Article Contents
| Clin Exp Pediatr > Volume 67(7); 2024 |
|
Abstract
Endocrine-disrupting chemicals (EDCs) are natural or synthetic chemicals that mimic, block, or interfere with the hormones in the body. The most common and well- studied EDCs are bisphenol A, phthalates, and persistent organic pollutants including polychlorinated biphenyls, polybrominated diphenyl ethers, per- and polyfluoroalkyl substances, other brominated flame retardants, organochlorine pesticides, dioxins, and furans. Starting in embryonic life, humans are constantly exposed to EDCs through air, diet, skin, and water. Fetuses and newborns undergo crucial developmental processes that allow adaptation to the environment throughout life. As developing organisms, they are extremely sensitive to low doses of EDCs. Many EDCs can cross the placental barrier and reach the developing fetal organs. In addition, newborns can be exposed to EDCs through breastfeeding or formula feeding. Pre- and postnatal exposure to EDCs may increase the risk of childhood diseases by disrupting the hormone-mediated processes critical for growth and development during gestation and infancy. This review discusses evidence of the relationship between pre- and postnatal exposure to several EDCs, childbirth, and neurodevelopmental outcomes. Available evidence suggests that pre- and postnatal exposure to certain EDCs causes fetal growth restriction, preterm birth, low birth weight, and neurodevelopmental problems through various mechanisms of action. Given the adverse effects of EDCs on child development, further studies are required to clarify the overall associations.
Graphical abstract. Association between pre- and postnatal exposure to endocrine-disrupting chemicals and birth and neurodevelopmental outcomes
A functional endocrine system is required to coordinate the actions of the hormones that regulate the body’s physiological and behavioral activities [1]. Hormones produced by the endocrine glands are transported to target cells to regulate body development, growth, reproduction, metabolism, immunity, and behavior by binding to cellular receptors [2]. However, some environmental chemicals, termed endocrine-disrupting chemicals (EDCs) [3], directly interfere with the production, release, binding, transport, and elimination of hormones in the body, thereby altering their effects on target cells [4].
The most extensively studied EDCs include bisphenol A (BPA) and phthalate, which are plastics and plasticizers. Other common EDCs are persistent organic pollutants (POPs) [4]. BPA is an organic chemical used in the manufacture of epoxy resins, polycarbonates, and polyvinyl chloride plastics [5]. It is widely used in plastic bottles, feeding bottles, plastic kitchenware, electronic materials, paints, thermal papers, medical and dental materials, and the inner surface coatings of food and beverage cans [6]. Phthalates are chemicals used in medical and building materials, toys, personal care products, plastic kitchenware, and food packaging [7]. POPs, which are pesticides, industrial chemicals, and byproducts of industrial processes, are organic chemicals that can persist longer in the environment [8]. These include polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), per- and polyfluoroalkyl substances (PFAS), other brominated flame retardants, organochlorine pesticides (OCPs), dioxins, furans, and others [9]. EDCs can enter the human body through ingestion, inhalation, and dermal absorption via leakage into the environment, food, and consumer products [4]. The sources of exposure to common EDCs in humans are listed in Table 1.
The incidence rates of noncommunicable diseases, particularly birth defects, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), asthma, obesity, diabetes, and childhood cancers, have increased over the last 30 years. There is a growing concern that there is a strong association between childhood diseases and exposure to industrial chemicals and other environmental toxins acting as EDC [10]. Pre- and postnatal exposure to EDCs can have profound effects on health during infancy, childhood, and adulthood by causing irreversible changes in differentiated tissues [11]. Phthalates, phenols, perfluorinated compounds, flame retardants, PCBs, and OCPs can cross the placental barrier and reach the developing fetal organs [11,12]. In addition, newborns may be exposed to EDCs through breastfeeding and formula feeding as well as inhalation and dermal absorption [11]. The early life stages from fertilization to 2 years of age are critical developmental windows characterized by the maturation and epigenetic programming of neuronal, metabolic, and immune pathways as well as endocrine, reproductive, and other systems [4]. However, sensitivity to EDCs increases during such critical periods (embryo, fetus, and neonate) because of rapid cellular proliferation/differentiation, immature metabolism, and inadequate detoxification mechanisms [13]. Many EDCs accumulate in the adipose tissues because of their lipid solubility; therefore, the long-term effects of pre and postnatal exposure are observed in later years [14]. Thus, this review discusses the evidence linking pre and postnatal exposure to several EDCs to various childbirth and neurodevelopmental outcomes.
We searched for publications published between 2018 and 2023 in PubMed, Google Scholar, and Science Direct using some keyword combinations. The following terms were used to describe exposure: prenatal exposure, postnatal exposure, EDCs, infant, newborn, neonate, pregnancy, BPA, phthalates, POPs, PCBs, PBDEs, PFAS, OCPs, dioxins, and furans. These terms were matched with the following keywords describing birth and neurodevelopmental outcomes: birth outcomes, fetal growth, birth weight, birth length, birth size, neurodevelopment, neurotoxicity, neurobehavior, mental development, cognitive develop- ment, psychomotor development, language development, ASD, attention deficit, and hyperactivity disorder.
The placenta is considered both a filter for the passage of EDCs and an endocrine organ acting as a conduit between the mother and fetus to maintain fetal homeostasis. EDCs affect pregnancy as direct hormonal agonists/antagonists affecting endocrine functions as well as indirectly by disrupting maternal, placental, and fetal homeostasis [15,16]. Environmental exposure to EDCs impairs placental function [17,18]. The placental barrier is not completely impermeable to harmful substances; therefore, exposure to environmental triggers can permanently reprogram normal physiological responses that affect both intrauterine and postpartum life [17]. Prenatal exposure to EDCs can result in epigenetic changes that alter fetal programming and increase the risk of certain noncommunicable diseases in postnatal life as suggested by the Developmental Origins of Health and Disease hypothesis [19].
Prenatal exposure to EDCs leads to preterm birth, fetal intrauterine growth retardation, changes in birth weight and size, small-for-gestational-age status, large-for- gestational-age status, older gestational age, macrosomia, and congenital disorders, all of which may have negative consequences [20-23]. EDCs can interfere with the insulin- like growth factor (IGF) system that is a critical growth regulator in fetal development [24].
The IGF system represents a particularly critical growth regulator for fetal development, and EDCs can interfere with this system [25]. Correlations between in utero exposure to EDCs and birth outcomes have been reported in epidemiological studies, but results are conflicting [26,27]. Table 2 shows the effects of EDCs on birth outcomes.
BPA is found in human plasma, urine, amniotic fluid, follicular fluid, placental tissue, breast milk, umbilical cord blood, and adipose tissue [28-30]. In utero exposure to BPA can affect fetal growth through multiple hormone-mediated mechanisms by mimicking estrogen, inhibiting androgen production, altering thyroid signaling, and inducing oxidative stress [31-33]. A high amount of BPA in trophoblast cells during the first trimester of pregnancy reportedly inhibits cellular growth and affects deoxyribonucleic acid (DNA) methylation [34]. However, the effect of prenatal exposure to BPA on postnatal growth remains unclear [35].
BPA is transported through the placenta and affects placental growth by increasing beta human chorionic gonadotropin levels [36,37]. BPA can easily cross the placenta and is associated with preterm birth [38]. At the same time, prenatal exposure to BPA may cause disease onset in childhood and adulthood by changing fetal epigenetic programming [39]. Combined prenatal exposure to BPA from dietary and nondietary sources (especially when the first-half exposure occurs) may contribute to fetal growth restriction [40-42].
Although in utero exposure to BPA may adversely affect placental development and function and lead to inadequate fetal growth and adverse birth outcomes, some meta-analyses reported no association between fetal BPA exposure and birth weight, height, or head circumference [43-47]. No association between fetal exposure to BPA and gestational age at birth was also reported [46,47].
Due to concerns about the potential negative effects of BPA, it is increasingly being replaced by other bisphenols, such as bisphenol S (BPS) and bisphenol F (BPF). However, whether these bisphenols have fewer adverse health effects than BPA remains unclear [48]. Higher maternal BPS concentrations, particularly in the first trimester, are reportedly associated with greater fetal head circumference and weight, suggesting that BPS exposure enhances fetal growth. In utero BPS exposure affects maternal hormone levels, which may affect fetal growth differently depending on time of exposure [49-51]. Two studies investigating maternal urine BPF concentrations during pregnancy showed an inverse relationship with birth weight [32,52]. However, other studies reported no relationship between BPF exposure and birth weight [48,50,53,54]. Although fetal growth appears to be affected by BPF and BPS exposure, the data remain inconsistent.
Phthalates cross the placental barrier and impair placental growth and development [55]. Due to its immature metabolism, the fetus is more vulnerable to phthalate metabolite exposure during pregnancy. Higher phthalate levels may be present in pregnant women, especially through dietary changes occurring during pregnancy and the widespread use of body care products [56]. Exposure to phthalates during pregnancy reportedly increases the risk of adverse pregnancy outcomes and has deleterious effects on the offspring’s health, including during adulthood [57,58]. High phthalate concentrations in pregnant women may lead to adverse birth outcomes such as preterm birth, younger gestational age at birth, spontaneous abortion, a shorter anogenital distance, and a smaller birth size [59,60].
Various mechanisms have been proposed for the manner by which exposure to phthalates affects preterm birth [61]. One mechanism involves interference with placental function through trophoblast differentiation and placental steroidogenesis, which may increase the risk of preterm birth. This risk increases in individuals with certain genetic mutations through gene-environment interactions [62]. Evidence suggests that maternal exposure to phthalates can increase the levels of certain hormones. Another mechanism is that in utero exposure to phthalates causes epigenetic changes in the placenta, which may affect delivery time [63]. Phthalate exposure may lead to sex-specific differences by regulating peroxisome proliferator-activated receptor (PPAR) activity, which affects sex hormone metabolism and functions [64]. One proposed mechanism by which phthalates and bisphenols affect health is by affecting DNA methylation [65]. Studies examining the relationship between phthalate or bisphenol exposure during fetal life and DNA methylation in humans have reported inconsistent results. Candidate gene studies reported varying associations between fetal exposure to phthalates or BPA and DNA methylation of the IGF2 gene [66-70]. A systematic review and meta-analysis reported a positive association of prenatal phthalate exposure with preterm birth and a negative association with gestational age [71]. A recent meta-analysis of 59 studies suggested that maternal exposure to phthalates carries an increased risk of preterm birth [27,72,73].
Potential endocrine disruptors such as phthalates can interfere with hormone activity and affect birth outcomes [74,75]. Prenatal exposure to low-molecular-weight phthalate monoester metabolites is positively associated with gestational age and head circumference [76]. Exposure to mono-oxo-isonyl phthalate with high-molecular- weight phthalate monoesters reportedly reduces head circumference [77]. Maternal prenatal exposure to high- molecular-weight phthalates is associated with impaired fetal growth and birth size [78].
Exposure to phthalate metabolites such as bis (2-ethylhexyl) phthalate (DEHP), diethyl phthalate, dibutyl phthalate (DBP), butyl benzyl phthalate, di-isobutyl phthalate (DIBP), and di-isononyl phthalate may cause malfunction [61]. In a review, exposure to the most frequently studied phthalates, DEHP and its metabolites, was associated with reduced birth weight. Exposed pregnant women show a variety of changes reflecting a disruption in normal fetal growth with endocrine, placental, and epigenetic modifications and high oxidative stress, indicators of such deterioration [79]. The sex- and trimester- specific effects of DEHP exposure on fetal growth and birth outcomes have been demonstrated and confirmed in early childhood [80]. However, a recent systematic review and meta-analysis of 22 longitudinal and 17 cross- sectional studies showed that prenatal exposure to DEHP is associated with reduced body mass index (BMI) z scores in children [81].
The association of phthalates with birth weight appears to be metabolite-dependent. Especially in newborns with fetal growth retardation, mono (2-ethyl-5-hydroxyhexyl) phthalate and mono (2-ethyl-5-oxohexyl) phthalate urine concentrations are associated with IGF2 DNA methylation, the main regulator of placental and fetal growth [67,82].
POPs can cross the placental barrier and enter the fetal bloodstream [83]. Some studies have reported that low- dose prenatal POP exposure can destroy the developing fetal endocrine and immune systems, eventually leading to irreversible birth defects such as intrauterine growth retardation [84-86]. Although their manufacture and use have long been banned, these substances are still widely distributed in the environment because of their persistence. Exposure to various POPs is associated with changes in gene methylation, including those of the IGF2 gene [82].
PCBs can cross the placenta and adversely affect fetal development [87-89]. By disrupting hormonal balance, they can cause changes in the secondary sex ratio, increase the risk of preterm birth, cause major malformations, and change fetal growth [26]. In one study, different effects were observed according to the degree of chlorination, with low-chlorinated PCBs reportedly associated with lower luteinizing hormone and testosterone levels, lower gestational age, and smaller head circumference [90]. In addition, prenatal exposure to PCBs is associated with an increased risk of low birth weight [91,92], small-for-gestational-age status [93,94], and prolonged pregnancy [95,96]. High maternal blood PCB concentrations at the end of pregnancy are associated with reduced anogenital distances in male neonates [97].
Chronic exposure to PBDEs in pregnant women can have potential adverse effects on the developing fetus. The presence of PBDEs in maternal and umbilical cord blood suggests that they are transported to the fetus via the placental interface [98-100].
The chemical structure of PBDEs is very similar to that of thyroid hormones; therefore, they are thought to act as thyroid disruptors and influence birth outcomes by affecting thyroid homeostasis. By mimicking thyroid hormones, PBDEs can disrupt the necessary roles of these hormones in fetal growth and development [101-103]. The thyroid hormones triiodothyronine (T3) and thyroxine (T4) play important roles in fetal growth and development during pregnancy; therefore, PBDE-induced thyroid disruption may have downstream effects on birth outcomes [101,104]. PBDE exposure is associated with placental epigenetic dysregulation, altered messenger ribonucleic acid expression, and metabolomic disruptions [105-107]. Birth outcomes are extremely important indicators of future adult health; therefore, adverse birth outcomes are associated with several adult diseases, including obesity, hypertension, heart disease, diabetes, and stroke [108-110].
Higher PFAS concentrations are associated with an increased risk of low birth weight and preterm birth [114-116]. According to a recent meta-analysis, epidemiological evidence indicates that PFAS exposure during pregnancy is associated with adverse conditions such as preterm birth and small-for-gestational-age status. However, some researchers have reported that such exposure has none or an inverse relationship. These associations vary with outcomes and the specific PFAS studied. Because of the diversity of PFAS sources and pathways, PFAS exposure in pregnancy occurs globally but differs between countries [117,118].
Pesticide exposure is a risk factor for growth disorders in children living in agricultural areas [124]. Prenatal exposure to pesticides is associated with increased prematurity and preterm birth. Birth weight may be related to height and head circumference [125-127]. Conversely, the overall frequency of household pesticide exposure reportedly had no effect on body weight or height. However, significant associations exist between the use of fumigation insecticides and reduced body weight and between exposure to pyrethroid pesticides and the suppression of neonatal height growth, but this finding requires validation in other studies [128].
A recent systematic review found no consistent association between prenatal pesticide exposure and birth weight or height for any pesticide class. Prenatal exposure to organochlorine is reportedly associated with birth weight; however, the direction of this relationship remains unclear, with studies showing both positive and negative relationships. Additionally, there is no consistent evidence of an association between prenatal pesticide exposure, low birth weight, and preterm birth [129]. A meta-analysis reported that prenatal exposure to organophosphate pesticides was weakly associated with birth head circumference but not with birth weight or length [130]. Exposure to chlordecone, an OCP, was reportedly not associated with changes in birth weight [131].
In utero exposure to OCPs (dichlorodiphenyltrichloroeth ane [DDT], dichlorodiphenyldichloroethylene [DDE], and hexachlorobenzene [HCB]) may be associated with rapid weight gain in infancy [132,133] and later in childhood evidenced by a higher BMI [134,135]. Positive longitudinal associations have been reported between prenatal exposure to DDT and DDE in children and other obesity-related outcomes [136]. Prenatal DDE and DDT levels were significantly associated with increased newborn birth weight for both sexes. DDE exposure is positively associated with overweight status or a high BMI at 6, 12, or 14 months of age [132,133,137]. HCB exposure is significantly associated with increased newborn birth weight, especially in girls [138].
Owing to its complex structure, the brain is more sensitive to the negative effects of EDC exposure than other organs. The hypothalamus, cerebral cortex, and hippocampus, all of which are involved in neuroendocrine regulation, are the most vulnerable regions to EDCs [139]. Prenatal exposure to EDCs can affect fetal neurodevelopment, mainly through 2 different hormonal pathways. Until the second semester, the fetus is dependent on the transplacental transfer of maternal thyroid hormones. Maternal thyroid hormones play an important role in fetal brain development during the first trimester [140]. EDCs may affect the synthesis, bioavailability, function, and metabolism of thyroid hormones, resulting in neurodevelopmental problems in children [140,141]. The impaired action of thyroid and sex hormones can cause neurodevelopmental disorders [140]. Postnatal EDC exposure is also associated with neurodevelopmental and neurobehavioral problems in children; however, the mechanism underlying this effect remains unclear. EDCs exert neurotoxicity by interacting directly with nuclear hormone receptors such as estrogen, androgen, and thyroid. EDC exposure can also increase reactive oxygen species levels, oxidative stress, apoptosis, and epigenetic changes [139]. Pre- or postnatal EDC exposure can have lasting and lifelong neurodevelopmental outcomes including ASD, ADHD, and other cognitive and behavioral disorders [142]. However, the neurotoxic effects of EDCs are dependent on exposure type, duration, timing, frequency, and amount [139]. Table 3 shows the effects of EDCs on the nervous systems of children.
There is strong empirical evidence that pre and postnatal BPA exposure causes long-term behavioral changes in intelligence, language skills, depression, anxiety, sexual behavior, learning, and memory. An increasing number of studies have examined the relationship between pre and postnatal BPA exposure and neurodevelopment [13]. One study found that prenatal BPA exposure reduced intelligence quotient (IQ), verbal comprehension, and vocabulary [143]. In the Odense Child Cohort of mother-child pairs, higher prenatal BPA exposure was associated with lower vocabulary and language development scores [144].
There is strong evidence that BPA exposure during critical developmental windows leads to negative behaviors in children. Epidemiological studies have shown that pre- and postnatal BPA exposure may increase neurobehavioral problems such as anxiety, depression, self-control issues, metacognitive dysfunction, aggression, emotional dysregulation, impaired peer relationships, social-communication disorders, and internalizing and externalizing problems in children [140,143,145-151]. Although BPA exposure has potential adverse effects on children’s intelligence and behavioral development, the results of these studies differed according to child sex and age [143].
Previous studies reported epidemiological evidence of an association between maternal BPA exposure and ASD [152,153]. The fact that urine BPA levels are higher in children with autism than in healthy subjects supports these findings [154]. In addition, BPA exposure may play a role in the development of ADHD by affecting the catecholaminergic and serotonergic systems. A meta- analysis of animal and human studies noted that early pre- and postnatal BPA exposure was significantly associated with increased hyperactivity [155]. Case-controlled, cross- sectional, and longitudinal studies have suggested that ADHD symptoms are associated with pre and postnatal BPA exposure [149,150,156-159].
BPA exposure may affect child neurodevelopment by several mechanisms. Gonadal hormones play important roles in neurodevelopment. BPA is a structural analog of estrogen that binds to estrogen receptors (ERs), thereby affecting the balance of sex hormones. Therefore, the effects of BPA on neurodevelopment in children may be attributed to its endocrine-disrupting effects [160]. Moreover, BPA affects thyroid function by binding to thyroid receptors and subsequently disrupting thyroid hormones, which are essential for brain development in children [160]. Exposure to BPA during critical periods of development initiates a series of neurodevelopmental processes that permanently change the developing brain.
BPA can downregulate key neurodevelopmental transcription factors such as Sox2 and Pax6 that mediate neural stem cell activation and brain development. It may also affect neurogenesis by altering neural stem cell proliferation and differentiation. The cerebellum, hypothalamus, and hippocampus may also be susceptible to the neurotoxic effects of BPA. Permanent changes in children’s behavior are expected, a finding that is consistent with the impact of BPA on these brain regions [161]. Other mechanisms include reduction in synaptogenesis and synaptic protein expression, alterations in structural plasticity, and increased inflammation and oxidative stress [143,162]. Thus, BPA exposure can interfere with normal brain development as well as cognitive and behavioral functions.
Phthalates may cross the blood-placental barrier and adversely affect fetal brain development. Ongoing phthalate exposure during infancy and early childhood may contribute to poor long-term neurodevelopmental outcomes [163]. Numerous studies have suggested that phthalate exposure during these sensitive periods may be associated with altered behavioral, cognitive, and psychomotor outcomes [164-167]. Prenatal phthalate exposure can impair cognitive, social, motor, and emotional development [168]. Studies conducted in different countries have shown that prenatal DEHP, DBP, monobutyl phthalate, dibutyl phthalate, DIBP, and butyl benzyl phthalate exposure is associated with decreased language, verbal, communication, personal-social, cognitive, and psychomotor development; lower IQ; and learning difficulties during childhood [143,169-173].
A recent meta-analysis demonstrated a significant association between prenatal phthalate exposure and psychomotor outcomes in children [164]. However, some authors have highlighted the importance of altered sex-specific differences in infant and child neurodevelopment [166,174]. Prenatal phthalate exposure reportedly contributes to increased internalization and externalization problems, somatic complaints, aggressive behaviors, poor social relationships, peer relationship problems, anxiety, depression, and emotional symptoms in children [13,142,143,159]. In addition, the relationship between prenatal and early childhood phthalate exposure and children's behavior varies by sex [158].
Early phthalate exposure may affect developmental and behavioral outcomes in children; however, the results of these studies varied in terms of phthalate metabolites, affected brain areas, and sex [143]. Prenatal phthalate exposure may be associated with ASD, ADHD, and other specific behavioral problems [164,165,175,176]. In a population- based birth cohort of 1,064 women in Australia, elevated urine phthalate metabolite levels at 36 weeks’ gestation were associated with childhood autism [175]. A study of Norwegian mother-child pairs showed that prenatal phthalate exposure was correlated with the risk of ADHD during infancy [176].
Although the biological mechanisms underlying the role of phthalates in neurodevelopment remain unclear, several studies have proposed possible mechanisms. Phthalates can impair the homeostasis of sex hormones, thyroid hormones, calcium signaling, and lipid metabolism. Given the role of steroids and thyroid hormones in the brain and synaptic development, it is not surprising that early phthalate exposure affects children’s cognitive development and social competence [177]. Sex hormones are essential for neurodevelopment. Progesterone is essential for neurosteroid production, whereas estrogen plays important roles in brain development and neuroprotection. Androgens have crucial biological effects on early prenatal brain development and social cognition. Estrogens can affect neuroplasticity and neurogenesis in the hippocampus by binding to ERs and activating cell signaling pathways. Phthalate exposure adversely affects neurodevelopment by disrupting sex hormone homeostasis [178]. Phthalates are thought to primarily affect neurodevelopment by disrupting thyroid hormone homeostasis, which is crucial for fetal and infant brain development; thus, it can ultimately affect children’s cognitive and motor abilities [163].
At the same time, phthalates can reduce dopamine release by disrupting the D2 dopamine receptor, tyrosine hydroxylase, and calcium-dependent neurotransmitter homeostasis. Nicotinic acetylcholine receptor– mediated calcium signaling participates in various neurodevelopmental processes. Phthalate metabolites may interfere with calcium signaling coupled with nicotinic acetylcholine receptors [143]. Phthalates may impair functional plasticity within the hippocampus, which plays an important role in learning and memory [163]. Animal studies have associated phthalate exposure with increased lipid peroxidation, which can lead to motor neuron apoptosis and other brain disorders. The last mechanism may be that phthalate exposure affects the child’s neurodevelopment by causing oxidative stress [173].
PCBs have long been associated with neurological disorders [179]. Low-dose PCB exposure during critical periods has adverse effects on behavioral, physiological, neurobiological, and cognitive regulation [13]. A comprehensive summary of studies published in 1990–2018 stated that numerous epidemiological studies revealed negative associations between PCB exposure and childhood neurodevelopment [180]. It was concluded that PCB exposure during critical developmental periods led to impaired executive function and psychomotor coordination; learning disabilities; poor memory; low IQ; problems with fine motor skills such as attention, memory, and writing; and decreased language development [179-182]. A close negative relationship was found between intrauterine PCB exposure and verbal and memory scores in 4-year-old children [183]. Another study found a 3-point decrease in full-scale IQ and a 4-point decrease in verbal IQ for each increase in placental PCB concentration of 1 ng/g (wet weight) [184]. Epidemiological studies reported that PCBs have adverse effects on internalizing behaviors, such as anxiety, somatization, and depression, as well as externalizing behaviors, such as hostility and antisocial tendencies [185,186]. PCB exposure has adverse effects on children’s psychomotor, learning, memory, and neurobehavioral functions [187]. Additionally, current studies confirmed the association between developmental PCB exposure and ASD and ADHD [185,188-191]. In one study, cord serum PCB-153 levels were significantly associated with increased ADHD behaviors in 8-year-old children [192].
The mechanisms through which PCBs exert adverse effects on the brain remain unclear [139]. The hypothalamus appears to be an area of nervous system vulnerability to PCBs. PCB exposure during the prenatal period may decrease ERβ expression in the anteroventral periventricular nucleus. It can also alter the expression of brain-derived neurotrophic factor (BDNF) genes in the preoptic region of the hypothalamus in a sex- specific manner, suggesting that prenatal PCB exposure masculinizes the brain. Decreased BDNF expression, which regulates sensory neuron development, may be the underlying mechanism of neurobehavioral disorders [161]. PCBs may affect dopaminergic and serotonergic receptors, bind to aryl hydrocarbon receptors, interrupt neuroimmune function, and increase cytokine production [139].
Exposure to PBDEs before and after birth may adversely affect children’s cognitive development and motor function. Prenatal PBDE exposure may adversely affect cognitive functions associated with a lower IQ [179,193-198], reduced motor skills [194], poorer executive function [199,200], lower social and language development [201], and lower reading and attention skills [194, 197,202-205]. One study found that a 10-fold increase in the sum of 4 major PBDE congeners (BDE-47, -99, -100, and -153) in maternal serum at 16±3 weeks’ gestation was associated with a 6.2-point decrease in reading scores in 8-year-old children [197]. Similarly, Chao et al. [206] and Gascon et al. [207] reported that postnatal exposure to PBDEs, particularly BDE-209, via breast milk potentially delays the neurological and mental development of breastfed infants aged 0–18 months. Tsai et al. [208] also found that BDE-209 levels in breast milk were significantly negatively correlated with cognitive levels in 8- to 12-month-old infants.
Pre- and postnatal PBDE exposure is associated with externalizing problems, impaired self-control, and reduced social skills in childhood [201,202,205,207]. However, the Shanghai-Minhang Birth Cohort Study showed that prenatal PBDE exposure was associated with somatic complaints, introversion, and internalization problems in girls and somatic complaints and attention problems in boys [209]. These results indicate that the effects of PBDE exposure on neurobehavioral outcomes may differ by sex. A South Korean study reported that mothers exposed to higher PBDE levels had higher scores on all ADHD scales [210]. A study conducted in Norway found a relationship between different types of PBDEs in breast milk and ADHD [211].
The mechanisms underlying the neurotoxic effects of PBDEs are not fully understood, although it is clear that pre and postnatal PBDE exposure affects children’s neurodevelopment. Disruption of thyroid homeostasis is a suggested potential mechanism [204]. Thyroid hormones are involved in myelination, cerebellar development, glial cell proliferation, neuronal differentiation, and synapse formation [139]. As PBDEs have a chemical structure similar to that of T4, they may bind to thyroid hormone transport proteins and receptors. Therefore, PBDEs can reduce circulating T4 and T3 levels [102]. Abnormalities in thyroid hormone levels may be responsible for impaired neurodevelopment [139]. Another potential mechanism involves the direct effects of PBDEs on the brain. PBDEs can directly affect brain cells by inducing alterations in cellular migration and differentiation into neurons and oligodendrocytes, interfering with calcium signaling and protein kinase C pathways in neurons, and causing oxidative stress [204]. In addition, PBDE exposure may affect the gamma-aminobutyric acid (GABA) and glutamatergic neurotransmitter systems in the frontal cortex and cholinergic nicotinic receptors in the hippocampus [102]. Since GABA is an important neurotransmitter in the brain, its changes can interrupt neuronal activity, causing deficits in various cognitive processes such as hyperactivity and impulsivity, aggressive behaviors, impaired social behaviors, and low academic skills [139].
Evidence from animal and human studies suggests that PFAS exposure during early life may have short- and long-term neurodevelopmental effects [212]. Epidemiological studies reported that early life exposure to PFAS is associated with neurodevelopmental disorders such as executive dysfunction, cognitive dysfunction, motor disabilities, and language and communication impairments in children [200,212-216]. Various studies reported that prenatal PFAS exposure is associated with attention deficits, hyperactivity, impulsivity, and altered externalizing and internalizing behaviors [217,218]. Several hypothetical scenarios suggested weak inverse associations between prenatal PFAS exposure and ADHD in school-aged children [217,219]. Epidemiological studies reported null [220], positive [221,222], or negative [223] associations between ASD and PFAS exposure.
Several mechanisms have been proposed by which early life exposure to PFAS and their mixtures may adversely affect childhood neurodevelopment. PFAS affect neuronal differentiation and can alter neuroprotein levels in the hippocampus and cerebral cortex, which are important for normal brain development [224]. Some toxicological studies suggested that early PFAS exposure may lead to neurotoxic effects by altering synaptogenesis, cell death, and reactive oxygen species generation [225]. Possible mechanisms include the impaired expression of calcium-related signaling molecules in the hippocampus, changes in the cholinergic system, and the disruption of thyroid homeostasis [214]. The neuroprotective effects of PFAS can also be explained by the hypothesis that PFAS appears to be a partial agonist of PPARγ, agonists of which effectively attenuate oxidative stress, inflammation, and apoptosis in the central nervous system [225].
A growing body of evidence demonstrates a positive association between pre- and postnatal OCP exposure and neurodevelopmental disorders [226]. DDT and DDE in particular cause neurodevelopmental toxicity by crossing the placental barrier and contaminating the breast milk [226,227]. The Center for the Health Assessment of Mothers and Children of Salinas study showed that increased prenatal levels of DDT and its breakdown product, DDE, affected the neurodevelopmental performance of children aged 1–2 years. Higher levels of prenatal DDT exposure in particular are negatively associated with information processing speed, a possible risk factor for attention and learning problems in children [228]. The fine motor development of boys in Guadeloupe exposed to chlordecone, a permanent OCP formerly used in banana plantations, in utero was adversely affected [229]. Eskenazi et al. [194] reported that prenatal exposure to DDT and DDE was associated with decreased cognitive development in 12- to 24-month-old infants and impaired psychomotor development in 6- to 12-month-old infants. A study of 55 Taiwanese mother-infant pairs found that higher levels of DDT in breast milk were significantly associated with lower performance of 8- to 12-month-old infants in the cognitive, language, and social-emotional domains [230].
Prenatal pesticide exposure has also been associated with anxiety, depression, somatization, aggression, hyperactivity, and behavioral problems [140]. One study reported that DDE exposure negatively affected infant attention skills within the first 5 days after birth [231]. Additionally, prenatal exposure to OCPs such as DDT and DDE has been associated with developmental delays and a greater risk of ASD onset [229]. A Finnish prenatal study of autism and ASD determined that high maternal serum DDE levels correlated with ASD symptoms in infants [232]. Roberts et al. [233] reported an increased risk of ASD in infants whose mothers were exposed to DDT during the first trimester of pregnancy. The most well-known mechanism involves the effects of OCPs on thyroid hormones [234]. OCPs can also cause oxidative stress and DNA damage, resulting in long-term effects on neurodevelopment [235].
The adverse health effects of dioxin exposure in children may include negative neurological outcomes [236]. Epidemiological studies reported that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure was associated with subtle neurodevelopmental problems such as decreased cognition, motor, attention, social-emotional, learning, and language skills [182,237-239]. Autistic traits and poor cognitive and motor development have been observed in Vietnamese infants born in dioxin-contaminated areas after the wartime use of Agent Orange [240]. One study observed increased ADHD symptoms in children perinatally exposed to more TCDD [241]. Dioxins have been suggested to affect neurodevelopment via aryl hydrocarbon receptor–mediated signaling pathways [242].
This review examined the reported findings of birth and neurodevelopmental disorders following pre and postnatal exposure to 3 main classes of EDCs: BPA, phthalates, and POPs (PCB, PBDE, PFAS, OCP, dioxins, and furans). Humans are constantly exposed to EDCs through the air, nutrients, and water. By interfering with endocrine and neurological patterns, EDCs adversely affect general human health, particularly that of children. Humans are highly sensitive to EDC exposure during the intrauterine and early postpartum periods. Such exposures affect birth outcomes through various mechanisms. Birth outcomes such as fetal growth restriction, preterm birth, and low birth weight have been associated with EDC exposure, but results from epidemiological studies are conflicting. EDC exposure can also cause brain reprogramming by affecting behaviors in other areas, such as epigenetics. The time of exposure is an important factor in determining potential neurodevelopmental outcomes. Previous studies suggested that postnatal EDC exposure is associated with adverse neurobehavioral outcomes in children. Although possible mechanisms of action have been mentioned, the exact mechanisms are yet to be elucidated. Precautions should be taken to control, prevent, and limit contact with EDCs, especially since infants are at greater risk for changes in programming and/or malformations during the first 1,000 days of life.
Table 1.
Sources of endocrine-disrupting chemicals
Table 2.
Effects of endocrine-disrupting chemicals (EDCs) on birth outcomes
Table 3.
Effects of endocrine-disrupting chemicals (EDCs) on the nervous system in children
References
1. Darbre PD. The history of endocrine-disrupting chemicals. Curr Opin Endocr Metab Res 2019;7:26–33.

3. La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 2020;16:45–57.




4. Predieri B, Alves CA, Iughetti L. New insights on the effects of endocrine-disrupting chemicals on children. J Pediatr 2022;98:73–85.



5. Ramakrishna M, Girigoswami A, Chakraborty S, Girigoswami K. Bisphenol A-An overview on its effect on health and environment. Biointerface Res Appl Chem 2021;12:105–19.

6. Yesildemir O, Akdevelioglu Y, Duyan CamurdanA, Cuhaci Cakir B, Erdemli Kose SB, Arca Cakir D, et al. Estimated exposure to bisphenol A in breastfed and breastfed plus formula-fed infants in Turkey: a comparison study. Drug Chem Toxicol 2022;Dec 26 1. –11. doi: 10.1080/01480545.2022.2160456. [Epub].

7. Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. Gestational and childhood exposure to phthalates and child behavior. Environ Int 2020;144:106036.



8. Alharbi OM, Khattab RA, Ali I. Health and environmental effects of persistent organic pollutants. J Mol Liq 2018;263:442–53.

9. DiVall SA. The influence of endocrine disruptors on growth and development of children. Curr Opin Endocr Metab Res 2013;20:50–5.

10. Zlatnik MG. Endocrine-disrupting chemicals and reproductive health. J Midwifery Womens Health 2016;61:442–55.




11. Ghassabian A, Vandenberg L, Kannan K, Trasande L. Endocrine-disrupting chemicals and child health. Annu Rev Pharmacol Toxicol 2022;62:573–94.


12. Mitro SD, Johnson T, Zota AR. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep 2015;2:367–78.




13. Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: a review on the major concerns. Birth Defects Res C Embryo Today 2016;108:224–42.


14. Ünüvar T, Büyükgebiz A. Fetal and neonatal endocrine disruptors. J Clin Res Pediatr Endocrinol 2012;4:51–60.



15. Caserta D, Pegoraro S, Mallozzi M, Di Benedetto L, Colicino E, Lionetto L, et al. Maternal exposure to endocrine disruptors and placental transmission: a pilot study. Gynecol Endocrinol 2018;34:1001–4.


16. Padmanabhan V, Song W, Puttabyatappa M. Praegnatio perturbatio-impact of endocrine-disrupting chemicals. Endocr Rev 2021;42:295–353.




17. Gingrich J, Ticiani E, Veiga-Lopez A. Placenta disrupted: endocrine disrupting chemicals and pregnancy. Trends Endocrinol Metab 2020;31:508–24.



18. Gingrich J. The effects of endocrine disrupting chemicals on placental development and function [dissertation]. East Lansing (MI): Michigan State University,, 2020.
19. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986;327:1077–81.

20. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017;68:3–33.



21. Brown HL, Smith GN. Pregnancy complications, cardiovascular risk factors, and future heart disease. Obstet Gynecol 2020;47:487–95.

22. Eriksson JG. Developmental pathways and programming of diabetes: epidemiological aspects. J Endocrinol 2019;Apr 1 JOE-18-0680.R2. doi: 10.1530/JOE-18-0680. [Epub].

23. Guarner-Lans V, Rubio-Ruiz ME, Castrejón-Téllez V, Soto ME, Pérez-Torres I. Early programming of adult systemic essential hypertension. Int J Mol Sci 2020;21:1203.



24. Talia C, Connolly L, Fowler PA. The insulin-like growth factor system: a target for endocrine disruptors? Environ Int 2021;147:106311.


25. Kim S, Lee J, Park J, Kim HJ, Cho GJ, Kim GH, et al. Urinary phthalate metabolites over the first 15 months of life and risk assessment–CHECK cohort study. Sci Total Environ 2017;607:881–7.


26. Tang ZR, Xu XL, Deng SL, Lian ZX, Yu K. Oestrogenic endocrine disruptors in the placenta and the fetus. Int J Mol Sci 2020;21:1519.



27. Broe A, Pottegård A, Hallas J, Ahern TP, Lamont RF, Damkier P. Phthalate exposure from drugs during pregnancy and possible risk of preterm birth and small for gestational age. Eur J Obstet Gynecol Reprod Biol 2019;240:293–9.


28. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010;118:1055–70.



29. Chen M, Zhu P, Xu B, Zhao R, Qiao S, Chen X, et al. Determination of nine environmental phenols in urine by ultrahigh-performance liquid chromatography–tandem mass spectrometry. J Anal Toxicol 2012;36:608–15.


30. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 2002;17:2839–41.


31. Tomza-Marciniak A, Stępkowska P, Kuba J, Pilarczyk B. Effect of bisphenol A on reproductive processes: a review of in vitro, in vivo and epidemiological studies. J Appl Toxicol 2018;38:51–80.



32. Liang J, Liu S, Liu T, Yang C, Wu Y, Jennifer Tan HJ, et al. Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns. Chemosphere 2020;252:126422.


33. Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 2014;122:775–86.



34. Basak S, Srinivas V, Duttaroy AK. Bisphenol-A impairs cellular function and alters DNA methylation of stress pathway genes in first trimester trophoblast cells. Reprod Toxicol 2018;82:72–9.


35. Pergialiotis V, Kotrogianni P, Christopoulos-Timogiannakis E, Koutaki D, Daskalakis G, Papantoniou N. Bisphenol A and adverse pregnancy outcomes: a systematic review of the literature. J Matern Fetal Neonatal Med 2018;31:3320–7.


36. Mørck TJ, Sorda G, Bechi N, Rasmussen BS, Nielsen JB, Ietta F, et al. Placental transport and in vitro effects of Bisphenol A. Reprod Toxicol 2010;30:131–7.


37. Paulesu L, Rao CV, Ietta F, Pietropolli A, Ticconi C. hCG and its disruption by environmental contaminants during human pregnancy. Int J Mol Sci 2018;19:914.



38. Namat A, Xia W, Xiong C, Xu S, Wu C, Wang A, et al. Association of BPA exposure during pregnancy with risk of preterm birth and changes in gestational age: a meta-analysis and systematic review. Ecotoxicol Environ Saf 2021;220:112400.


39. Zulkifli S, Rahman AA, Kadir SHSA, Nor NSM. Bisphenol A and its effects on the systemic organs of children. Eur J Pediatr 2021;180:3111–27.



40. Vrachnis N, Loukas N, Vrachnis D, Antonakopoulos N, Zygouris D, Kοlialexi A, et al. A systematic review of bisphenol A from dietary and non-dietary sources during pregnancy and its possible connection with fetal growth restriction: investigating its potential effects and the window of fetal vulnerability. Nutrients 2021;13:2426.



41. Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. J Clin Endocrinol Metab 2015;100:1394–403.



42. Lee YM, Hong YC, Ha M, Kim Y, Park H, Kim HS, et al. Prenatal bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: from multiregional prospective birth cohort MOCEH study. Sci Total Environ 2018;612:1433–41.


43. Zbucka-Krętowska M, Łazarek U, Miltyk W, Sidorkiewicz I, Pierzyński P, Milewski R, et al. Simultaneous analysis of bisphenol A fractions in maternal and fetal compartments in early second trimester of pregnancy. J Perinat Med 2019;47:765–70.


44. Yang C, Song G, Lim W. A mechanism for the effect of endocrine disrupting chemicals on placentation. Chemosphere 2019;231:326–36.


45. Hu CY, Li FL, Hua XG, Jiang W, Mao C, Zhang XJ. The association between prenatal bisphenol A exposure and birth weight: a meta-analysis. Reprod Toxicol 2018;79:21–31.


46. Zhong Q, Peng M, He J, Yang W, Huang F. Association of prenatal exposure to phenols and parabens with birth size: a systematic review and meta-analysis. Sci Total Environ 2020;703:134720.


47. Zhou Z, Lei Y, Wei W, Zhao Y, Jiang Y, Wang N, et al. Association between prenatal exposure to bisphenol a and birth outcomes: a systematic review with meta-analysis. Medicine (Baltimore) 2019;98:e17672.


48. Sol CM, van Zwol-Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, Kannan K, et al. Maternal bisphenol urine concentrations, fetal growth and adverse birth outcomes: a population-based prospective cohort. Environ Health 2021;20:1–9.




49. Rochester JR, Bolden AL. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 2015;123:643–50.



50. Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Cordero JF, et al. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern Puerto Rico. Environ Res 2019;169:41–51.



51. Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study. Environ Int 2018;113:341–9.



52. Hu J, Zhao H, Braun JM, Zheng T, Zhang B, Xia W, et al. Associations of trimester-specific exposure to bisphenols with size at birth: a Chinese prenatal cohort study. Environ Health Perspect 2019;127:107001.



53. Goodrich JM, Ingle ME, Domino SE, Treadwell MC, Dolinoy DC, Burant C, et al. First trimester maternal exposures to endocrine disrupting chemicals and metals and fetal size in the Michigan Mother–Infant Pairs study. J Dev Orig Health Dis 2019;10:447–58.



54. Zhou B, Yang P, Deng YL, Zeng Q, Lu WQ, Mei SR. Prenatal exposure to bisphenol a and its analogues (bisphenol F and S) and ultrasound parameters of fetal growth. Chemosphere 2020;246:125805.


55. Zong T, Lai L, Hu J, Guo M, Li M, Zhang L, et al. Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J Hazard Mater 2015;297:25–33.


56. Wittassek M, Angerer J, Kolossa-Gehring M, Schäfer SD, Klockenbusch W, Dobler L, et al. Fetal exposure to phthalates–a pilot study. Int J Hyg Environ Health 2009;212:492–8.


57. Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. Urinary concentrations of phthalate metabolites and bisphenol A and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environ Health Perspect 2016;124:321–8.



58. Minatoya M, Araki A, Miyashita C, Sasaki S, Goto Y, Nakajima T, et al. Prenatal di-2-ethylhexyl phthalate exposure and cord blood adipokine levels and birth size: the Hokkaido study on environment and children's health. Sci Total Environ 2017;579:606–11.


59. Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernández MF, Garcia-Esteban R, et al. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environ Health Perspect 2016;124:521–8.



60. Gao H, Wang Yf, Huang K, Han Y, Zhu YD, Zhang QF, et al. Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study. Environ Res 2019;176:108530.


61. Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatrics 2014;168:61–7.



62. Chen CH, Jiang SS, Chang IS, Wen HJ, Sun CW, Wang SL. Association between fetal exposure to phthalate endocrine disruptor and genome-wide DNA methylation at birth. Environ Res 2018;162:261–70.


63. Ferguson KK, Rosen EM, Barrett ES, Nguyen RH, Bush N, McElrath TF, et al. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ Int 2019;133(Pt B): 105254.



64. Huang Q, Chen Q. Mediating roles of PPARs in the effects of environmental chemicals on sex steroids. PPAR Res 2017;2017:3203161.




65. Dutta S, Haggerty DK, Rappolee DA, Ruden DM. Phthalate exposure and long-term epigenomic consequences: a review. Front Genet 2020;11:405.



66. Goodrich JM, Dolinoy DC, Sánchez BN, Zhang Z, Meeker JD, Mercado-Garcia A, et al. Adolescent epigenetic profiles and environmental exposures from early life through periadolescence. Environ Epigenet 2016;2:1–11.



67. Zhao Y, Chen J, Wang X, Song Q, Xu HH, Zhang YH. Third trimester phthalate exposure is associated with DNA methylation of growth-related genes in human placenta. Sci Rep 2016;6:33449.




68. Montrose L, Padmanabhan V, Goodrich JM, Domino SE, Treadwell MC, Meeker JD, et al. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics 2018;13:301–9.



69. Choi YJ, Lee YA, Hong YC, Cho J, Lee KS, Shin CH, et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ Int 2020;143:105929.


70. LaRocca J, Binder AM, McElrath TF, Michels KB. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res 2014;133:396–406.



71. Zhong Q, Liu Hl, Fu H, Niu QS, Wu HB, Huang F. Prenatal exposure to phthalates with preterm birth and gestational age: a systematic review and meta-analysis. Chemosphere 2021;282:130991.


72. Wu Y, Wang J, Wei Y, Chen J, Kang L, Long C, et al. Maternal exposure to endocrine disrupting chemicals (EDCs) and preterm birth: a systematic review, meta-analysis, and metaregression analysis. Environ Pollut 2022;292(Pt A): 118264.


73. Santos S, Sol CM, van Zwol–Janssens C, Philips EM, Asimakopoulos AG, Martinez-Moral MP, et al. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ Int 2021;151:106443.


74. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health 2007;210:623–34.


75. Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates–the human biomonitoring approach. Mol Nutr Food Res 2011;55:7–31.



76. Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 2008;116:1092–7.



77. Polańska K, Ligocka D, Sobala W, Hanke W. Effect of environmental phthalate exposure on pregnancy duration and birth outcomes. Int J Occup Med Environ Health 2016;29:683–97.


78. Kamai EM, McElrath TF, Ferguson KK. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health 2019;18:1–30.




79. Vrachnis N, Loukas N, Vrachnis D, Antonakopoulos N, Christodoulaki C, Tsonis O, et al. Phthalates and fetal growth velocity: tracking down the suspected links. J Matern Fetal Neonatal Med 2022;35:4985–93.


80. Li J, Qian X, Zhou Y, Li Y, Xu S, Xia W, et al. Trimester-specific and sex-specific effects of prenatal exposure to di (2-ethylhexyl) phthalate on fetal growth, birth size, and early-childhood growth: a longitudinal prospective cohort study. Sci Total Environ 2021;777:146146.


81. Lee DW, Lim HM, Lee JY, Min KB, Shin CH, Lee YA, et al. Prenatal exposure to phthalate and decreased body mass index of children: a systematic review and meta-analysis. Sci Rep 2022;12:8961.




82. Kim S, Cho YH, Lee I, Kim W, Won S, Ku JL, et al. Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: a CHECK cohort study. Environ Int 2018;119:398–406.


83. Acosta-Maldonado B, Sánchez-Ramírez B, Reza-López S, Levario-Carrillo M. Effects of exposure to pesticides during pregnancy on placental maturity and weight of newborns: a cross-sectional pilot study in women from the Chihuahua State, Mexico. Hum Exp Toxicol 2009;28:451–9.



84. Frazier LM. Reproductive disorders associated with pesticide exposure. J Agromedicine 2007;12:27–37.

85. Pathak R, Mustafa M, Ahmed T, Ahmed RS, Tripathi A, Guleria K, et al. Intra uterine growth retardation: association with organochlorine pesticide residue levels and oxidative stress markers. Reprod Toxicol 2011;31:534–9.


86. Schoeters G, Den Hond E, Dhooge W, Van Larebeke N, Leijs M. Endocrine disruptors and abnormalities of pubertal development. Basic Clin Pharmacol Toxicol 2008;102:168–75.


87. Vizcaino E, Grimalt JO, Fernández-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int 2014;65:107–15.


88. Grandjean P, Bellinger D, Bergman Å, Cordier S, Davey-Smith G, Eskenazi B, et al. The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol 2008;102:73–5.


90. Tang M, Yin S, Zhang J, Chen K, Jin M, Liu W. Prenatal exposure to polychlorinated biphenyl and umbilical cord hormones and birth outcomes in an island population. Environ Pollut 2018;237:581–91.


91. Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology 2005;16:648–56.


92. Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, De Boer M, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlor odiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ Health Perspect 2012;120:162–70.


93. Govarts E, Iszatt N, Trnovec T, de Cock M, Eggesbø M, Murinova LP, et al. Prenatal exposure to endocrine disrupting chemicals and risk of being born small for gestational age: pooled analysis of seven European birth cohorts. Environ Int 2018;115:267–78.


94. Lauritzen HB, Larose TL, Øien T, Sandanger TM, Odland JØ, Van De Bor M, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case–cohort study. Pediatr Res 2017;81:33–42.




95. Kezio KL, Liu X, Cirillio PM, Kalantzi OI, Wang Y, Petreas MX, et al. Prenatal polychlorinated biphenyl exposure is associated with decreased gestational length but not birth weight: archived samples from the Child Health and Development Studies pregnancy cohort. Environ Healt 2012;11:49.
96. Lignell S, Aune M, Darnerud PO, Hanberg A, Larsson SC, Glynn A. Prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may influence birth weight among infants in a Swedish cohort with background exposure: a cross-sectional study. Environ Healt 2013;12:44.


97. Sheinberg R, Siegel EL, Keidar R, Mandel D, Lubetzky R, Kohn E, et al. Associations between intrauterine exposure to polychlorinated biphenyls on neonatal ano-genital distance. Reprod Toxicol 2020;96:67–75.


98. Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Healt 2010;9:32.




99. Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, et al. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One 2013;8:e62526.



100. Ruis MT, Rock KD, Hall SM, Horman B, Patisaul HB, Stapleton HM. PBDEs concentrate in the fetal portion of the placenta: implications for thyroid hormone dysregulation. Endocrinology 2019;160:2748–58.




101. Forhead AJ, Fowden AL. Thyroid hormones in fetal growth and prepartum maturation. J Endocrinol 2014;221:R87–103.


102. Costa LG, de Laat R, Tagliaferri S, Pellacani C. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 2014;230:282–94.



103. Zhao Y, Song Q, Ge W, Jin Y, Chen S, Zhao Y, et al. Associations between in utero exposure to polybrominated diphenyl ethers, pathophysiological state of fetal growth and placental DNA methylation changes. Environ Int 2019;133(Pt B): 105255.


104. Bernasconi S, Sartori C, Merli S, Lazzeroni P, Cesari S, Street M. Thyroid hormones in fetal development. In: Bona G, De Luca F, Monzani A, editors. Thyroid diseases in childhood: recent advances from basic science to clinical practice. Berlin: Springer, 2015:15-25.
105. Zhao X, Peng S, Xiang Y, Yang Y, Li J, Shan Z, et al. Correlation between prenatal exposure to Polybrominated Diphenyl ethers (PBDEs) and infant birth outcomes: a meta-analysis and an experimental study. Int J Environ Res Public Health 2017;14:268.



106. Park HR, Elkin ER, Castillo-Castrejon M, Loch-Caruso R. Brominated diphenyl ether-47 differentially regulates cellular migration and invasion in a human first trimester trophoblast cell line. Reprod Toxicol 2020;93:191–8.



107. Wang Y, Wang Q, Zhou L, Zeng Z, Zhao C, You L, et al. Metabolomics insights into the prenatal exposure effects of polybrominated diphenyl ethers on neonatal birth outcomes. Sci Total Environ 2022;836:155601.


108. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr 2004;23(6 Suppl): 588S–595S.


109. Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E, Cosmi E. Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 2014;237:391–9.


111. Chen L, Wang C, Cui C, Ding G, Zhou Y, Jin J, et al. Prenatal exposure to polybrominated diphenyl ethers and birth outcomes. Environ Pollut 2015;206:32–7.


112. Eick SM, Hom Thepaksorn EK, Izano MA, Cushing LJ, Wang Y, Smith SC, et al. Associations between prenatal maternal exposure to per-and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ Health 2020;19:100.




113. Lopez-Espinosa MJ, Costa O, Vizcaino E, Murcia M, Fernandez-Somoano A, Iñiguez C, et al. Prenatal exposure to polybrominated flame retardants and fetal growth in the INMA Cohort (Spain). Environ Sci Technol 2015;49:10108–16.



114. Sagiv SK, Rifas-Shiman SL, Fleisch AF, Webster TF, Calafat AM, Ye X, et al. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol 2018;187:793–802.



115. Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health 2018;15:1832.



116. Workman CE, Becker AB, Azad MB, Moraes TJ, Mandhane PJ, Turvey SE, et al. Associations between concentrations of perfluoroalkyl substances in human plasma and maternal, infant, and home characteristics in Winnipeg, Canada. Environ Pollut 2019;249:758–66.


117. Blake BE, Fenton SE. Early life exposure to per-and polyfluoroalkyl substances (PFAS) and latent health outcomes: a review including the placenta as a target tissue and possible driver of peri-and postnatal effects. Toxicology 2020;443:152565.



118. Gao X, Ni W, Zhu S, Wu Y, Cui Y, Ma J, et al. Per-and polyfluoroalkyl substances exposure during pregnancy and adverse pregnancy and birth outcomes: a systematic review and meta-analysis. Environ Res 2021;201:111632.


119. Du G, Hu J, Huang H, Qin Y, Han X, Wu D, et al. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem 2013;32:353–60.


120. Starling AP, Liu C, Shen G, Yang IV, Kechris K, Borengasser SJ, et al. Prenatal exposure to per-and polyfluoroalkyl substances, umbilical cord blood DNA methylation, and cardio-metabolic indicators in newborns: the healthy start study. Environ Health Perspect 2020;128:127014.



121. Chang CJ, Barr DB, Ryan PB, Panuwet P, Smarr MM, Liu K, et al. Per-and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: a meet-in-the-middle approach. Environ Int 2022;158:106964.



122. Szilagyi JT, Avula V, Fry RC. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator–activated receptors (PPARs). Curr Environ Health Rep 2020;7:222–30.




123. Ku MS, Pan WC, Huang YT, Hsieh WS, Hsu YH, Chen PC, et al. Associations between prenatal exposure to perfluoroalkyl substances, hypomethylation of MEST imprinted gene and birth outcomes. Environ Pol 2022;304:119183.

124. Kartin A, Subagio HW, Hadisaputro S, Kartasurya MI, Suhartono S, Budiyono B. Pesticide exposure and stunting among children in agricultural areas. Int J Occup Environ Med 2019;10:17–29.




125. Migeot V, Albouy-Llaty M, Carles C, Limousi F, Strezlec S, Dupuis A, et al. Drinking-water exposure to a mixture of nitrate and low-dose atrazine metabolites and small-forgestational age (SGA) babies: a historic cohort study. Environ Res 2013;122:58–64.


126. Gemmill A, Gunier RB, Bradman A, Eskenazi B, Harley KG. Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environ Health Perspec 2013;121737–43.

127. Béranger R, Hardy EM, Binter AC, Charles MA, Zaros C, Appenzeller BM, et al. Multiple pesticides in mothers' hair samples and children's measurements at birth: results from the French national birth cohort (ELFE). Int J Hyg Environ Health 2020;223:22–33.


128. Matsuki T, Ebara T, Tamada H, Ito Y, Yamada Y, Kano H, et al. Association between prenatal exposure to household pesticides and neonatal weight and length growth in the Japan environment and children’s study. Int J Environ Res Public Health 2020;17:4608.



129. Bliznashka L, Roy A, Jaacks LM. Pesticide exposure and child growth in low-and middle-income countries: a systematic review. Environ Res 2022;215(Pt 1): 114230.



130. Khoshhali M, Davoodi S, Ebrahimpour K, Shoshtari-Yeganeh B, Kelishadi R. The association between maternal exposure to organophosphate pesticides and neonatal anthropometric measures: a systematic review and meta-analysis. J Res Med Sci 2020;25:79.



131. Hervé D, Costet N, Kadhel P, Rouget F, Monfort C, Thomé JP, et al. Prenatal exposure to chlordecone, gestational weight gain, and birth weightin a Guadeloupean birth cohort. Environ Res 2016;151:436–44.


132. Mendez MA, Garcia-Esteban R, Guxens M, Vrijheid M, Kogevinas M, Goñi F, et al. Prenatal organochlorine compound exposure, rapid weight gain, and overweight in infancy. Environ Health Perspect 2011;119:272–8.



133. Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goni F, et al. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy. Obesity 2014;22:488–96.



134. Warner M, Ye M, Harley K, Kogut K, Bradman A, Eskenazi B. Prenatal DDT exposure and child adiposity at age 12: the CHAMACOS study. Environ Res 2017;159:606–12.



135. Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagaña X, Robinson O, et al. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: a multi-pollutant approach. Environ Health Perspect 2015;123:1030–7.



136. Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, et al. Association of prenatal exposure to persistent organic pollutants with obesity and cardiometabolic traits in early childhood: the Rhea mother– child cohort (Crete, Greece). Environ Health Perspect 2015;123:1015–21.



137. Yang C, Fang J, Sun X, Zhang W, Li J, Chen X, et al. Prenatal exposure to organochlorine pesticides and infant growth: a longitudinal study. Environ Int 2021;148:106374.


138. Cabrera-Rodríguez R, Luzardo OP, Almeida-González M, Boada LD, Zumbado M, Acosta-Dacal A, et al. Association between prenatal exposure to multiple persistent organic pollutants (POPs) and growth indicators in newborns. Environ Res 2019;171:285–92.


139. Ramírez V, Gálvez-Ontiveros Y, González-Domenech PJ, Baca MÁ, Rodrigo L, Rivas A. Role of endocrine disrupting chemicals in children's neurodevelopment. Environ Res 2022;203:111890.


140. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 2020;8:703–18.



141. Ghassabian A, Trasande L. Disruption in thyroid signaling pathway: a mechanism for the effect of endocrine-disrupting chemicals on child neurodevelopment. Front Endocrinol (Lausanne) 2018;9:204.



142. Di Pietro G, Forcucci F, Chiarelli F. Endocrine disruptor chemicals and children’s health. Int J Mol Sci 2023;24:2671.



143. Minatoya M, Kishi R. A review of recent studies on bisphenol A and phthalate exposures and child neurodevelopment. Int J Environ Res Public Health 2021;18:3585.



144. Jensen TK, Mustieles V, Bleses D, Frederiksen H, Trecca F, Schoeters G, et al. Prenatal bisphenol A exposure is associated with language development but not with ADHD-related behavior in toddlers from the Odense Child Cohort. Environ Res 2019;170:398–405.


145. Perera F, Nolte ELR, Wang Y, Margolis AE, Calafat AM, Wang S, et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ Res 2016;151:195–202.



146. Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, et al. Early life bisphenol A exposure and neurobehavior at 8 years of age: identifying windows of heightened vulnerability. Environ Int 2017;107:258–65.



147. Huang Z, Fu W, Dou L, Bao H, Wu W, Su P, et al. Prenatal bisphenol A exposure and early childhood behavior and cognitive function: a chinese birth cohort study. Neuroendocrinology 2022;112:311–23.



148. Lim YH, Bae S, Kim BN, Shin CH, Lee YA, Kim JI, et al. Prenatal and postnatal bisphenol A exposure and social impairment in 4-year-old children. Environ Health 2017;16:79.




149. Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009;117:1945–52.



150. Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011;128:873–82.




151. Evans SF, Kobrosly RW, Barrett ES, Thurston SW, Calafat AM, Weiss B, et al. Prenatal bisphenol A exposure and maternally reported behavior in boys and girls. Neurotoxicology 2014;45:91–9.



152. Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 2012;354:74–84.



153. Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011;32:261–7.



154. Qian X, Li J, Xu S, Wan Y, Li Y, Jiang Y, et al. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ Int 2019;131:105023.


155. Rochester JR, Bolden AL, Kwiatkowski CF. Prenatal exposure to bisphenol A and hyperactivity in children: a systematic review and meta-analysis. Environ Int 2018;114:343–56.


156. Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant DevelopmentII in a population of young urban children. Environ Res 2017;152:51–8.



157. Casas M, Forns J, Martínez D, Avella-García C, Valvi D, Ballesteros-Gómez A, et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ Res 2015;142:671–9.


158. Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 2013;126:43–50.



159. Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, et al. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 years. Environ Health Perspect 2017;125:097014.



160. Pan R, Wang C, Shi R, Zhang Y, Wang Y, Cai C, et al. Prenatal bisphenol A exposure and early childhood neurodevelopment in Shandong, China. Int J Hyg Environ Health 2019;222:896–902.


161. Nesan D, Kurrasch DM. Gestational exposure to common endocrine disrupting chemicals and their impact on neurodevelopment and behavior. Annu Rev Physiol 2020;82:177–202.


162. Liu J, Martin LJ, Dinu I, Field CJ, Dewey D, Martin JW. Interaction of prenatal bisphenols, maternal nutrients, and toxic metal exposures on neurodevelopment of 2-year-olds in the APrON cohort. Environ Int 2021;155:106601.


163. Jankowska A, Nazareth L, Kaleta D, Polanska K. Review of the existing evidence for sex-specific relationships between prenatal phthalate exposure and children’s neurodevelopment. Int J Environ Res Public Health 2021;18:13013.



164. Lee DW, Kim MS, Lim YH, Lee N, Hong YC. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: a systematic review and metaanalysis. Environmental Res 2018;167:558–66.

165. Bornehag CG, Lindh C, Reichenberg A, Wikström S, Hallerback MU, Evans SF, et al. Association of prenatal phthalate exposure with language development in early childhood. JAMA Pediatrics 2018;172:1169–76.



166. Zhang Q, Chen XZ, Huang X, Wang M, Wu J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology 2019;73:199–212.


167. Praveena SM, Munisvaradass R, Masiran R, Rajendran RK, Lin CC, Kumar S. Phthalates exposure and attention-deficit/hyperactivity disorder in children: a systematic review of epidemiological literature. Environ Sci Pollut Res 2020;27:44757–70.


168. Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 2012;120:290–5.



169. Jankowska A, Polańska K, Koch HM, Pälmke C, Waszkowska M, Stańczak A, et al. Phthalate exposure and neurodevelopmental outcomes in early school age children from Poland. Environ Res 2019;179(Pt B): 108829.


170. Won EK, Kim Y, Ha M, Burm E, Kim YS, Lim H, et al. Association of current phthalate exposure with neurobehavioral development in a national sample. Int J Hyg Environ Health 2016;219:364–71.


171. Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One 2014;9:e114003.



172. Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ 2013;461:386–90.

173. Park S, Zimmerman E, Huerta-Montañez G, Rosario-Pabón Z, Vélez-Vega CM, Cordero JF, et al. Gestational exposure to phthalates and phthalate replacements in relation to neurodevelopmental delays in early childhood. Toxics 2023;11:65.



174. Palanza P, Paterlini S, Brambilla MM, Ramundo G, Caviola G, Gioiosa L, et al. Sex-biased impact of endocrine disrupting chemicals on behavioral development and vulnerability to disease: of mice and children. Neurosci Biobehav Rev 2021;121:29–46.


175. Ponsonby AL, Symeonides C, Saffery R, Mueller JF, O’Hely M, Sly PD, et al. Prenatal phthalate exposure, oxidative stress-related genetic vulnerability and early life neurodevelopment: a birth cohort study. Neurotoxicology 2020;80:20–8.


176. Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SS, et al. Prenatal phthalates, maternal thyroid function, and risk of attention-deficit hyperactivity disorder in the Norwegian mother and child cohort. Environ Health Perspect 2018;126:057004.



177. Jankowska A, Polańska K, Hanke W, Wesołowska E, Ligocka D, Waszkowska M, et al. Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years–Polish Mother and Child Cohort. Environ Res 2019;177:108626.


178. Chen HK, Wang SL, Chang YH, Sun CW, Wu MT, Chen ML, et al. Associations between maternal phthalate exposure and neonatal neurobehaviors: the Taiwan maternal and infant cohort study (TMICS). Environ Pollut 2023;319:120956.


179. Okoro HK, Ige JO, Iyiola OA, Pandey S, Lawal IA, Zvinowanda C, et al. Comprehensive reviews on adverse health effects of human exposure to endocrine-disrupting chemicals. Fresenius Environ Bull 2017;26:4623–36.
180. Pessah IN, Lein PJ, Seegal RF, Sagiv SK. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 2019;138:363–87.




181. Berghuis SA, Bos AF, Sauer PJ, Roze E. Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome. Arch Toxicol 2015;89:687–709.



182. Caspersen IH, Aase H, Biele G, Brantsæter AL, Haugen M, Kvalem HE, et al. The influence of maternal dietary exposure to dioxins and PCBs during pregnancy on ADHD symptoms and cognitive functions in Norwegian preschool children. Environ Int 2016;94:649–60.


183. Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, et al. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in Arctic Quebec. Neurotoxicology 2010;31:17–25.


184. Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect 2008;116:1416–22.



185. Lyall K, Croen LA, Sjödin A, Yoshida CK, Zerbo O, Kharrazi M, et al. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environ Health Perspect 2017;125:474–80.



186. Ruel MVM, Bos AF, Soechitram SD, Meijer L, Sauer PJJ, Berghuis SA. Prenatal exposure to organohalogen compounds and children’s mental and motor development at 18 and 30 months of age. Neurotoxicology 2019;72:6–14.


187. Klocke C, Lein PJ. Evidence implicating non-dioxin-like congeners as the key mediators of polychlorinated biphenyl (PCB) developmental neurotoxicity. Int J Mol Sci 2020;21:1013.



188. Granillo L, Sethi S, Keil KP, Lin Y, Ozonoff S, Iosif AM, et al. Polychlorinated biphenyls influence on autism spectrum disorder risk in the MARBLES cohort. Environ Res 2019;171:177–84.



189. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017;38:81–102.



190. Rosenquist AH, Høyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V, et al. Prenatal and postnatal PCB-153 and p, p′- DDE exposures and behavior scores at 5–9 years of age among children in Greenland and Ukraine. Environ Health Perspect 2017;125:107002.



191. De Cock M, Maas YG, Van De Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatrica 2012;101:811–8.


192. Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, Korrick SA. Measured prenatal and estimated postnatal levels of polychlorinated biphenyls (PCBs) and ADHD-related behaviors in 8-year-old children. Environ Health Perspect 2015;123:888–94.



193. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 2017;13:161–73.




194. Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect 2013;121:257–62.



195. Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, et al. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect 2017;125:086001.



196. Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, et al. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 2017;58:75–83.



197. Zhang H, Yolton K, Webster GM, Sjödin A, Calafat AM, Dietrich KN, et al. Prenatal PBDE and PCB exposures and reading, cognition, and externalizing behavior in children. Environ Health Perspect 2017;125:746–52.



198. Vuong AM, Braun JM, Yolton K, Xie C, Webster GM, Sjödin A, et al. Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children. Environ Res 2017;153:83–92.



199. Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, et al. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol 2015;52:151–61.



200. Vuong AM, Yolton K, Webster GM, Sjödin A, Calafat AM, Braun JM, et al. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res 2016;147:556–64.



201. Ding G, Yu J, Cui C, Chen L, Gao Y, Wang C, et al. Association between prenatal exposure to polybrominated diphenyl ethers and young children's neurodevelopment in China. Environ Res 2015;142:104–11.


202. Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, et al. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in US children through 5 years of age: the HOME study. Environ Health Perspect 2014;122:856–62.



203. Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 2010;118:712–9.



204. Liang H, Vuong AM, Xie C, Webster GM, Sjödin A, Yuan W, et al. Childhood polybrominated diphenyl ether (PBDE) serum concentration and reading ability at ages 5 and 8 years: the HOME Study. Environ Int 2019;122:330–9.



205. Shy CG, Huang HL, Chang-Chien GP, Chao HR, Tsou TC. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol 2011;87:643–8.



206. Chao HR, Tsou TC, Huang HL, Chang-Chien GP. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res 2011;70:596–600.


207. Gascon M, Fort M, Martínez D, Carsin AE, Forns J, Grimalt JO, et al. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect 2012;120:1760–5.



208. Tsai MH, Chao HR, Hsu WL, Tsai CC, Lin CW, Chen CH. Analysis of polybrominated diphenyl ethers and lipid composition in human breast milk and their correlation with infant neurodevelopment. Int J Environ Res Public Health 2021;18:11501.



209. Ji H, Liang H, Wang Z, Miao M, Wang X, Zhang X, et al. Associations of prenatal exposures to low levels of Polybrominated Diphenyl Ether (PBDE) with thyroid hormones in cord plasma and neurobehavioral development in children at 2 and 4 years. Environ Int 2019;131:105010.


210. Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, et al. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2 years of age-CHECK cohort study. Sci Total Environ 2018;624:377–84.


211. Lenters V, Iszatt N, Forns J, echová E, Ko an A, Legler J, et al. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: a multi-pollutant analysis of a Norwegian birth cohort. Environ Int 2019;125:33–42.


212. Zhou Y, Li Q, Wang P, Li J, Zhao W, Zhang L, et al. Associations of prenatal PFAS exposure and early childhood neurodevelopment: Evidence from the Shanghai MaternalChild Pairs Cohort. Environ Int 2023;173:107850.


213. Skogheim TS, Villanger GD, Weyde KVF, Engel SM, Surén P, Øie MG, et al. Prenatal exposure to perfluoroalkyl substances and associations with symptoms of attention-deficit/hyperactivity disorder and cognitive functions in preschool children. Int J Hyg Environ Health 2020;223:80–92.



214. Spratlen MJ, Perera FP, Lederman SA, Rauh VA, Robinson M, Kannan K, et al. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ Pollut 2020;263(Pt B): 114444.



215. Yao Q, Vinturache A, Lei X, Wang Z, Pan C, Shi R, et al. Prenatal exposure to per-and polyfluoroalkyl substances, fetal thyroid hormones, and infant neurodevelopment. Environ Res 2022;206:112561.


216. Ojo AF, Peng C, Ng JC. Assessing the human health risks of per-and polyfluoroalkyl substances: A need for greater focus on their interactions as mixtures. J Hazard Mater 2021;407:124863.


217. Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, et al. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years–a prospective study. Environ Health 2015;14:2.


218. Vuong AM, Yolton K, Xie C, Dietrich KN, Braun JM, Webster GM, et al. Childhood exposure to per-and polyfluoroalkyl substances (PFAS) and neurobehavioral domains in children at age 8 years. Neurotoxicol Teratol 2021;88:107022.



219. Luo J, Xiao J, Gao Y, Ramlau-Hansen CH, Toft G, Li J, et al. Prenatal exposure to perfluoroalkyl substances and behavioral difficulties in childhood at 7 and 11 years. Environ Res 2020;191:110111.



220. Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case–control study in the Danish National Birth Cohort. Environ Health Perspect 2015;123:367–73.



221. Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, et al. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case-control study. Mol Autism 2019;10:1.




222. Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. Prenatal maternal serum concentrations of per-and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environ Health Perspect 2018;126:017001.



223. Oh J, Bennett DH, Calafat AM, Tancredi D, Roa DL, Schmidt RJ, et al. Prenatal exposure to per-and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ Int 2021;147:106328.



224. Harris MH, Oken E, Rifas-Shiman SL, Calafat AM, Bellinger DC, Webster TF, et al. Prenatal and childhood exposure to per-and polyfluoroalkyl substances (PFAS) and child executive function and behavioral problems. Environ Res 2021;202:111621.



225. Xie Z, Tan J, Fang G, Ji H, Miao M, Tian Y, et al. Associations between prenatal exposure to perfluoroalkyl substances and neurobehavioral development in early childhood: a prospective cohort study. Ecotoxicol Environ Saf 2022;241:113818.


226. Saravi SSS, Dehpour AR. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci 2016;145:255–64.


227. Fenster L, Eskenazi B, Anderson M, Bradman A, Hubbard A, Barr DB. In utero exposure to DDT and performance on the Brazelton neonatal behavioral assessment scale. Neurotoxicology 2007;28:471–7.


228. Gaspar FW, Harley KG, Kogut K, Chevrier J, Mora AM, Sjödin A, et al. Prenatal DDT and DDE exposure and child IQ in the CHAMACOS cohort. Environ Int 2015;85:206–12.



229. Etiemble J, Cordier S. Pesticides and neurodevelopment in children. Environ Risques Sante 2022;21:51–65.

230. Kao CC, Que DE, Bongo SJ, Tayo LL, Lin YH, Lin CW, et al. Residue levels of organochlorine pesticides in breast milk and its associations with cord blood thyroid hormones and the offspring’s neurodevelopment. Int J Environ Res Public Health 2019;16:1438.



231. Sagiv SK, Nugent JK, Brazelton TB, Choi AL, Tolbert PE, Altshul LM, et al. Prenatal organochlorine exposure and measures of behavior in infancy using the Neonatal Behavioral Assessment Scale (NBAS). Environ Health Perspect 2008;116:666–73.



232. Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomäki S, Surcel HM, McKeague IW, Kiviranta HA, et al. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: a pilot study. Neurotoxicol Teratol 2013;38:1–5.



233. Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 2007;115:1482–9.



234. Yamazaki K, Itoh S, Araki A, Miyashita C, Minatoya M, Ikeno T, et al. Associations between prenatal exposure to organochlorine pesticides and thyroid hormone levels in mothers and infants: The Hokkaido study on environment and children's health. Environ Res 2020;189:109840.


235. Jeddy Z, Kordas K, Allen K, Taylor EV, Northstone K, Flanders WD, et al. Prenatal exposure to organochlorine pesticides and early childhood communication development in British girls. Neurotoxicology 2018;69:121–9.



236. Yim G, Minatoya M, Kioumourtzoglou MA, Bellavia A, Weisskopf M, Ikeda-Araki A, et al. The associations of prenatal exposure to dioxins and polychlorinated biphenyls with neurodevelopment at 6 Months of age: Multi-pollutant approaches. Environ Res 2022;209:112757.



237. Nishijo M, Anh NTN, Maruzeni S, Nakagawa H, Van Luong H, Anh TH, et al. Dioxin exposure in breast milk and infant neurodevelopment in Vietnam. Occup Environ Med 2013;70:656–62.


238. The TP, Ngoc TP, Van TH, Nishijo M, Ngoc NT, Thi HV, et al. Effects of perinatal dioxin exposure on learning abilities of 8-year-old children in Vietnam. Int J Hyg Environ Health 2020;223:132–41.


239. Pham TT, Nishijo M, Nguyen ATN, Tran NN, Van Hoang L, Tran AH, et al. Perinatal dioxin exposure and the neurodevelopment of Vietnamese toddlers at 1 year of age. Sci Total Environ 2015;536:575–81.


240. Nishijo M, Pham T, Nguyen A, Tran N, Nakagawa H, Hoang L, et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin in breast milk increases autistic traits of 3-year-old children in Vietnam. Mol Psychiatry 2014;19:1220–6.








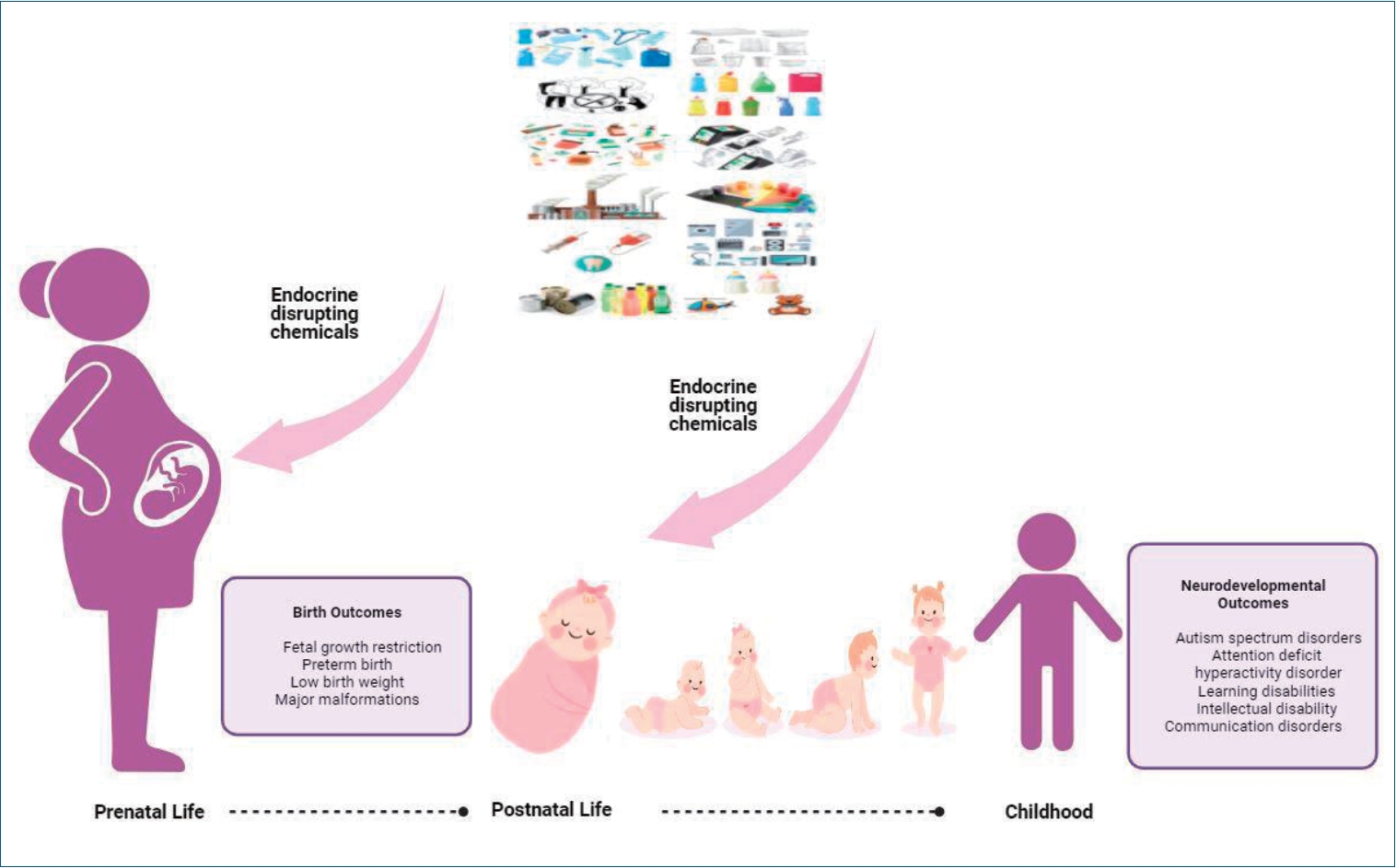
 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link PubMed
PubMed Download Citation
Download Citation


