Beyond COVID-19: meeting the challenge of evolving pediatric invasive group A streptococcal disease
Article information
Key message
Pediatric for invasive group A Streptococcus has resurged globally with increasing severity and toxin-mediated presentations. Beta-lactams remain the first-line treatment, but linezolid has emerged as a safe alternative in cases refractory to β-lactams. Early intravenous immunoglobulin use may improve outcomes in severe streptococcal toxic shock syndrome cases, while C-reactive protein and procalcitonin aid early risk stratification. Integrating global surveillance and individualized therapy is crucial in the postpandemic era.
The resurgence of pediatric invasive Streptococcus pyogenes (group A Streptococcus [iGAS]) infections after the coronavirus disease 2019 pandemic has drawn attention worldwide [1]. It has been reported in several countries, including the United Kingdom, Spain, Japan, and the United States, with the emergence of hypervirulent emm1 and emm12 lineages, particularly the M1UK variant. In Korea, a recent national research report documented a substantial postpandemic resurgence of GAS infections, with scarlet fever cases increasing by more than tenfold from 2022 to 2024, the majority occurring in children under 10 years of age [2]. The report identified 383 iGAS cases between 2015 and 2024, with an estimated mortality rate of approximately 14%, including the detection of the M1UK lineage in 2020 and 2024. This postpandemic pattern reflects both an “immunity gap” from prolonged infection control measures and possible strain evolution leading to increased toxin-mediated disease.
Buricchi et al. [3] recently provided valuable insights from an Italian tertiary pediatric center, where 46 children with confirmed or probable iGAS were analyzed over 2 years, from September 2022 to September 2024. Pneumonia with pleural effusion or empyema was the predominant presentation; 35% required pediatric intensive care, and the mortality rate reached 4.3%. The study highlighted that while standard β-lactam and clindamycin therapy remains central, linezolid and intravenous immunoglobulin (IVIG) as alternative or adjunctive treatments are becoming increasingly relevant in refractory or severe cases.
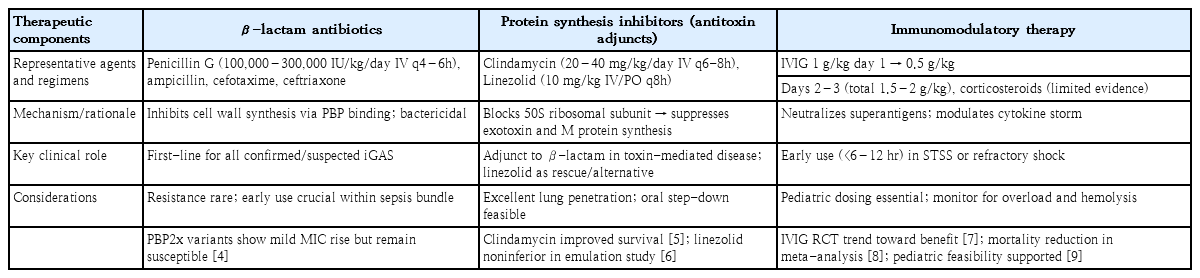
Table 1 summarizes the current antibiotic and adjunctive therapeutic strategies for invasive S. pyogenes infections, highlighting the evolving roles of toxin-targeted agents and immunomodulatory approaches. While β-lactams remain universally effective, increasing attention is warranted for emerging pbp2x mutations associated with modest increases in minimum inhibitory concentrations [4]. Protein synthesis inhibitors, particularly clindamycin and linezolid, play essential roles in suppressing toxins and in lung involvement. β-lactam plus clindamycin combination therapy remains the cornerstone for invasive S. pyogenes infections, particularly in cases of necrotizing fasciitis and streptococcal toxic shock syndrome (STSS). The toxin-suppressive properties of clindamycin have been demonstrated in experimental and clinical settings, and extensive multicenter cohort analyses have confirmed lower mortality rates with its adjunctive use [5]. More recently, linezolid has emerged as a viable alternative with comparable efficacy and better tolerability for toxin-driven or pulmonary presentations [6]. In the cohort of Buricchi et al. [3], linezolid was successfully used in cases refractory to β-lactams and clindamycin, supporting its role as a secondary adjunct in pediatric iGAS.
IVIG has long been used in STSS because of its ability to neutralize superantigens and modulate immune dysregulation. A landmark European randomized trial by Darenberg et al. [7] suggested a trend toward a survival benefit despite limited power. A subsequent meta-analysis by Parks et al. [8] further indicated that polyspecific IVIG combined with clindamycin reduced mortality rates in STSS. In children, recent feasibility trials demonstrated that early administration (within 6–12 hours of shock recognition) is logistically achievable and may improve outcomes [9]. The finding of Buricchi et al. [3] that delayed IVIG use in irreversible shock failed to confer benefits echoes this principle. However, pediatric evidence remains limited, and further studies are warranted to determine optimal timing and patient selection. The use of corticosteroids, which are sometimes used to treat hyperinflammation, is not supported by consistent pediatric evidence and should not be routinely chosen.
Elevated C-reactive protein and procalcitonin (PCT) levels, as demonstrated by Buricchi et al. [3], can aid early identification of children at risk of intensive care admission. Meta-analyses further support PCT's superior diagnostic performance for invasive bacterial infections [10]. These markers can complement, although not replace, clinical judgment and microbiological confirmation.
The postpandemic reemergence of pediatric iGAS underscores the need to adapt surveillance and therapeutic paradigms. Evidence from Italy and other regions converges on a model of individualized, toxin-targeted therapy anchored in β-lactams, enhanced by protein synthesis inhibitors such as linezolid, and supplemented by timely immunomodulation when indicated. Continued international collaboration and pediatric-specific trials are essential for translating these evolving strategies into standardized evidence-based care. Advances in rapid diagnostics to support antibiotic stewardship and the progress of multiple GAS vaccine candidates entering early clinical evaluations represent essential opportunities. Focused efforts to refine pediatric diagnostic and therapeutic pathways and establish the safety and immunogenicity profiles of future vaccines are critical for reducing the global burden of GAS.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
HWK is the only contributing author listed for this manuscript.