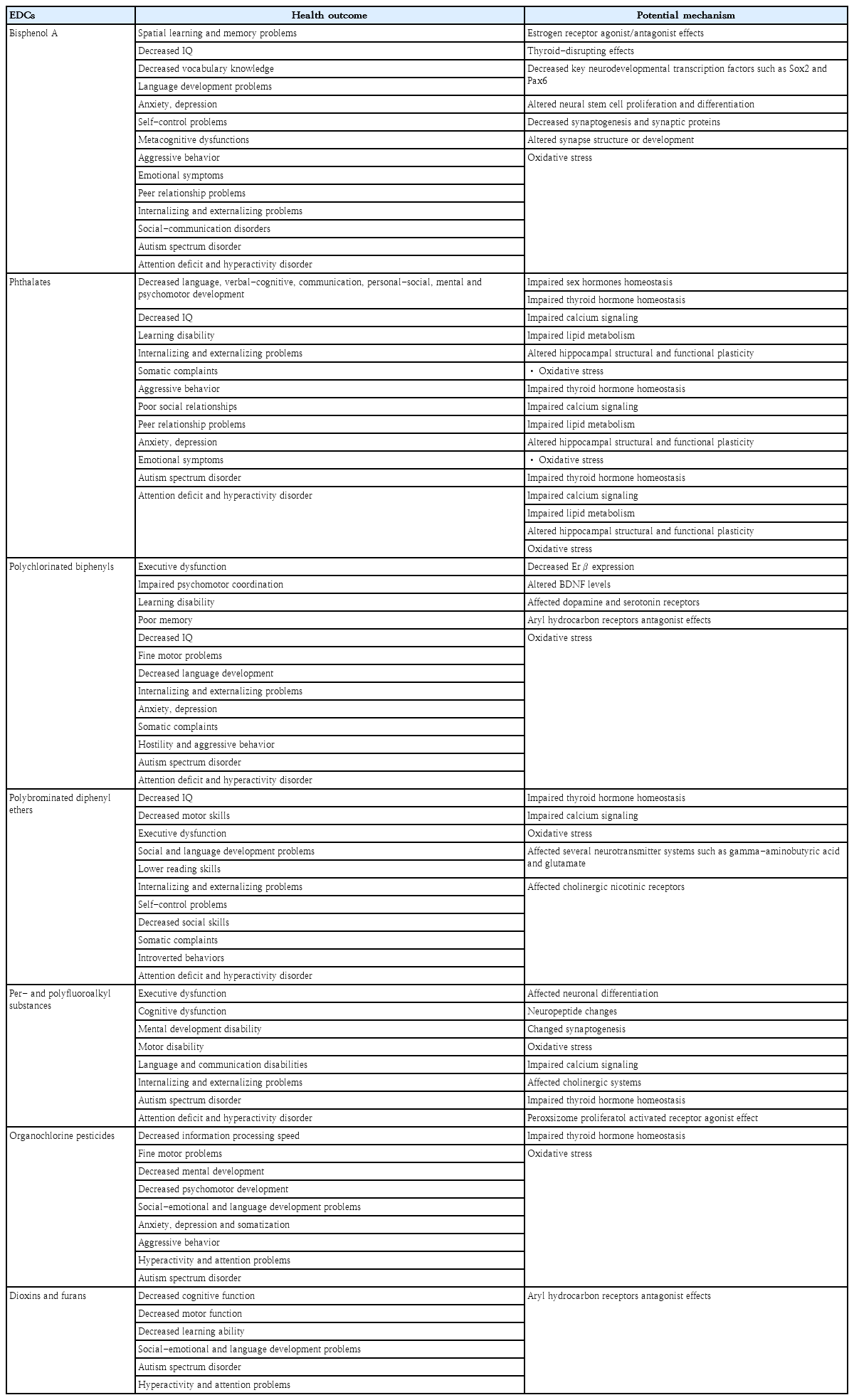
Owing to its complex structure, the brain is more sensitive to the negative effects of EDC exposure than other organs. The hypothalamus, cerebral cortex, and hippocampus, all of which are involved in neuroendocrine regulation, are the most vulnerable regions to EDCs [139]. Prenatal exposure to EDCs can affect fetal neurodevelopment, mainly through 2 different hormonal pathways. Until the second semester, the fetus is dependent on the transplacental transfer of maternal thyroid hormones. Maternal thyroid hormones play an important role in fetal brain development during the first trimester [140]. EDCs may affect the synthesis, bioavailability, function, and metabolism of thyroid hormones, resulting in neurodevelopmental problems in children [140,141]. The impaired action of thyroid and sex hormones can cause neurodevelopmental disorders [140]. Postnatal EDC exposure is also associated with neurodevelopmental and neurobehavioral problems in children; however, the mechanism underlying this effect remains unclear. EDCs exert neurotoxicity by interacting directly with nuclear hormone receptors such as estrogen, androgen, and thyroid. EDC exposure can also increase reactive oxygen species levels, oxidative stress, apoptosis, and epigenetic changes [139]. Pre- or postnatal EDC exposure can have lasting and lifelong neurodevelopmental outcomes including ASD, ADHD, and other cognitive and behavioral disorders [142]. However, the neurotoxic effects of EDCs are dependent on exposure type, duration, timing, frequency, and amount [139]. Table 3 shows the effects of EDCs on the nervous systems of children.
1) Bisphenol A
There is strong empirical evidence that pre and postnatal BPA exposure causes long-term behavioral changes in intelligence, language skills, depression, anxiety, sexual behavior, learning, and memory. An increasing number of studies have examined the relationship between pre and postnatal BPA exposure and neurodevelopment [13]. One study found that prenatal BPA exposure reduced intelligence quotient (IQ), verbal comprehension, and vocabulary [143]. In the Odense Child Cohort of mother-child pairs, higher prenatal BPA exposure was associated with lower vocabulary and language development scores [144].
There is strong evidence that BPA exposure during critical developmental windows leads to negative behaviors in children. Epidemiological studies have shown that pre- and postnatal BPA exposure may increase neurobehavioral problems such as anxiety, depression, self-control issues, metacognitive dysfunction, aggression, emotional dysregulation, impaired peer relationships, social-communication disorders, and internalizing and externalizing problems in children [140,143,145-151]. Although BPA exposure has potential adverse effects on children’s intelligence and behavioral development, the results of these studies differed according to child sex and age [143].
Previous studies reported epidemiological evidence of an association between maternal BPA exposure and ASD [152,153]. The fact that urine BPA levels are higher in children with autism than in healthy subjects supports these findings [154]. In addition, BPA exposure may play a role in the development of ADHD by affecting the catecholaminergic and serotonergic systems. A meta- analysis of animal and human studies noted that early pre- and postnatal BPA exposure was significantly associated with increased hyperactivity [155]. Case-controlled, cross- sectional, and longitudinal studies have suggested that ADHD symptoms are associated with pre and postnatal BPA exposure [149,150,156-159].
BPA exposure may affect child neurodevelopment by several mechanisms. Gonadal hormones play important roles in neurodevelopment. BPA is a structural analog of estrogen that binds to estrogen receptors (ERs), thereby affecting the balance of sex hormones. Therefore, the effects of BPA on neurodevelopment in children may be attributed to its endocrine-disrupting effects [160]. Moreover, BPA affects thyroid function by binding to thyroid receptors and subsequently disrupting thyroid hormones, which are essential for brain development in children [160]. Exposure to BPA during critical periods of development initiates a series of neurodevelopmental processes that permanently change the developing brain.
BPA can downregulate key neurodevelopmental transcription factors such as Sox2 and Pax6 that mediate neural stem cell activation and brain development. It may also affect neurogenesis by altering neural stem cell proliferation and differentiation. The cerebellum, hypothalamus, and hippocampus may also be susceptible to the neurotoxic effects of BPA. Permanent changes in children’s behavior are expected, a finding that is consistent with the impact of BPA on these brain regions [161]. Other mechanisms include reduction in synaptogenesis and synaptic protein expression, alterations in structural plasticity, and increased inflammation and oxidative stress [143,162]. Thus, BPA exposure can interfere with normal brain development as well as cognitive and behavioral functions.
2) Phthalates
Phthalates may cross the blood-placental barrier and adversely affect fetal brain development. Ongoing phthalate exposure during infancy and early childhood may contribute to poor long-term neurodevelopmental outcomes [163]. Numerous studies have suggested that phthalate exposure during these sensitive periods may be associated with altered behavioral, cognitive, and psychomotor outcomes [164-167]. Prenatal phthalate exposure can impair cognitive, social, motor, and emotional development [168]. Studies conducted in different countries have shown that prenatal DEHP, DBP, monobutyl phthalate, dibutyl phthalate, DIBP, and butyl benzyl phthalate exposure is associated with decreased language, verbal, communication, personal-social, cognitive, and psychomotor development; lower IQ; and learning difficulties during childhood [143,169-173].
A recent meta-analysis demonstrated a significant association between prenatal phthalate exposure and psychomotor outcomes in children [164]. However, some authors have highlighted the importance of altered sex-specific differences in infant and child neurodevelopment [166,174]. Prenatal phthalate exposure reportedly contributes to increased internalization and externalization problems, somatic complaints, aggressive behaviors, poor social relationships, peer relationship problems, anxiety, depression, and emotional symptoms in children [13,142,143,159]. In addition, the relationship between prenatal and early childhood phthalate exposure and children's behavior varies by sex [158].
Early phthalate exposure may affect developmental and behavioral outcomes in children; however, the results of these studies varied in terms of phthalate metabolites, affected brain areas, and sex [143]. Prenatal phthalate exposure may be associated with ASD, ADHD, and other specific behavioral problems [164,165,175,176]. In a population- based birth cohort of 1,064 women in Australia, elevated urine phthalate metabolite levels at 36 weeks’ gestation were associated with childhood autism [175]. A study of Norwegian mother-child pairs showed that prenatal phthalate exposure was correlated with the risk of ADHD during infancy [176].
Although the biological mechanisms underlying the role of phthalates in neurodevelopment remain unclear, several studies have proposed possible mechanisms. Phthalates can impair the homeostasis of sex hormones, thyroid hormones, calcium signaling, and lipid metabolism. Given the role of steroids and thyroid hormones in the brain and synaptic development, it is not surprising that early phthalate exposure affects children’s cognitive development and social competence [177]. Sex hormones are essential for neurodevelopment. Progesterone is essential for neurosteroid production, whereas estrogen plays important roles in brain development and neuroprotection. Androgens have crucial biological effects on early prenatal brain development and social cognition. Estrogens can affect neuroplasticity and neurogenesis in the hippocampus by binding to ERs and activating cell signaling pathways. Phthalate exposure adversely affects neurodevelopment by disrupting sex hormone homeostasis [178]. Phthalates are thought to primarily affect neurodevelopment by disrupting thyroid hormone homeostasis, which is crucial for fetal and infant brain development; thus, it can ultimately affect children’s cognitive and motor abilities [163].
At the same time, phthalates can reduce dopamine release by disrupting the D2 dopamine receptor, tyrosine hydroxylase, and calcium-dependent neurotransmitter homeostasis. Nicotinic acetylcholine receptor– mediated calcium signaling participates in various neurodevelopmental processes. Phthalate metabolites may interfere with calcium signaling coupled with nicotinic acetylcholine receptors [143]. Phthalates may impair functional plasticity within the hippocampus, which plays an important role in learning and memory [163]. Animal studies have associated phthalate exposure with increased lipid peroxidation, which can lead to motor neuron apoptosis and other brain disorders. The last mechanism may be that phthalate exposure affects the child’s neurodevelopment by causing oxidative stress [173].
3) Polychlorinated biphenyls
PCBs have long been associated with neurological disorders [179]. Low-dose PCB exposure during critical periods has adverse effects on behavioral, physiological, neurobiological, and cognitive regulation [13]. A comprehensive summary of studies published in 1990–2018 stated that numerous epidemiological studies revealed negative associations between PCB exposure and childhood neurodevelopment [180]. It was concluded that PCB exposure during critical developmental periods led to impaired executive function and psychomotor coordination; learning disabilities; poor memory; low IQ; problems with fine motor skills such as attention, memory, and writing; and decreased language development [179-182]. A close negative relationship was found between intrauterine PCB exposure and verbal and memory scores in 4-year-old children [183]. Another study found a 3-point decrease in full-scale IQ and a 4-point decrease in verbal IQ for each increase in placental PCB concentration of 1 ng/g (wet weight) [184]. Epidemiological studies reported that PCBs have adverse effects on internalizing behaviors, such as anxiety, somatization, and depression, as well as externalizing behaviors, such as hostility and antisocial tendencies [185,186]. PCB exposure has adverse effects on children’s psychomotor, learning, memory, and neurobehavioral functions [187]. Additionally, current studies confirmed the association between developmental PCB exposure and ASD and ADHD [185,188-191]. In one study, cord serum PCB-153 levels were significantly associated with increased ADHD behaviors in 8-year-old children [192].
The mechanisms through which PCBs exert adverse effects on the brain remain unclear [139]. The hypothalamus appears to be an area of nervous system vulnerability to PCBs. PCB exposure during the prenatal period may decrease ERβ expression in the anteroventral periventricular nucleus. It can also alter the expression of brain-derived neurotrophic factor (BDNF) genes in the preoptic region of the hypothalamus in a sex- specific manner, suggesting that prenatal PCB exposure masculinizes the brain. Decreased BDNF expression, which regulates sensory neuron development, may be the underlying mechanism of neurobehavioral disorders [161]. PCBs may affect dopaminergic and serotonergic receptors, bind to aryl hydrocarbon receptors, interrupt neuroimmune function, and increase cytokine production [139].
4) Polybrominated diphenyl ethers
Exposure to PBDEs before and after birth may adversely affect children’s cognitive development and motor function. Prenatal PBDE exposure may adversely affect cognitive functions associated with a lower IQ [179,193-198], reduced motor skills [194], poorer executive function [199,200], lower social and language development [201], and lower reading and attention skills [194, 197,202-205]. One study found that a 10-fold increase in the sum of 4 major PBDE congeners (BDE-47, -99, -100, and -153) in maternal serum at 16±3 weeks’ gestation was associated with a 6.2-point decrease in reading scores in 8-year-old children [197]. Similarly, Chao et al. [206] and Gascon et al. [207] reported that postnatal exposure to PBDEs, particularly BDE-209, via breast milk potentially delays the neurological and mental development of breastfed infants aged 0–18 months. Tsai et al. [208] also found that BDE-209 levels in breast milk were significantly negatively correlated with cognitive levels in 8- to 12-month-old infants.
Pre- and postnatal PBDE exposure is associated with externalizing problems, impaired self-control, and reduced social skills in childhood [201,202,205,207]. However, the Shanghai-Minhang Birth Cohort Study showed that prenatal PBDE exposure was associated with somatic complaints, introversion, and internalization problems in girls and somatic complaints and attention problems in boys [209]. These results indicate that the effects of PBDE exposure on neurobehavioral outcomes may differ by sex. A South Korean study reported that mothers exposed to higher PBDE levels had higher scores on all ADHD scales [210]. A study conducted in Norway found a relationship between different types of PBDEs in breast milk and ADHD [211].
The mechanisms underlying the neurotoxic effects of PBDEs are not fully understood, although it is clear that pre and postnatal PBDE exposure affects children’s neurodevelopment. Disruption of thyroid homeostasis is a suggested potential mechanism [204]. Thyroid hormones are involved in myelination, cerebellar development, glial cell proliferation, neuronal differentiation, and synapse formation [139]. As PBDEs have a chemical structure similar to that of T4, they may bind to thyroid hormone transport proteins and receptors. Therefore, PBDEs can reduce circulating T4 and T3 levels [102]. Abnormalities in thyroid hormone levels may be responsible for impaired neurodevelopment [139]. Another potential mechanism involves the direct effects of PBDEs on the brain. PBDEs can directly affect brain cells by inducing alterations in cellular migration and differentiation into neurons and oligodendrocytes, interfering with calcium signaling and protein kinase C pathways in neurons, and causing oxidative stress [204]. In addition, PBDE exposure may affect the gamma-aminobutyric acid (GABA) and glutamatergic neurotransmitter systems in the frontal cortex and cholinergic nicotinic receptors in the hippocampus [102]. Since GABA is an important neurotransmitter in the brain, its changes can interrupt neuronal activity, causing deficits in various cognitive processes such as hyperactivity and impulsivity, aggressive behaviors, impaired social behaviors, and low academic skills [139].
5) Per- and polyfluoroalkyl substances
Evidence from animal and human studies suggests that PFAS exposure during early life may have short- and long-term neurodevelopmental effects [212]. Epidemiological studies reported that early life exposure to PFAS is associated with neurodevelopmental disorders such as executive dysfunction, cognitive dysfunction, motor disabilities, and language and communication impairments in children [200,212-216]. Various studies reported that prenatal PFAS exposure is associated with attention deficits, hyperactivity, impulsivity, and altered externalizing and internalizing behaviors [217,218]. Several hypothetical scenarios suggested weak inverse associations between prenatal PFAS exposure and ADHD in school-aged children [217,219]. Epidemiological studies reported null [220], positive [221,222], or negative [223] associations between ASD and PFAS exposure.
Several mechanisms have been proposed by which early life exposure to PFAS and their mixtures may adversely affect childhood neurodevelopment. PFAS affect neuronal differentiation and can alter neuroprotein levels in the hippocampus and cerebral cortex, which are important for normal brain development [224]. Some toxicological studies suggested that early PFAS exposure may lead to neurotoxic effects by altering synaptogenesis, cell death, and reactive oxygen species generation [225]. Possible mechanisms include the impaired expression of calcium-related signaling molecules in the hippocampus, changes in the cholinergic system, and the disruption of thyroid homeostasis [214]. The neuroprotective effects of PFAS can also be explained by the hypothesis that PFAS appears to be a partial agonist of PPARγ, agonists of which effectively attenuate oxidative stress, inflammation, and apoptosis in the central nervous system [225].
6) Organochlorine pesticides
A growing body of evidence demonstrates a positive association between pre- and postnatal OCP exposure and neurodevelopmental disorders [226]. DDT and DDE in particular cause neurodevelopmental toxicity by crossing the placental barrier and contaminating the breast milk [226,227]. The Center for the Health Assessment of Mothers and Children of Salinas study showed that increased prenatal levels of DDT and its breakdown product, DDE, affected the neurodevelopmental performance of children aged 1–2 years. Higher levels of prenatal DDT exposure in particular are negatively associated with information processing speed, a possible risk factor for attention and learning problems in children [228]. The fine motor development of boys in Guadeloupe exposed to chlordecone, a permanent OCP formerly used in banana plantations, in utero was adversely affected [229]. Eskenazi et al. [194] reported that prenatal exposure to DDT and DDE was associated with decreased cognitive development in 12- to 24-month-old infants and impaired psychomotor development in 6- to 12-month-old infants. A study of 55 Taiwanese mother-infant pairs found that higher levels of DDT in breast milk were significantly associated with lower performance of 8- to 12-month-old infants in the cognitive, language, and social-emotional domains [230].
Prenatal pesticide exposure has also been associated with anxiety, depression, somatization, aggression, hyperactivity, and behavioral problems [140]. One study reported that DDE exposure negatively affected infant attention skills within the first 5 days after birth [231]. Additionally, prenatal exposure to OCPs such as DDT and DDE has been associated with developmental delays and a greater risk of ASD onset [229]. A Finnish prenatal study of autism and ASD determined that high maternal serum DDE levels correlated with ASD symptoms in infants [232]. Roberts et al. [233] reported an increased risk of ASD in infants whose mothers were exposed to DDT during the first trimester of pregnancy. The most well-known mechanism involves the effects of OCPs on thyroid hormones [234]. OCPs can also cause oxidative stress and DNA damage, resulting in long-term effects on neurodevelopment [235].
7) Dioxins and furans
The adverse health effects of dioxin exposure in children may include negative neurological outcomes [236]. Epidemiological studies reported that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure was associated with subtle neurodevelopmental problems such as decreased cognition, motor, attention, social-emotional, learning, and language skills [182,237-239]. Autistic traits and poor cognitive and motor development have been observed in Vietnamese infants born in dioxin-contaminated areas after the wartime use of Agent Orange [240]. One study observed increased ADHD symptoms in children perinatally exposed to more TCDD [241]. Dioxins have been suggested to affect neurodevelopment via aryl hydrocarbon receptor–mediated signaling pathways [242].
Conclusions
This review examined the reported findings of birth and neurodevelopmental disorders following pre and postnatal exposure to 3 main classes of EDCs: BPA, phthalates, and POPs (PCB, PBDE, PFAS, OCP, dioxins, and furans). Humans are constantly exposed to EDCs through the air, nutrients, and water. By interfering with endocrine and neurological patterns, EDCs adversely affect general human health, particularly that of children. Humans are highly sensitive to EDC exposure during the intrauterine and early postpartum periods. Such exposures affect birth outcomes through various mechanisms. Birth outcomes such as fetal growth restriction, preterm birth, and low birth weight have been associated with EDC exposure, but results from epidemiological studies are conflicting. EDC exposure can also cause brain reprogramming by affecting behaviors in other areas, such as epigenetics. The time of exposure is an important factor in determining potential neurodevelopmental outcomes. Previous studies suggested that postnatal EDC exposure is associated with adverse neurobehavioral outcomes in children. Although possible mechanisms of action have been mentioned, the exact mechanisms are yet to be elucidated. Precautions should be taken to control, prevent, and limit contact with EDCs, especially since infants are at greater risk for changes in programming and/or malformations during the first 1,000 days of life.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.