Prednisolone impairs trabecular bone score changes in adolescents with 21-hydroxylase deficiency
Article information
Abstract
Background
Individuals with 21-hydroxylase deficiency (21OHD) require lifelong glucocorticoid (GC) therapy, which increases their risk of fragility fractures. However, fractures in GC-treated individuals can occur at normal bone mineral density (BMD) levels, suggesting an alteration in the bone microarchitecture.
Purpose
To evaluate trabecular bone microarchitecture and its changes in adolescents with 21OHD.
Methods
We enrolled 38 adolescents with 21OHD for whom complete clinical data and baseline and follow-up lumbar spine BMD (LSBMD) measurements were available. The mean duration was 1.5±0.6 years. Trabecular bone score (TBS), an indirect measurement of bone microarchitecture, was analyzed using iNsight software version 3.0. Impaired BMD and TBS were defined as z scores ≤ -1.5.
Results
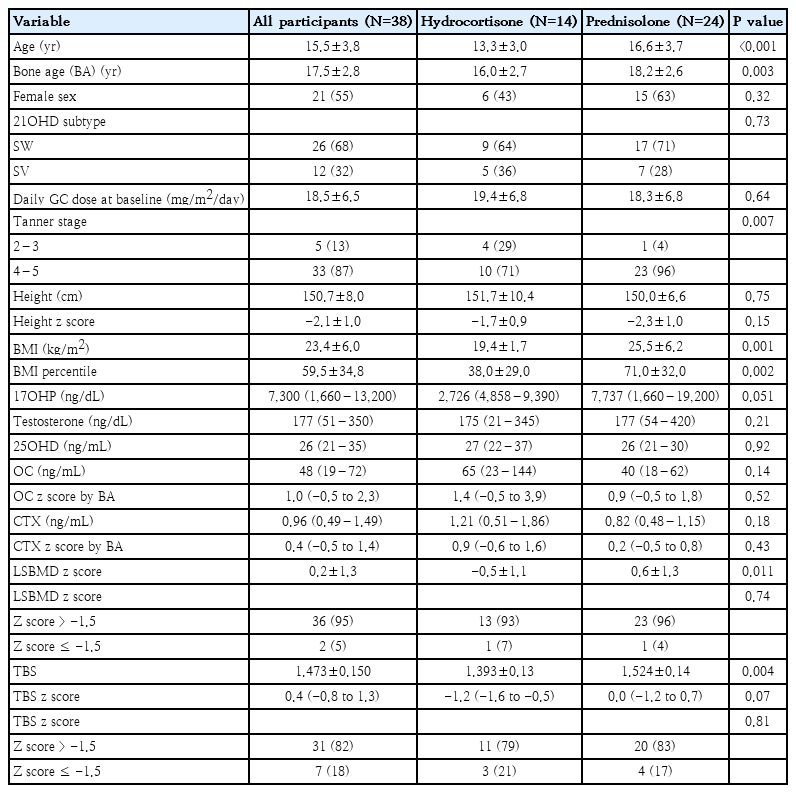
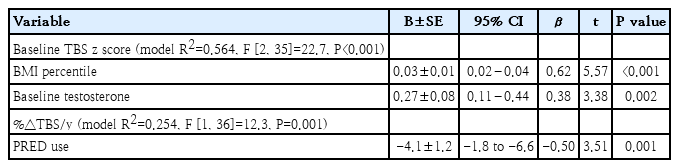
At baseline, participants (55% female; 68% salt- wasting type; mean age, 15.2±3.8 years; bone age, 17.5± 2.8 years; mean GC dose, 18.5±6.5 mg/m2/day) had the prevalence of impaired BMD and TBS of 5% and 18%, respectively. During follow-up, adolescents with 21OHD receiving prednisolone showed a lower annual percentage change in TBS than those who received hydrocortisone (P=0.028). A stepwise regression analysis showed that body mass index percentile (P<0.001) and testosterone concentration (P=0.002) were independent positive predictors of the baseline TBS z score, whereas prednisolone use was the only negative predictor of the annual percentage change in TBS (P=0.002).
Conclusion
Adolescents with 21OHD have a high prevalence of impaired bone microarchitecture. Furthermore, prednisolone therapy is associated with impaired bone microarchitecture development, suggesting that hydrocortisone may better preserve bone microarchitecture and should be considered the first-line treatment for this population.
Key message
Question: What is the prevalence of an impaired trabecular bone score (TBS), a measure of bone microarchitecture, in adolescents with 21-hydroxylase deficiency (21OHD)? Do prednisolone and hydrocortisone affect TBS differently in this patient population?
Finding: Impaired TBS was observed in 18% of participants. Prednisolone use negatively impacted TBS change.
Meaning: Impaired TBS is prevalent among adolescents with 21OHD. Prednisolone impairs trabecular bone microarchitecture development.
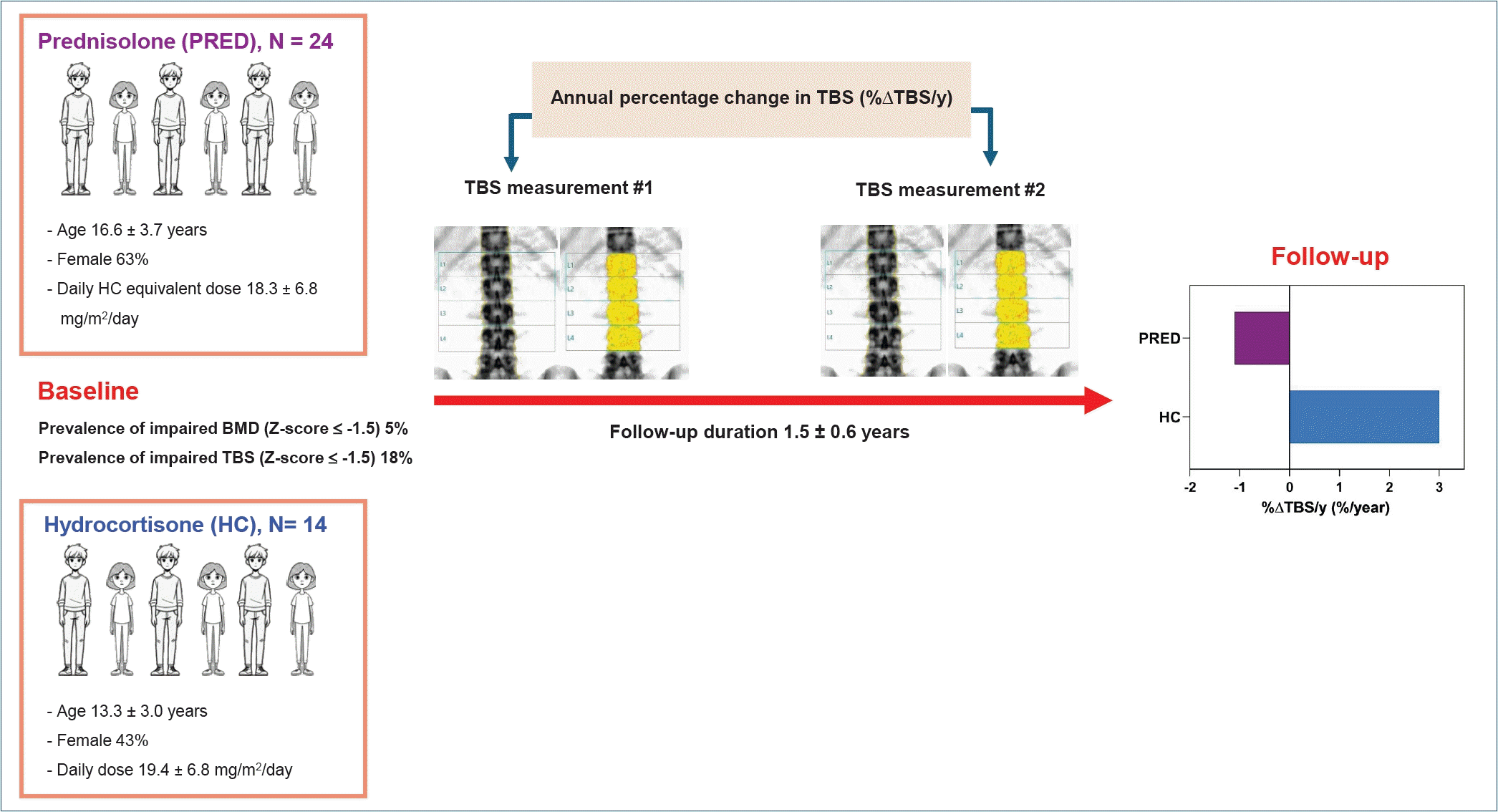
Graphical abstract. TBS, trabecular bone score; BMD, bone mineral density.
Introduction
Congenital adrenal hyperplasia (CAH) is a genetic disorder caused by defects in adrenal steroidogenic enzymes, with classic 21-hydroxylase deficiency (21OHD) being the most prevalent form accounting for 95% of individuals with CAH [1]. This enzymatic defect results in decreased synthesis of glucocorticoid (GC) and mineralocorticoid as well as increased androgen production. Due to the lack of an effective GC preparation or dosing regimen that mimics the normal cortisol circadian rhythm, individuals with 21OHD often require supraphysiologic doses of GC2) to suppress adrenocorticotropic hormone (ACTH) and androgen production [3]. As such, individuals with 21OHD have an increased risk of GC-induced adverse effects such as cardiovascular disease and osteoporosis [4].
Fracture rates are elevated in individuals with 21OHD across all age groups [4]. While several studies have demonstrated decreased bone mineral density (BMD) in individuals with 21OHD compared with healthy controls [5-7], it is important to note that their BMD z scores or T scores typically remained above the conventional thresholds for diagnosing low BMD or osteoporosis [7-9]. Moreover, it is widely recognized that GC-induced osteoporotic fractures can occur at BMD levels higher than those observed in other forms of osteoporosis [10]. These findings suggest an alteration in bone microarchitecture, another determinant of bone strength, may play a role in the pathogenesis of GC-induced fractures [11]. Recently, a growing body of research has indicated that trabecular bone score (TBS), a measure of bone microarchitecture based on a grey-level variation of lumbar spine dual-energy x-ray absorptiometry (DXA) images, is a promising predictor for primary and secondary osteoporotic fractures in adults, independent of BMD or FRAX tool [12]. However, the use of TBS in pediatrics remains limited to certain conditions such as anorexia nervosa [13], type 1 diabetes [14], and inflammatory bowel disease [15]. While many studies have investigated BMD in individuals with 21OHD [5,9,16], there is little information on TBS in children and adolescents with classic 21OHD. Therefore, the present study aimed to assess TBS and its changes in adolescents with classic 21OHD. Additionally, we sought to evaluate the impact of GC type and dose on baseline and follow-up TBS.
Methods
1. Subjects
A total of 38 adolescents with classic 21OHD were enrolled for the TBS analysis. Thirty-three participants were included from our previous retrospective cohort9) where their BMD data were reported, and 5 participants were from the retrospective chart review of adolescents with 21OHD who were followed up at the Pediatric Endocrine Clinic, Khon Kaen University Hospital during January 2021 to December 2023. Inclusion criteria for this study were: (1) ages 8–24 years, (2) Tanner stage >1, (3) diagnosed with classic 21OHD by serum 17OHP ≥10,000 ng/dL and cortisol <18 μg/dL after a 250-μg ACTH stimulation test [17], (4) having both baseline and follow-up lumbar spine (LS) BMD measurements, (5) bone age (BA) measurement within 3–6 months of baseline LSBMD measurement, (6) complete clinical data regarding Tanner stage, body weight (BW), height, GC dose and regimen, (7) available data of baseline osteocalcin (OC) and C-telopeptide of type-1 collagen (CTX), and (8) complete data of baseline and follow-up concentrations of 17-hydroxyprogesterone (17OHP) and testosterone, which were measured every 3–6 months between the 2 LSBMD scans. Participants were excluded if they received medications affecting bone metabolism except GCs, vitamin D, or calcium supplements. Participants were classified as having salt-wasting (SW) or simple virilizing (SV) 21OHD based on hyponatremia, hypokalemia, or metabolic acidosis at the initial visit. This study was approved by the Khon Kaen University Ethics Committee in Human Research (HE671131).
2. GC dose calculation
The daily dose of hydrocortisone (HC) for participants was converted into mg per body surface area for each visit from baseline to follow-up. For those using prednisolone (PRED), the dose was presented in HC equivalent, with 1 mg of PRED considered equivalent to 4 mg of HC. The cumulative and average daily GC dose was subsequently determined using the following equations:
3. BA assessment
BA radiography was performed for each participant within 3–6 months of the baseline LSBMD measurement and rated using Greulich and Pyle method18) by the first author (PW).
4. Physical examination
Participant clinical data were obtained from electronic medical records. Our clinic followed a standard protocol for measuring height and BW. Height was measured with a stadiometer to the nearest of 0.1 cm, and BW was measured using a calibrated electronic scale to the nearest of 0.1 kg. Participant height was converted into z score by BA using the Thai growth chart. Body mass index (BMI) percentiles were calculated according to participant BA, using the World Health Organization growth reference. The breast or genital Tanner stages were assessed by pediatric endocrinologists (PW and OP) at every visit.
5. DXA scan
1) LSBMD measurement
Baseline and follow-up LSBMD were assessed using GE Lunar Prodigy Advance (GE Healthcare, Madison, WI, USA) and Hologic Discovery A (Hologic Inc., Bedford, MA, USA) DXA scanners in 30 and 8 participants, respectively. For data acquisition and analyses, we used the enCORE software version 18 (SP1) for the Lunar Prodigy Advance, and software version 13.3.0.1 for the Hologic Discovery A system. The precision of the Lunar and Hologic densitometer for LSBMD measurement was 0.022 g/cm2 and 0.028 g/cm2, respectively. The areal LSBMD (g/cm2) was evaluated on LS 1–4. The annual percentage change in LSBMD (%ΔLSBMD/yr) was calculated as follow:
LSBMD z scores were generated using BA according to the normative reference data for LS 2–4 in Thai children [19]. For participants older than 18 years or with a BA greater than 18 years, BMD z scores were calculated using BMD reference data at the age of 18 years. Due to the difference in densitometers used in our study, we used the previously published equation20) to convert the Hologic BMD values to Lunar BMD values, enabling the comparison of BMD z scores using the reference range for Thai children.
2) TBS measurement
The TBS iNsight software (Med-Imaps, Pessac, France) version 3.0 was used to analyze TBS from participant DXA images of LS 1–4. TBS values were converted into z scores based on BA using pediatric reference data for each densitometer [21,22]. The annual percentage change in TBS (%ΔTBS/yr) was calculated as follows:
6. Biochemical measures
Laboratory data were retrieved from the electronic medical records. Participant serum samples were drawn between 7–9 AM for 17OHP, testosterone, total 25-hydroxyvitamin D2/D3 (25OHD), OC, and CTX measurement. 17OHP was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS, API 4000 triple-quadrupole mass spectrometer, Applied Biosystems/MDS SCIEX). Testosterone, 25OHD, OC, and CTX concentrations were measured with electrochemiluminescence immunoassay on an automatic Roche Cobas e601 analyzer (Roche Diagnostics, Mannheim, Germany). 25OHD had a measuring range of 3–70 ng/mL with the reported repeatability and intermediate precision of <6.9% and <13.2%, respectively. OC had the measuring range of 0.5–300 ng/mL with the reported repeatability and intermediate precision of <1.4% and <2.4%, respectively. CTX had the measuring range of 0.01–6.0 ng/mL with the reported repeatability and intermediate precision of <4.8% and <5.8%, respectively. OC and CTX concentrations were expressed as z scores by BA as described in our previous study [9]. Average concentrations of 17OHP, testosterone, and 25OHD over the follow-up period were also calculated.
7. Statistical analysis
The analyses were performed in IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). The distributions of all variables were assessed before analysis. Descriptive statistics are presented as number (percentage), mean±standard deviation, or median (interquartile range [IQR]), as appropriate. Participants were stratified according to types of GC used. Group proportions were compared by the chi-square or Fisher exact test. Normally distributed data were compared using the t test or 1-way analysis of covariate. The Mann-Whitney U test was used for skewed continuous data. In this study, impaired BMD was defined as a z score of ≤-1.5, acknowledging that fractures can occur even with BMD z scores above -2 in GC-induced osteoporosis [10]. In adults, a TBS of ≤1.35 indicates partially degraded bone microarchitecture, which corresponds to a TBS z score of -1 to -2.23) Consequently, we arbitrarily defined TBS z scores of ≤-1.5 as impaired TBS. The Spearman method was used for the correlation analysis. Variables shown to be significantly correlated with baseline TBS z score and %ΔTBS/yr were included in stepwise linear regression models to identify independent predictors. Further, we also examined the influence of sex, BA, and GC type and dose on baseline TBS z score in the regression models. For %ΔTBS/yr, we examined the influence of sex, BA, average daily GC dose, average testosterone concentration, and %ΔLSBMD/y. Analyses were considered exploratory and hypothesis generating. P values <0.05 were considered statistically significant.
Results
1. Baseline participant characteristics
Baseline participant clinical data, biochemical profile and DXA scan measures are shown in Table 1. At baseline, 14 and 24 adolescents with classic 21OHD received HC and PRED, respectively. Six (43%) and 15 female participants (63%) were in the HC and PRED groups, respectively. There were no significant differences in 21OHD subtype and daily GC dose between the groups. Participants in the PRED vs. HC group was older (P<0.001), had more advanced BA (P=0.003) and pubertal stage (P=0.007), and had higher BMI percentile (P=0.002). However, height and height z score were not different between the 2 groups. The 2 groups had no differences in serum concentrations of 17OHP, testosterone, and 25OHD, and OC and CTX z scores. While participants receiving PRED exhibited higher LSBMD z score (P=0.011), their TBS z scores were similar to those receiving HC (P=0.07). Eight participants (21%) had impaired bone health, with 1 (12.5%) having impaired BMD only, 6 (75%) having impaired TBS only, and 1 (12.5%) having impaired both BMD and TBS. In participants with impaired TBS only, their BMD z scores ranged from -0.7 to 1.0.
2. Follow-up clinical, biochemical, and DXA-related data
Over the follow-up period, the HC versus PRED group received similar average daily GC doses and cumulative GC doses (Table 2). The HC versus PRED group had no differences in average concentrations of 17OHP, testosterone and 25OHD. However, despite having similar GC doses and biochemical concentrations, the HC group exhibited higher %ΔTBS/yr and %ΔLSBMD/yr, and had lower prevalence of negative TBS change (%ΔTBS/yr <0; P=0.008). %ΔTBS/yr remained significantly higher in HC group after adjusting for BA, sex, and BMI percentile (2.7±1.2%/yr vs. -0.9±0.8%/yr, P=0.028) (Fig. 1).
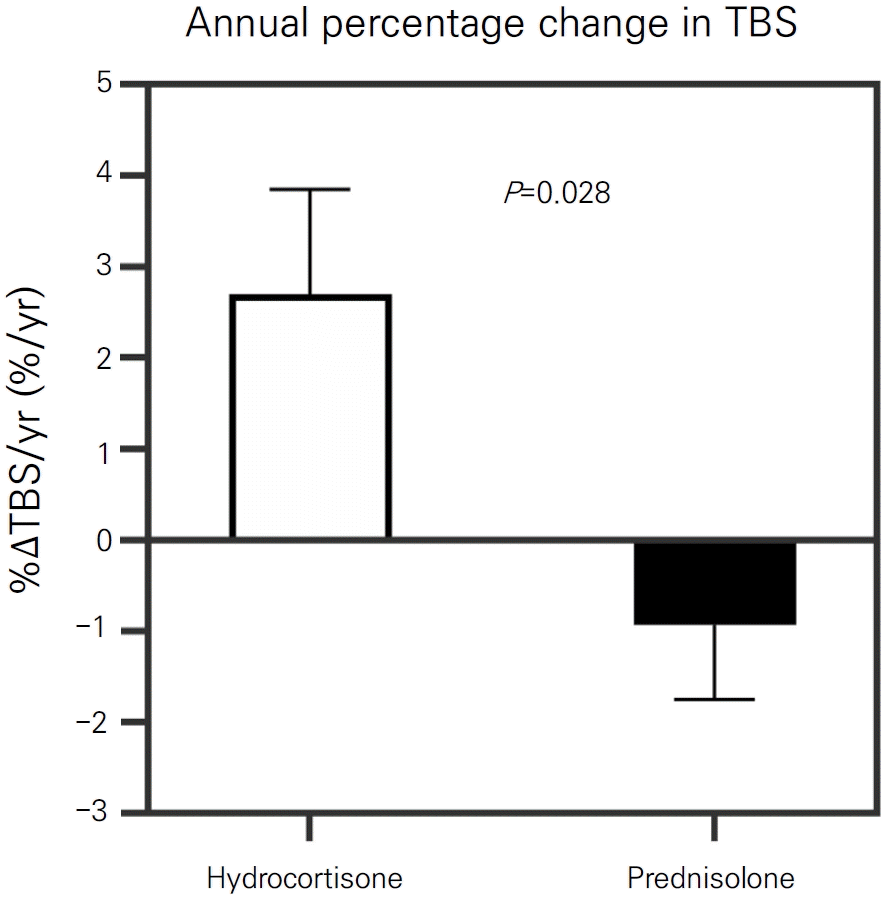
Annual percentage changes in TBS in adolescents with 21OHD receiving hydrocortisone versus prednisolone. Data are shown by group according to use of hydrocortisone or prednisolone. The bar and whisker graphs indicate the least squares mean and standard error of the mean, respectively. Analysis of covariance was used to examine the difference in %ΔTBS/yr with adjustment for bone age, sex, and body mass index percentile. TBS, trabecular bone score; %ΔTBS/yr, annual percentage change in trabecular bone score.
3. Associations between baseline and the change in TBS, and clinical data
1) Correlation analysis
Spearman correlations are shown in Table 3. At baseline, TBS z score was positively associated with BMI percentile (P<0.001), LSBMD z score (P=0.002), and testosterone (P=0.039). At follow-up, %ΔTBS/yr was negatively correlated with baseline age (P=0.021), BA (P=0.041), BMI percentile (P=0.017), and Tanner stage (P=0.013), but not with %ΔLSBMD/yr (P=0.059), GC dose or any average biochemical concentrations.
2) Stepwise linear regression analysis
Stepwise linear regression analysis was conducted to identify independent predictors of TBS z score and %ΔTBS/yr (Table 4). At baseline, BMI percentile (P<0.001) and testosterone concentration (P=0.002) emerged as significant independent predictors for TBS z score. During follow-up, PRED use was identified as the only negative predictor for %ΔTBS/yr (P=0.001). Further analyses revealed that sex, BA, and GC type and dose were not significant predictors for baseline TBS z score, while sex, BA, average daily GC dose, average testosterone concentration, and %ΔLSBMD/yr were not predictors for %ΔTBS/yr (data not shown).
Discussion
In this study, we investigated baseline and follow-up TBS, and examined factors associated with TBS change (%ΔTBS/yr) in adolescents with classic 21OHD. Our results showed that at baseline, while only 5% of participants with 21OHD had impaired BMD (z scores ≤-1.5), 18% of participants exhibited impaired TBS (z scores ≤-1.5). At follow-up, participants receiving PRED had decreased %ΔTBS/yr compared to those receiving HC. In stepwise regression models, we found that PRED was the only independent predictor for impaired TBS change. These suggest PRED has a negative impact on TBS development, thus, HC may be the preferred GC formulation for preserving development of bone microarchitecture in adolescents with 21OHD.
It is well-established that measuring TBS alongside BMD enhances fracture risk assessment in adults receiving chronic GC therapy [11,24]. This is due to the preferential impact of GCs on trabecular bone microarchitecture. A study involving 46 young adults with 21OHD has demonstrated a 24% prevalence of abnormal TBS z score (≤-2) and 4% for abnormal BMD z score. Consistent with these findings, our baseline results showed a higher prevalence of impaired TBS (18%) compared to impaired BMD (5%) in adolescents with 21OHD. Most adolescents with impaired TBS had BMD z score within the normal range, with only one participant showing a congruent decrease in both TBS and BMD. Other studies in individuals exposed to supraphysiologic doses of GCs such as those with rheumatologic diseases [25] or endogenous Cushing syndrome [26] have similarly found that abnormal TBS is more prevalent than decreased BMD [25]. Collectively, these findings suggest that TBS evaluation may enhance the detection of impaired bone health in adolescents with 21OHD.
In many countries, PRED is commonly prescribed for fully-grown adolescents and adults with adrenal insufficiency due to its cost-effectiveness, widespread availability and ease of administration. Previous studies have demonstrated that, in contrast to higher daily doses of HC (≥25–30 mg [27]), PRED even at low doses (2.5–5 mg [28]), which are equivalent to 10–20 mg of HC, increases fracture risk. While decreased BMD in individuals with 21OHD has been associated with PRED use compared to HC [7,9,16], there have been no in vivo or in vitro studies specifically addressing the effects of different forms of GCs on bone microarchitecture. Our study is the first to demonstrate that adolescents with 21OHD receiving PRED, as opposed to HC, exhibited a smaller change in TBS, despite receiving similar doses of GC. Furthermore, PRED was the only independent predictor for decreased TBS change. In adults using HC for autoimmune adrenalitis, their TBS was similar to that of healthy controls [29]. Another study found no association between the use of dual-release HC (median [IQR] dose of 13.2 [10.3–16.2] mg/m2) and TBS changes [30]. Taken together, our findings and those of other studies suggest that TBS degradation may be a crucial link between PRED use and increased fracture risk. Further research is needed to investigate the mechanisms underlying abnormal bone microarchitecture in different forms of GCs.
Adolescents using PRED also exhibited a higher proportion of TBS decline (i.e., negative TBS change). Kalkwarf and colleagues demonstrated a trajectory for TBS development in healthy children and adolescents, showing that TBS being steady during prepubertal years, rising greatly at the onset of the pubertal growth spurt, gradually increasing thereafter, and finally reaching a plateau at 19–20 years of age [31]. Therefore, a TBS decline in our adolescents receiving PRED might indicate abnormal development of trabecular microarchitecture. Further, the annual TBS decline rate (0.9%) in these adolescents is faster than the age-dependent decline in adults (0.11%–0.27%/yr) [23]. On the other hand, adolescents using HC had a mean annual increase in TBS (2.7%/yr) similar to that found in healthy adolescents reported by Kalkwarf (2.9%–3.4%/yr at chronological age of 14–16 years) [31]. As such, using HC in fully-grown adolescents and adults with 21OHD may preserve bone microarchitecture. However, our study did not find any association between the cumulative or average daily dose of GC and changes in TBS. Previous studies have shown mixed results including significant association [24,32] and no association with GC dose [11,29,32]. It is important to note that in most studies observing no association, the mean daily dose of PRED ranged from 3.3–6.5 mg (equivalent to ~8–16 mg/m2/day of HC) [29,32], while only one study involved participants using a higher PRED dose of 14.5 mg (equivalent to ~36 mg/m2/day of HC) [11].
We investigated other factors associated with both baseline and the change in TBS in adolescents with 21OHD. At baseline, we demonstrated that testosterone was independently associated with TBS z score. In men and male rats, testosterone promotes both BMD acquisition and trabecular bone number by inhibiting trabecular bone resorption [33]. Upon follow-up, we demonstrated that advancing age, BA and Tanner stage were negatively correlated with TBS change. Notably, our participants exhibited markedly advanced BA and Tanner stage at study entry. Such findings are consistent with previous studies in children showing that the magnitude of TBS increase diminishes with age [31,34]. Our study also identified BMI as an independent predictor for TBS z score at baseline. However, during follow-up, this association was not significant in regression models. Data regarding the association between BMI and the TBS change vary across studies. It appears that the association between BMI and TBS is influenced by developmental stage [31,34], sex [24,34], and health status [13,15,21]. Among the pediatric population regardless of health status, BMI is positively associated with TBS [15,31,34]. In GC-treated adults, many studies found a negative correlation between TBS and BMI [11,35]. Further studies are needed to investigate the effect of BMI across specific conditions.
Our study has limitations. First, due to the retrospective design, we could not ascertain participants' medication compliance, which may influence the dose-response relationship with TBS. Additionally, some relevant data, such as physical activity and dietary intake, were not available for analysis. Second, the small sample size, lack of healthy controls, and short follow-up duration may limit the generalizability of our findings. The study population is also limited to Thai adolescents, and while this provides valuable insights into this specific demographic, the findings may not be fully applicable to other ethnic groups. Third, there is no normative data for TBS in Thai children, nor is there a consensus definition for abnormal TBS in pediatrics, thus, the TBS z score results should be interpreted with caution.
In conclusion, impaired bone microarchitecture is prevalent in adolescents with 21OHD. TBS measurement may enhance the identification of impaired bone health in 21OHD. Furthermore, PRED therapy is independently associated with impaired bone microarchitecture development in adolescents with 21OHD, suggesting HC may better preserve bone microarchitecture and be considered as the first-line treatment in this patient population.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
Khon Kaen University, Faculty of Medicine Invitation Research Grant [IN64118].
Acknowledgments
Part of this work was accepted for a poster presentation at the European Calcified Tissue Society Meeting 2024 in Marseille, France, 25-28 May 2024. We extend our gratitude to the patients and their families for their participation in this study. We also acknowledge the support of the Khon Kaen University Faculty of Medicine and the Mekong Health Science Research Institute Biobank Project.
Author contribution
Conceptualization: PW; Formal Analysis: PW, YR; Investigation: PW, PN; Methodology: PW, PN, YR, NW; Project Administration: PW, PN; Writing – Original Draft: PW; Writing – Review & Editing: PW, CP, OP