All issues > Volume 53(10); 2010
Treatment of obstructive sleep apnea in children
- Corresponding author: Young Min Ahn, M.D. Department of Pediatrics, Eulji General Hospital, Eulji University, 171-1 Hagye-dong, Nowon-gu, Seoul 138-775, Korea. Tel: +82.2-970-8228, Fax: +82.2-976-5441, aym3216@eulji.ac.kr
- Received September 02, 2010 Accepted September 28, 2010
- Abstract
-
Obstructive sleep apnea (OSA) in children is a frequent disease for which optimal diagnostic methods are still being defined. Treatment of OSA in children should include providing space, improving craniofacial growth, resolving all symptoms, and preventing the development of the disease in the adult years. Adenotonsillectomy (T&A) has been the treatment of choice and thought to solve young patient's OSA problem, which is not the case for most adults. Recent reports showed success rates that vary from 27.2% to 82.9%. Children snoring regularly generally have a narrow maxilla compared to children who do not snore. The impairment of nasal breathing with increased nasal resistance has a well-documented negative impact on early childhood maxilla-mandibular development, making the upper airway smaller and might lead to adult OSA. Surgery in young children should be performed as early as possible to prevent the resulting morphologic changes and neurobehavioral, cardiovascular, endocrine, and metabolic complications. Close postoperative follow-up to monitor for residual disease is equally important. As the proportion of obese children has been increasing recently, parents should be informed about the weight gain after T&A. Multidisciplinary evaluation of the anatomic abnormalities in children with OSA leads to better overall treatment outcome.
- Introduction
- Introduction
Obstructive sleep apnea (OSA), alternative name sleep-disordered breathing (SDB), refers to a group of respiratory conditions that are exacerbated during sleep due to increased upper airway resistance, disruption of ventilation, and sleep discontinuity1, 2). Contemporary descriptions of the condition begin with the report of Guilleminault3) in 1976 on 8 children with OSA accompanied by additional symptoms, such as hypertension, hyperactivity, and daytime somnolence, which have contributed to the present understanding that the clinical manifestations and treatments for OSA are substantially different in children compared with adults.The overall prevalence of parent-reported snoring, by any definition, in a meta-analysis was 7.45%4). Although the definition and measurement of OSA vary, a recent review of literature4) suggested the prevalence of OSA from 0.1% to 13%, and most studies report 1% to 4% diagnosed by varying criteria on diagnostic studies. Korean research reported that 4.3% of elementary school children snore almost every day5), and the prevalence of habitual snoring (>3 per week) was 7.1% among elementary school children6), and the prevalence of habitual snoring and OSA among high school children is 11.2% and 0.9% respectively7). OSA occurs at all ages, with the peak prevalence from 2 to 8 years of age, when tonsils and adenoids are largest in relation to the upper airway size1, 2 ,8). If unrecognized and untreated, OSAS can lead to neurobehavioral, growth, and cardiovascular sequelae in childhood1, 2). Adenoidectomy and/or tonsillectomy (T&A) are most often performed as the first-line treatment in childhood OSA1, 2). While it is generally believed that T&A is successful in most cases, recent reports showed success rates that vary from 27.2% to 82.9%9-13). New treatment modalities have been described in the last few years for childhood SDB beyond T&A14-16). The purpose of this article is to summarize current knowledge on available treatments for OSA in children to improve pediatric OSA management.
- Craniofacial morphological changes secondary to upper airway obstruction
- Craniofacial morphological changes secondary to upper airway obstruction
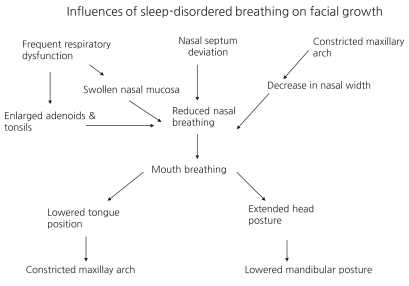
By the age of 4 years the craniofacial skeleton has attained 60% of adult size, and by the age of 12 years, it is 90% of adult size8). Craniofacial anomaly results from both genetic and environmental determinants. The observation of familial aggregates of SDB17, 18), and that an African American or Far Eastern origin is a risk factor for SDB, strongly suggests the importance of genes in linking the craniofacial skeleton and SDB19). However, environmental factors are also important determinants of craniofacial growth and development. Studies in monkeys and children have shown that upper airway obstruction with mouth breathing can induce craniofacial anomalies, which can be improved or normalized after SDB treatments such T&A20-22). Children who do not improve tend to have a narrower oropharyngeal airspace and mandibular retrognathia23, 24). SDB can induce craniofacial anomalies, which likely further increase the risk of SDB. This necessitates the early detection and treatment of SDB in children. It is suggested that continuation of SDB leads to worsening of the craniofacial pattern year by year25) (Fig. 1).
- Treatment of OSA in Children
- Treatment of OSA in Children
Treatment of OSA should include providing space for ventilation, improvement of cranio-facial growth, improvement all symptoms, and preventing the development of adult OSA.
- Surgical Treatment
- Surgical Treatment
- 1. Adenoidectomy and Tonsillectomy
- 1. Adenoidectomy and Tonsillectomy
1) Indications for T&A
1) Indications for T&A
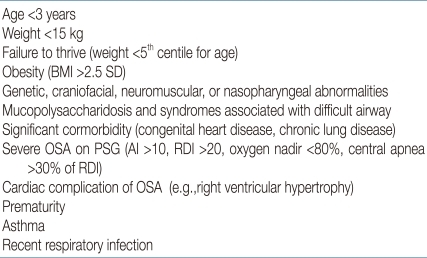
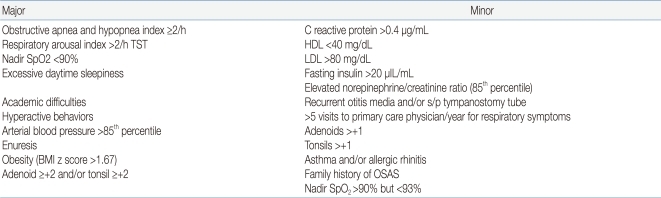
The indication for T&A in children with adenotonsillar hypertrophy is straightforward in the presence of OSA proven by polysomnography (PSG) or in snoring children with an abnormal, full-night pulse oxymetry recording1, 2, 26). The American Academy of Pediatrics (AAP) Clinical Practice Guidelines state that all children should be screened for snoring and recommended PSG as the gold standard for diagnosis of OSA because patient history and physical examination are poor discriminators of primary snoring and OSAS2). The prevalence of snoring was reported to be 6-12%, and the prevalence of OSAS was about 1-3%4). Testing 10% of children is a lot for PSG, but unnecessary surgery poses problems such as high cost and suffering in the patient. In a survey of the members of American Society of Pediatric Otolaryngology, only 10% of children undergoing T&A had PSG27). The recent guidelines2) that envisage the use of PSG as a reference method to differentiate primary snoring from OSAS are inapplicable in clinical practice due to cost of the test and the lack of laboratories that study sleep in the pediatric age group27, 28). Also, there is currently no definitive set of PSG criteria that will reliably discriminate those children who have OSAS and require treatment1, 2, 4, 28-32). Therefore, for otherwise healthy children with a history consistent with nighttime snoring; restless sleep; daytime symptoms including somnolence, behavior changes, and poor cognitive performance; and a physical exam consistent with adenotonsillar hypertrophy, with or without witnessed apnea, it is reasonable to proceed with T&A without prior PSG30, 31). Doubt in the diagnosis and being categorized as a high-risk individual for surgery are the most common indications for PSG28-30). Preoperative PSG is needed when the child has medical comorbidity, age <3 years, morbid obesity, small tonsils and adenoids, and history and physical findings that do not correlate with the degree of airway obstruction1, 2, 28-31) (Table 1).T&A is generally accepted as the first-line treatment in associated medical conditions such as abnormal control of upper airway muscles, e.g., cerebral palsy, Down syndrome, and obesity, even in the absence of clear adenotonsillar hypertrophy. However, persistence or recurrence of snoring and SDB after T&A is also common in these conditions1, 2).The PSG diagnosis of OSAS in children is defined as apnea hypopnea index (AHI) >1/h, according to the criteria of the American Academy of Sleep Medicine33), but there have been different criteria for childhood OSAS such as apnea index >1/h or AHI ≥1.5/h in several studies1, 2, 4, 34). This does not mean that every child with an AHI ≥1.5/h will benefit from treatment29-32). There is good consensus that moderate to severe PSG abnormality (AHI ≥5) should be treated with T&A1, 32, 35). However, for mild PSG abnormality, T&A is controversial and should depend on clinical symptoms and physical examination1, 2, 29-32).Gozal35, 36) suggested his criteria for diagnosis of pediatric OSA that requires treatment, like Jones criteria for the diagnosis of rheumatic fever, which included a combination approach of nocturnal PSG derived cut-off, and the presence of specific symptoms and measurable outcomes (biomarker, functional measures, etc.) (Table 2). It is not validated and revised continuously.2) Age, when to treat
2) Age, when to treat
The age limit for T&A may be related to fear about more frequent upper airway infections and postoperative complications. Children under 3 years old had more postoperative complications1, 2, 28, 37).OSAS occurs in children of all ages but peaks between 2 and 8 years, coinciding with the peak age of lymphoid hyperplasia8). Usually, children over 3 years or >15 kg could be operated without respiratory complications28). However, T&A can be performed in the presence of severe airway obstruction without any age restriction. Moreover, age is not a contraindication for T&A if OSA is present37, 38). Moreover, younger children under 3 years of age would have more severe OSAS, and surgery in younger children even with craniofacial abnormalities, syndromic features, and neurodevelopmental disorders had shown a more significant decrease in AHI after intervention than medical treatment38). This validates the safety and efficacy of surgical treatment in higher-acuity, young, tertiary-center patients with good respiratory investigation and support37-39).3) Tonsillectomy or adenoidectomy, or both?
3) Tonsillectomy or adenoidectomy, or both?
Both the tonsils and the adenoid should be removed, even if one or the other appears to be more enlarged, because OSAS is caused by both structural and neuromuscular abnormality and the treatment should widen the airway as much as possible1). There were anecdotal reports of the high persistence or recurrence of OSA if adenoidectomy or tonsillectomy alone was done instead of T&A1). Nieminen found improvement of OSA after tonsillectomy in 73% of children with previous adenoidectomy40). This suggests that adenoidectomy alone was insufficient. However, tonsillectomy alone is recommended for velopharyngeal incompetence such as submucous cleft palate1).4) Outcome
4) Outcome
The results of published data on success rate of T&A in curing OSA are highly variable, ranging from 24%10) to 100%11). A systematic review of all published series in 200611) reported an overall success rate of 82.9% for uncomplicated pediatric patients. In a recent meta-analysis of current literatures, the success rate was 66.3% when cure was defined as an AHI <113). In a recent multicenter retrospective study12), only 27.2% of children normalized their respiratory patterns during sleep as determined by the post-T&A PSG. Contrary to popular belief, these data demonstrate that pediatric sleep apnea is often not cured by T&A defined by PSG criteria, although there is a statistically significant AHI improvement in nearly all patients undergoing T&A11). High-risk groups of residual disease include patients with young or old age, morbid obesity, craniofacial syndrome, Down syndrome, neuromuscular disorders, and severe preoperative sleep apnea9-13). Preoperative AHI and BMI percentile are important factors that should be considered in the diagnosis and treatment plan of pediatric patients10, 12). Close postoperative follow-up to monitor for residual disease is important.For long-term outcome, there also appeared to be increased risk of snoring and desaturation on PSG 12 years later in children treated for OSA25).5) Risk of T&A, morbidity and mortality
5) Risk of T&A, morbidity and mortality
T&A continues to be associated with significant morbidity. The perioperative complication rate was 8.8%, and the unplanned admission rate was 8.0%28). Mortality of T&A can be as low as 1 in 10,000-35,0001, 16). Acute general complications of T&A include hemorrhage, respiratory decompensation, anesthetic complication, pain, and poor oral intake. Postoperative hemorrhage has been reported in up to 3-8%, higher in older children, and return to surgery has been reported in 0.8% to 1.8% of patients28, 41). Respiratory complication is increased when T&A is undertaken for airway obstruction relative to other indications. Postoperative respiratory complications has been reported in 4.4-15.1% in overall T&A, increased up to 23-36% in routine OSA cases and 60% in urgently performed cases. These complications result from transient worsening of OSA secondary to postoperative edema and increased secretion, respiratory depression from anesthetic agents or the use of oxygen with a blunted hypoxic drive and the occurrence of postoperative pulmonary edema28, 42). The major risk factors for post-operative respiratory complications in children include young age (2-3 years), high preoperative AHI, low nadir oxygen saturation (<70-80%), and the presence of concurrent medical problems (obesity, prematurity, cerebral palsy) or craniofacial disorders (Table 3). T&A is currently performed as an outpatient day-surgery, but high-risk patients should be hospitalized for continuous cardiopulmonary/oxygen monitoring38). Continuous positive airway pressure (CPAP) can be used in the perioperative period to stabilize patients before T&A and to treat postoperative complications39, 41). Late complications of T&A may be nasopharyngeal stenosis and velopharyngeal incompetence. Velopharyngeal incompetence is transient, and mostly resolves with time1).Tonsillotomy is less painful, but has a greater recurrence rate than tonsillectomy. Therefore, it should be avoided in SDB children with concomitant history of recurrent tonsillitis1).
- 2. Alternative Surgical Treatment
- 2. Alternative Surgical Treatment
1) Other soft tissue reduction procedures
1) Other soft tissue reduction procedures
- (1) Septoplasty and turbinectomy
- (1) Septoplasty and turbinectomy
With the increasing understanding of the progression of allergic rhinitis to SBD and the interplay between nasal obstruction and facial mal-developmen, which further predisposes to OSA, maximizing nasal patency quickly and safely should be considered in children with SDB. Septoplasy or turbinectomy may improve nasal airflow in older children. Due to the impact on facial growth, concerns about violating cartilage growth centers in the septum, no direct treatment of nasal septum deviation can be performed in children, however, inferior turbinate reduction is another viable option in treatment of allergic rhinitis to improve nasal flow and decrease nasal resistance much faster than nasal steroidal medication in older children1, 15, 42).- (2) Uvulopalatopharyngoplasty (UVPP)
- (2) Uvulopalatopharyngoplasty (UVPP)
UVPP is a surgical procedure involving the excision of the uvula and posterior portion of the palate and tonsils, and trimming and reorientation of the tonsillar pillar. In adults, it has a success rate of approximately 50%. UVPP is not commonly performed in children, because most children with sleep apnea do not demonstrate the redundant tissue found in adults. UVPP is reserved for children with abnormal upper airway neuromuscular tone, such as those with cerebral palsy or Down syndrome, and in obese children with high Mallampati scores or redundant lateral pharyngeal tissue1, 43).- (3) Lingual tonsillectomy
- (3) Lingual tonsillectomy
Enlargement of lingual tonsils is increasingly being recognized as a cause of OSA , especially in Down syndrome, obese children, residual OSA after T&A44). In about 35%, previous T&A is associated with an increased risk of overgrowth of the lingual tonsils. Endoscopic-assisted coblation lingual tonsillectomy is an effective technique for the treatment of lingual tonsillar hypertrophy causing persistent OSA in some children. It is important to identify enlargement of the lingual tonsils as a cause of OSA because it is one of the surgically curable causes of OSA. Especially when symptom recurs after T&A, lingual tonsillar hypertrophy should be suspected45).
An example of such procedures is radiofrequency ablation of the tongue base, which is only rarely undertaken in children, such as those with Down syndrome or other causes of macroglossia.2) Craniofacial surgery
2) Craniofacial surgery
Craniofacial surgery is indicated in complex patients who do not tolerate CPAP or who would also benefit from the cosmetic effect of surgery, particularly those with craniofacial anomalies1).3) Tracheostomy
3) Tracheostomy
- 3. Orthodontic treatment
- 3. Orthodontic treatment
1) Rapid maxillary expansion (RME)
1) Rapid maxillary expansion (RME)
RME is an orthodontic procedure that uses a fixed appliances with expansion screw anchored on selected teeth, causing skeletal expansion of the upper jaw by application of orthopedic force to the midpalatal suture in maxillary widening46). The procedure consists of an active increase of the palatal transverse diameters by progressive opening of the midpalatal suture (1 mm/d) during 10-20 days, followed by a fixed retention time of 6-12 months. Eventually, its indication was extended to OSA in children with or without T&A47).2) Mandibular distraction procedures and/or mandibular midline osteotomy
2) Mandibular distraction procedures and/or mandibular midline osteotomy
Distraction osteogenesis consists of slowly moving the mandible or midface in the direction desired by using a distraction device. It can be performed in children with severe micrognathia, including infants with Pierre Robin syndrome or hemifacial microsomia. Because the procedure take advantage of the rapid healing and capacity of growth in the pediatric skeleton, osteotomy with distraction of bone is widely accepted as the procedure of choice in the early management of airway obstruction caused by craniofacial disproportion48).
An appropriate treatment of upper airway anatomic abnormalities must be achieved as early and as completely as possible. It is much easier to use orthodontic treatment in children than in adults46) (Fig. 2).
- Medical therapies
- Medical therapies
- 1. Continuous positive airway pressure
- 1. Continuous positive airway pressure
Nasal positive airway pressure therapy to maintain upper airway patency is the main treatment of choice in adults with OSA. However, due to the different pathophysiology of childhood OSA, CPAP is reserved only for moderate to severe cases of OSA in children who are not eligible for T&A (noneligibility criteria includes neuromuscular diseases, obesity, severe bleeding tendency, craniofacial abnormality, idiopathic OSAS, and small tonsils and adenoids in adolescents)1, 2, 49). CPAP has also been prescribed for children whose SBD persisted despite T&A.CPAP is not curative and often needs to be used indefinitely. Thus, compliance with long-term CPAP therapy is a major issue, and behavioral intervention is helpful50). The remaining significant problems are related to the lack of optimal equipment for young children and frequent difficulties with regard to initial adaptation to the nasal mask and compliance to treatment.The most common side effects of CPAP use are nasal symptoms, including nasal congestion, rhinorrhea, dryness, and epistaxis, which are caused by the cooling and drying effect of CPAP, which, in turn, alters the nasal mucosa and impedes mucociliary clearance. Side effects, such as mask leak, skin irritation, and pressure sores, are generally mild and self-limited; however, the development of midface hypoplasia following prolonged use of nasal CPAP has been reported as a rare long-term complication1, 2, 51).- 2. Antibiotics
- 2. Antibiotics
Broad-spectrum antibiotics may offer temporary improvement52). However, the use of antibiotics does not seem to obviate the need for surgery in most cases with adenotonsillar hypertrophy.- 3. Anti-inflammatory Therapies
- 3. Anti-inflammatory Therapies
Upper airway inflammation is an important component of SDB in children. Chronic snoring causes repetitive vibration of upper airway soft tissues, inducing mechanical stress resulting in upper airway inflammation. Moreover, chronic systemic inflammation with SDB may contribute to upper airway inflammation. Such understanding has generated new therapeutic concepts in children with SDB. Nasal corticosteroids exert lympholytic action to treat inflammation and upper airway edema53). Nasal fluticasone for 6 weeks significantly decreased both adenoid size and AHI53). In an open-labeled study, the leukotriene receptor antagonist montelukast was found to be clinically effective in reducing disease severity in children with mild OSA54). In a subsequent study, a combination of intranasal steroids and leukotriene modifier for 12 weeks was found to be useful in children with residual OSA after adenotonsillectomy55). However, there was no long-term follow-up result more than 12 weeks after discontinuation of the medication.- 4. Weight Loss
- 4. Weight Loss
Weight loss is recommended for all obese children1, 56). After T&A, even obese children with OSA will demonstrate increase in weight gain57). After treatment of OSA, initial severe OSA, obesity, and those with a positive family history of OSA, initial high BMI, and African-American race, Mallampati score, and have other anatomical abnormalities of upper airway confer an independent increased risk for recurrence or persistence of SDB after T&A9-13). This implies that good weight control is important after surgical treatment. Postoperative PSG and CPAP are frequently recommended after T&A in obese children. Bariatric surgery is an effective weight reduction treatment for morbidly obese adults and children with severe apnea who failed other treatments.- 5. Oral Appliance
- 5. Oral Appliance
Tongue retraining or mandibular repositioning devices are widely used in adults with OSA to increase the oropharyngeal space, but most are cumbersome and poorly tolerated by children58).- 6. Supplemental Oxygen
- 6. Supplemental Oxygen
Supplemental oxygen abolishes desaturation episodes associated with OSA, but it does not treat the underlying obstruction and may worsen hypoventilation59). If supplemental oxygen is considered in SDB children with severe desaturation, it must be used for a few days only, while awaiting urgent T&A, and should be monitored for ETCO2.- 7. Environmental Control
- 7. Environmental Control
- Follow Up
- Follow Up
All children should be reevaluated clinically after surgery. Significant improvement may occur soon after surgery and, traditionally, OSA improves 6-8 weeks after T&A28). Those with severe OSA preoperatively continue to have risk factors for persistent OSA and thus require postoperative PSG9, 10). Adenoidal recurrence may occur, especially in very young children. If children develop a recurrence of OSA symptoms, lateral PNS X-ray or direct visualization of the adenoid by endoscopy could check the adenoidal regrowth. Revision adenoidectomy is required. Adhesion and contraction of posterior pharyngeal wall were sometimes found1, 28).
- Conclusion
- Conclusion
OSA in children is a frequent disease with a 1-4% prevalence rate. It imposes significant morbidity, causing neurobehavioral, cardiovascular, and endocrine complications. The impairment of nasal breathing with increased nasal resistance has a well-documented negative impact on early childhood maxillomandibular development, making the upper airway smaller and might lead to adult OSA after puberty. Optimal diagnostic methods and criteria are still being defined.T&A should be considered a first-line therapy. For the majority of children with OSA, T&A improved upper airway obstructive sign and symptoms, quality of life, and behavioral and cognition problems, and decreased polysomnographic abnormality. However, recent published data on the success rates of T&A in curing pediatric OSAS are highly variable. Close postoperative follow-up to monitor for residual disease is equally important. Parents should be informed about the weight gain after T&A, and reduction of body weight is considered primarily for obese children. Management with ongoing CPAP therapy and/or other surgical interventions and anti-inflammatory therapies are also considered. Initially for severe cases, multidisciplinary team approach is recommended to plan the optimal therapy.
- References
- 1. Sheldon SH, Ferber R, Kryger MH. Principles and practice of Pediatric sleep medicine. 2005;1st ed. Philadelphia: Elsevier Saunders Co, :197–267.2. Section on pediatric pulmonology and subcommittee on obstructive sleep apnea syndrome. American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704–712.
[Article] [PubMed]3. Guilleminault C, Eldridge FL, Simmons FB, Dement WC. Sleep apnea in eight children. Pediatrics 1976;58:23–30.
[Article] [PubMed]4. Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–252.
[Article] [PubMed] [PMC]5. Cho SJ, Kim EY, Rho YI, Yang ES, Park YB. Prevalence and associated factors of snoring in school-aged children. J Korean Pediatr Soc 2002;45:1340–1345.6. Seo WS, Koo BH, Kim MJ, Rho YH, Sung HM, Shin JH. Preliminary Study of Children's Sleep Problems in an Elementary School in Daegu. J Korean Acad Child Adolesc Psychiatry 2008;19:156–161.7. Shin C, Joo S, Kim J, Kim T. Prevalence and correlates of habitual snoring in high school students. Chest 2003;124:1709–1715.
[Article] [PubMed]8. Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:253–262.
[Article] [PubMed] [PMC]9. Guilleminault C, Huang YS, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: A prospective survey. Otolaryngol Head Neck Surg 2007;136:169–175.
[Article] [PubMed]10. Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O'Brien LM, Ivanenko A, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 2006;149:803–808.
[Article] [PubMed]11. Brietzke SE, Gallagher D. The effectiveness of tonsillectomy and adenoidectomy in the treatment of pediatric obstructive sleep apnea/hypopnea syndrome: a meta-analysis. Otolaryngol Head Neck Surg 2006;134:979–984.
[Article] [PubMed]12. Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, et al. Adenotonsillectomy outcomes in treatment of OSA in Children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676–683.
[Article] [PubMed]13. Friedman M, Wilson M, Lin HC, Chang HW. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol-Head Neck Surg 2009;140:800–808.
[Article] [PubMed]14. Kuhle S, Urschitz MS, Eitner S, Poets CF. Interventions for obstructive sleep apnea in children: a systematic review. Sleep Med Rev 2009;13:123–131.
[Article] [PubMed]15. Guilleminault C, Biol D, Li K, Quo S. A prospective study on the surgical outcomes of children with sleep-disordered breathing. Sleep 2004;27:95–100.
[Article] [PubMed]16. Praud JP, Dorion D. Obstructive sleep disordered breathing in children; Beyond Adenotonsillectomy. Pediatr Pulmonol 2008;43:837–843.
[Article] [PubMed]17. Sundquist J, Li X, Friberg D, Hemminki K, Sundquist K. Obstructive sleep apnea syndrome in siblings: an 8-year Swedish follow-up study. Sleep 2008;31:817–823.
[Article] [PubMed] [PMC]18. Schwab R, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, et al. Family aggregation of upper airway soft tissue structure in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 2006;173:453–463.
[Article] [PubMed]19. Guilleminault C, Quo SD. Sleep-disordered breathing. A view at the beginning of the new millennium. Dent Clin North Am 2001;45:643–656.
[PubMed]20. Vargervik K, Miller AJ, Chierici G, Harvold E, Tomer BS. Morphologic response to changes in neuromuscular patterns experimentally induced by altered modes of respiration. Am J Orthod 1984;85:115–124.
[Article] [PubMed]21. Valera FC, Travitzki LV, Mattar SE, Matsumoto MA, Elias AM, Anselmo-Lima WT. Muscular, functional and orthodontic changes in preschool children with enlarged adenoids and tonsils. Int J Pediatr Otorhinolaryngol 2003;67:761–770.
[Article] [PubMed]22. Zettergren-Wijk L, Forsberg CM, Linder-Aronson S. Changes in dentofacial morphology after adeno-/tonsillectomy in young children with obstructive sleep apnea - a 5-year follow-up study. Eur J Orthod 2006;28:319–326.
[Article] [PubMed]23. Guilleminault C, Parinen M, Praud MA, Quera-Salva MA, Powell N, Reily R, et al. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr 1989;114:997–999.
[Article] [PubMed]24. Tasker C, Crosby JH, Stradling JR. Evidence for persistence of upper airway narrowing during sleep, 12 years after adenotonsillectomy. Arch Dis Child 2002;86:34–37.
[Article] [PubMed] [PMC]25. Guilleminault C, Lee JH, Chan A. Pediatric obstructive sleep apnea syndrome. Arch Pediatr Adolesc Med 2005;159:775–785.
[Article] [PubMed]26. Nixon GM, Kermack AS, Davis GM, Manoukian JJ, Brown KA, Brouillette RT. Planning adenotonsillectomy in children with obstructive sleep apnea: the role of overnight oxymetry. Pediatrics 2004;113:e19–e25.
[Article] [PubMed]27. Mitchell RB, Pereira KD, Freidman NR. Sleep-disordered breathing in children: Survey of current practice. Laryngoscope 2006;116:956–958.
[Article] [PubMed]28. Waters KA, Cheng AT. Adenotonsillectomy in the context of obstructive sleep apnea. Paediatr Respir Rev 2009;10:25–31.
[Article] [PubMed]29. Marcus CL. Childhood obstructive sleep apnea: to treat or not to treat, that is the question. Thorax 2010;65:4–5.
[Article] [PubMed]30. Yellon RF. Is polysomnography required prior to tonsillectomy and adenoidectomy for diagnosis of obstructive sleep apnea versus mild sleep disordered breathing in children? Laryngoscope 2010;120:868–869.
[Article] [PubMed]31. Ameli F, Brocchetti F, Semino L, Fibbi A. Adenotonsillectomy in obstructive sleep apnea syndrome. Proposal of a surgical decision-taking algorithm. Int J Pediatr Otorhinolaryngol 2007;71:729–734.
[Article] [PubMed]32. Marcus CL. Childhood obstructive sleep apnea syndrome: unanswered questions. Chest 2008;134:1114–1115.
[Article] [PubMed]33. International Classification of Sleep Disorders-ICSD. 2005;2nd ed. Westchester, IL: American Academy of Sleep Medicine.34. Traeger N, Schultz B, Pollack AN, Mason T, Marcus C, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol 2005;40:22–30.
[Article] [PubMed]35. Gozal D, Kheirandish-Gozal L. New approaches to the diagnosis of sleep-disordered breathing in children. Sleep Med 2010;11:708–713.
[Article] [PubMed]36. Kheirandish-Gozal L, Gozal D. The multiple challenges of obstructive sleep apnea in children : diagnosis. Curr Opin Pediatr 2008;20:650–653.
[Article] [PubMed]37. Brigance JS, Mitamoto C, Schilt P, Houston D, Wiebke JL, Govan D. Surgical management of obstructive sleep apnea in infants and young children. Otolaryngol Head Neck Surg 2009;140:912–916.
[Article] [PubMed]38. Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in children under 3 years. Otolaryngol Head Neck Surg 2005;132:681–684.
[Article] [PubMed]39. Robb PJ, Bew S, Kubba H, Murphy N, Primhak R, Rollin AM, et al. Tonsillectomy and adenoidectomy in children with sleep-related breathing disorders: consensus statement of a UK multidisciplinary working party. Ann R Coll Surg Engl 2009;91:371–373.
[Article] [PubMed] [PMC]40. Walkers P, Gilles D. Post-tonsillectomy hemorrahage rates: Are they technique-dependent? Otolaryngol Head Neck Surg 2007;136(4 Suppl): S27–S31.
[Article] [PubMed]41. Statham MM, Elluru RG, Buncher R, Kaira M. Adenotonsillectomy for obstructive sleep apnea syndrome in young children :prevalence of pulmonary complications. Arch Otolaryngol Head Neck Surg 2006;132:476–480.
[Article] [PubMed]42. Sullivan S, Li K, Guilleminault C. Nasal obstruction in children with sleep-disordered breathing. Ann Acad Med Singapore 2008;37:645–648.
[Article] [PubMed]43. Kosko JR, Derkay CS. Uvulopalatopharyngoplasty: treatment of obstructive sleep apnea in neurologically impaired pediatric patients. Int J Pediatr Otorhinolaryngol 1995;32:241–246.
[Article] [PubMed]44. Lin AC, Koltai PJ. Persistent pediatric obstructive sleep apnea and lingual tonsillectomy. Otolaryngol Head Neck Surg 2009;141:81–85.
[Article] [PubMed]45. Park CH, Shim HJ, Kim EJ, Ahn YM. A Case of treatment in obstructive sleep apnea syndrome in children with lingual tonsillar hypertrophy. Korean J Otorhinolaryngol-Head Neck Surg 2008;51:562–565.46. Guilleminault C, Li KK. Maxillomandibular expansion for the treatment of sleep disordered breathing. Laryngoscope 2004;114:893–896.
[Article] [PubMed]47. Villa MP, Malagola C, Pagani J, Montesano M, Rizzoli A, Guilleminault C, et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12month follow-up. Sleep Med 2007;8:128–134.
[Article] [PubMed]48. Guilleminault C, Quo S, Huynh NT, Li K. Orthodontic expansion treatment and adenotonsillectomy in the treatment of obstructive sleep apnea in prepubertal children. Sleep 2008;31:953–957.
[Article] [PubMed] [PMC]49. Marcus CL, Ward SL, Mallory GB, Rosen CL, Beckerman RC, Weese-Mayer DE, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr 1995;127:88–94.
[Article] [PubMed]50. Downey R III, Perkin RM, MacQuarrie J. Nasal continuous positive airway pressure use in children with obstructive sleep apnea younger than 2 years of age. Chest 2000;117:1608–1612.
[Article] [PubMed]51. Marcus CL, Rosen G, Ward SL, Halbower AC, Sterni L, Lutz J, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics 2006;117:e442–e451.
[Article] [PubMed]52. Li KK, Riley RW, Guilleminault C. An unreported risk in the use of home nasal continuous positive airway pressure and home nasal ventilation in children: mid-face hypoplasia. Chest 2000;117:916–918.
[Article] [PubMed]53. Sclafani AP, Ginsburg J, Shah MK, Dolitsky JN. Treatment of symptomatic chronic adenotonsillar hypertrophy with amoxicillin/clavulanate potassium: short- and long-term results. Pediatrics 1998;101:675–681.
[Article] [PubMed]54. Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr 2001;138:838–844.
[Article] [PubMed]55. Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364–370.
[Article] [PubMed] [PMC]56. Kheirandish L, Goldbart AD, Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in children. Pediatrics 2006;117:e61–e66.
[Article] [PubMed]57. Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc 2008;5:274–282.
[Article] [PubMed] [PMC]58. Kudoh F, Sanai A. Effect of tonsillectomy and adenoidectomy on obese children with sleep-associated breathing disorders. Acta Otolaryngol Suppl 1996;523:216–218.
[PubMed]
Table 2
Proposed Criteria for the Diagnosis of Pediatric Obstructive Sleep Apnea that Requires Treatment35)

 About
About Browse articles
Browse articles For contributors
For contributors