All issues > Volume 53(12); 2010
Neonatal innate immunity and Toll-like receptor
- Corresponding author: Hye Sun Yoon, M.D. Department of Pediatrics, Eulji Hospital, 280-1 Hagye-1 dong, Nowon-gu, Seoul 139-711, Korea. Tel: +82.2-970-8228, Fax: +82.2-976-5441, yhs3211@eulji.ac.kr
- Received November 15, 2010 Accepted November 30, 2010
- Abstract
-
The innate immune response is the first line of defense against microbial infections. Innate immunity is made up of the surface barrier, cellular immunity and humoral immunity. In newborn, immunologic function and demands are different to adults. Neonatal innate immunity specifically suppresses Th1-type immune responses, and not Th2-type immune responses, which are enhanced. And the impaired response of macrophages is associated with the defective innate immunity in newborn period. Toll-like receptors (TLRs) play a key roles in the detection of invading pathogens and in the induction of innate immune responses. In newborn, the expression of TLRs is age dependent, so preterm has low expression of TLRs. Also, there are defects in signaling pathways downstream of TLRs. As a consequence, the defects of TLRs activity cause the susceptibility to infection in the neonatal period.
- Introduction
- Introduction
Neonatal immunity is immature. Both defective adaptive immunity and innate immunity contribute to the impaired neonatal host defense. Adaptive immune response is impaired at birth due minimal preexisting antigen exposure and due to the impaired functions of effector B and T cells1). And adaptive immunity is slowly developing system after exposure of microorganisms. In contrast, innate immune response does not require immunologic experience to function well. And innate immunity is the critical first-line barrier of host defense, bridging the interval between exposure to the pathogen and the specific response of the adaptive immune system2). Therefore, a neonate is largely dependent on passively acquired antibodies and defective innate immune responses in the defense against microorganisms3). This review describes the current understanding of neonatal innate immunity and its important receptors, Toll-like receptors (TLRs).
- Neonatal innate immunity
- Neonatal innate immunity
- 1. Surface barrier
- 1. Surface barrier
For the immune system of surface barrier, the mucous membrane and skin have tissue macrophages and dendritic cells act as the first-line defense mechanism against the entry of microorganism. When microorganism enters to the body, these cells under the skin first recognize pathogen and are activated for secretion of inflammatory cytokines and recruitment of many immune cells. After birth, the neonatal skin and gut are rapidly colonized with microbial floras. The newborn skin has protective mechanism including vernix caseosa and protective epithelial cells. The vernix caseosa consists of lysozyme, α-defensins, ubiquitin, psoriasin and antimicrobial free fatty acids. It can act with immune cells to kill the microorganisms6).The gastrointestinal tract is an important immune organ. It could secret peptides and proteins and other host defenses. Innate immunity plays a central role in intestinal immune defense against invading pathogens. Also, It serves as a bridge to the activation of the adaptive immune system. Some kinds of intestinal mucosal surface receptors serve as the pathogen recognition receptors in the innate immune defense system. Secreted bactericidal peptides or defensins produced by the intestinal epithelia represent another crucial element of innate mucosal immune defense. Mutations in pattern recognition receptors and dysfunction of secretory bactericidal peptides may impair host immune defenses leading to an invasion of pathogens resulting in chronic inflammation of the gut7). Neonatal intestinal immunity can be significantly modified by breastfeeding. Breast milk contains diverse immunological factors, including innate immune molecules such as the antimicrobial proteins and peptides, lactoferrin and lysozyme8).- 2. Cellular immunity
- 2. Cellular immunity
Mononuclear inflammatory cells, particularly mast cells and tissue macrophages, play a major role in the cellular immunity of host defense against any microbes that break the physical barriers such as intact skin. Mast cells acts by releasing tumor necrosis factor-alpha (TNF-α). TNF-α recruits other innate host defense cells such as polymorphonuclear leukocytes (PMNs), monocytes and dendritic cells. Also, it can instruct dendritic cells to process and present microbial antigenic material to T lymphocytes. This is a initial step in the generation of a specific immune response4).Circulating monocytes differentiate into mononuclear macrophages which can release of many proinflammatory cytokines. In the newborn period, the function and the number of monocyte and mononuclear macrophage are normal. In vitro studies of cord blood from healthy term baby have effective phagocytosis activity similar to adult. But the functional maturation of TLR expression on the immune cell is main problem for the defective immune system.Also, selective impairment of specific immune system existed. For example, interferon-gamma (IFN-γ) production is decreased in the newborn period9). Overall, neonatal cellular immune system expresses selective impairments in Th1-type immune response production and Th2-type immune response enhancement. Th1 and Th2-type immune response derived from proliferating helper T cells. Proliferating helper T cells differentiate into two major subtypes of cells known as Th1 and Th2 cells (also known as type 1 and type 2 helper T cells, respectively). Th1 cells produce IFN-γ, interleukin (IL)-2, and tumor necrosis factor-beta (TNF-β), which activate macrophages and are responsible for cell-mediated immunity and phagocyte-dependent protective responses. By contrast, Th2 cells produce IL-4, IL-5, IL-10, and IL-13, which are responsible for strong antibody production, eosinophil activation, and inhibition of several macrophage functions, thus providing phagocyte-independent protective responses. Th1-type immune system relate to the alloimmune reaction between mother and fetus, and major cause of fetal loss. So, during pregnancy, placenta-derived Th2-type immune cytokines antagonize Th1-type immune responses, there are neonatal Th2-type immune polarization10). Therefore, during the newborn period, Th1-type immune cytokines such as TNF, IFN-α, IFN-γ, IL-12, IL-1b are decreased. In contrast, Th2-type immune cytokines such as IL-6, IL-8, IL-10, and IL-23 increased during newborn period.Also, macrophages from newborn are hyporesponsive to activation by IFN-γ. The impaired production of Th1-type immune cytokines and the impaired response of macrophages are associated to the defective innate immunity in newborn period.
Innate immunity is a natural host defense mechanism that operates effectively without exposure to a microorganism or its antigen. This system is made up of the surface barrier (such as skin and mucous membrane), many cells (such as neutrophil, monocyte, macrophage, dendritic cell, and natural killer cell) and humoral factors (such as complement)4). There are limitations of exposure to antigens in utero and defective adaptive immunity in the newborn period, newborn must rely on their innate immune system for protection5).
- Toll like receptor
- Toll like receptor
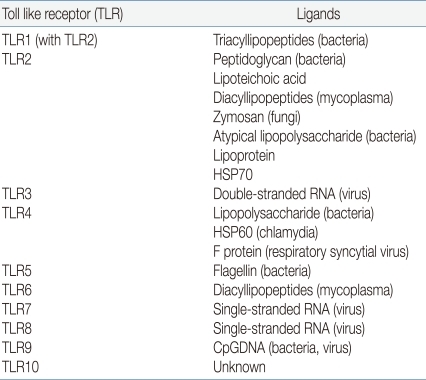
In mammals, a key factor of the innate immune system is a family of TLRs. Toll receptors were originally identified in Drosophila11) and not only function in determination of dorsal-ventral development in the fly embryo but also for antimicrobial function. In adult Drosophila, the Toll receptor recognize microorganism and activate a signaling pathway for induction of antifungal and antibacterial peptides about infection. In 1997, Janeway et al. identified TLR4, a human homologue of Toll receptor12). They found out that the activation of TLR4 leads to activation of various inflammatory cytokines such as nuclear factor-κB (NF-κB), IL-1, IL-6, and IL-8. He first suggested the existence of a set of factors which he named pattern recognition receptor (PRR), were capable of detecting pathogen-associated molecular patterns (PAMPs). TLRs act as a main PRR to the various PAMPs of microorganisms13). If a pathogen enters to the body, TLR recognizes microorganism PAMPs, and activates the signal pathway by utilization of an adaptor protein and results in activation of the transcription factor NF-κB and expression of inflammatory cytokine genes14,15). As a result, various cytokines, chemokines and colony stimulating factors produced and these factors have a crucial role in the induction of immune response. Another key role is a link between detection and recognition of microbial pathogens and the activation of the adaptive immune system16). TLRs are one kind of transmembrane receptors composed of extracellular leucine-rich repeat (LRR) motifs and a cytoplasmic Toll/interleukin-1 receptor (TIR) homology domain. They are expressed on the surface of monocytes, macrophages, dendritic cells and epithelial cells or in the cytoplasm of cells from different tissues. In humans, 10 TLRs have emerged to mediate the activation of innate effector cells. Some of the TLRs reside at the cell surface, and some of them are located in the endoplasmatic reticulum. TLR1, 2, 4, 5 and 6 are present on the cell membrane which recognize mainly microbial membrane components such as lipids, lipoproteins and proteins, whereas TLR3, 7, 8 and 9 appear to be confined to intracellular compartments where they recognize microbial nucleic acid. Each TLR has different ligand recognition and has a distinct function in terms of PAMP recognition and immune response (Table 1).TLR4 responds to bacterial lipopolysaccharide (LPS), an outer membrance component of Gram-negative bacteria. In addition to LPS, TLR4 is involved in recognition of respiratory syncytial virus fusion proteins17). TLR2 form a heterodimer with TLR1 or TLR6 recognize various microbial components, such as peptidoglycans, lipoprotein, and lipoteichoic acid from Gram-positive bacteria. TLR5 is involved in the recognition of flagellin protein component of bacterial flagella. TLR5 is expressed and functional to the basolateral site of the epithelial cell in intestine. TLR3, 7, 9 recognize the genomic RNA of virus including respiratory syncytial virus. TLR3 triggers mainly antiviral immune responses17).In newborn, basal expression of TLR, the accessory protein, and the adaptor protein are equivalent to adult level, but activation by the TLR appears to be reduced18). This reduction is age dependent pattern. One study with monocytes from very low birth weight infant (VLBWI) found that decreased surface expression of TLR4, lower mRNA expression of TLR4 and reduced cytokine production after LPS stimulation in gestational age less than 30 weeks19). Thus, monocytes in premature infants can be considered phenotypically and functionally immature. The other characteristic is impaired TLR4-mediated signaling in LPS-exposed newborn monocytes. Recent study provided evidence that TLR4-mediated, NF-κB dependent transcriptional activation of neonatal monocytes macrophages was depressed20). As a consequence, the defects of TLR activity cause the susceptibility to infection in the neonatal period.
- TLR associated neonatal researches
- TLR associated neonatal researches
So far, there are little human researches about association with neonatal diseases and TLRs. Most researches are animal based. Among them, the relation with TLRs and neonatal sepsis is most advanced part21-23). TLR2 and TLR4 expression is enhanced in preterm delivery with chorioamnionitis, and recent studies showed an association between preterm birth and TLR polymorphisms24). Recent study showed polymorphisms in TLR2, TLR5, IL10, and PLA2G2A genes were associated with preterm sepsis25).The direct role of TLRs in neonatal brain injury is suspected26,27). The strong innate immune response in the central nervous system (CNS) is critical for pathogen elimination and vital to host survival. However, it is evident that chronic or acute dysregulated inflammation in the CNS can cause tissue damage and neurodegeneration. Animal studies described a relation between the TLR4 agonist LPS and brain injury in newborn animals28). In necrotizing enterocolitis (NEC), increased proinflammatory cytokines are found in intestinal samples, suggesting that these mediators play a role in NEC development. One study in a rat NEC model showed overexpression of TLR2, as well as NFκB in intestinal epithelial cells that was correlated with the severity of mucosal damage29,30).Also, the intestinal expression of TLRs and cytokines precedes histological injury in the experimental NEC31). NEC is also associated with increased expression of TLR4 in the intestinal mucosa and physiological stressors such as exposure to LPS and hypoxia. These findings demonstrate a critical role for TLR4 in the development of NEC through effects on enterocyte injury and repair32).
- Conclusion
- Conclusion
The innate immune system in neonate is immature which results in increased susceptibility to various infections. On the other hand, newborn innate immunity has a characteristic feature that the balance of Th1 and Th2 type immunity and impairment of TLRs signaling downstream pathway. However, the functional state of the various components of innate immunity in newborn largely unknown and only recently a number of studies have assessed this feature of the innate immune system. Thus, more researches need to include the discovery of neonatal immune mechanism so that prevention and treatment of neonatal infections can more safely be targeted.
- References
- 1. Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553–564.
[Article] [PubMed]2. Marodi L. Neonatal innate immunity to infectious agents. Infect Immun 2006;74:1999–2006.
[Article] [PubMed] [PMC]3. Belderbos M, Levy O, Bont L. Neonatal innate immunity in allergy development. Curr Opin Pediatr 2009;21:762–769.
[Article] [PubMed]4. Clapp DW. Developmental regulation of the immune system. Semin Perinatol 2006;30:69–72.
[Article] [PubMed]5. Kenzel S, Henneke P. The innate immune system and its relevance to neonatal sepsis. Curr Opin Infect Dis 2006;19:264–270.
[Article] [PubMed]6. Tollin M, Bergsson G, Kai-Larsen Y, Lengqvist J, Sjövall J, Griffiths W, et al. Vernix caseosa as a muti-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci 2005;62:2390–2399.
[Article] [PubMed] [PMC]7. Levy O. Innate immunity of the newborn:basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379–390.
[Article] [PubMed]8. Maródi L, Káposzta R, Campbell DE, Polin RA, Csongor J, Johnston RB Jr. Candidacidal mechanisms in the human neonate. Impaired INF-gamma activation of macrophages in newborn infants. J Immunol 1994;153:5643–5649.
[PubMed]9. Marodi L. Innate cellular immune reponses in newborns. Clin Immunol 2006;118:137–144.
[Article] [PubMed]10. Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, et al. Skewed pattern of toll-like receptor 4-mediated cytokine production in human neonatal blood:low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol 2009;133:228–237.
[Article] [PubMed] [PMC]11. Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 1985;42:779–789.
[Article] [PubMed]12. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Dorosophila Toll protein signals activation of adaptive immunity. Nature 1997;388:394–397.
[Article] [PubMed]13. Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med 2006;84:712–725.
[Article] [PubMed]14. Fleer A, Krediet TG. Innate immunity: Toll-like receptors and some more. Neonatology 2007;92:145–157.
[Article] [PubMed]15. Lakhani SA, Bogue CW. Toll-like receptor signaling in sepsis. Curr Opin Pediatr 2003;15:278–282.
[Article] [PubMed]16. Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev 2010;68:38–61.
[Article] [PubMed]17. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–382.
[Article] [PubMed]18. Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009;183:7150–7160.
[Article] [PubMed] [PMC]19. Förster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, et al. Monocyte Toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res 2005;58:121–124.
[Article] [PubMed]20. Hasegawa K, Ichiyama T, Isumi H, Nakata M, Sase M, Furukawa S. NF-kappa B activation in peripheral blood mononuclear cells in neonatal asphyxia. Clin Exp Immunol 2003;132:261–264.
[Article] [PubMed] [PMC]21. Viemann D, Dubbel G, Schileiefenbaum S, Harms E, Sorg C, Roth J. Expression of toll-like receptors in neonatal sepsis. Pediatr Res 2005;58:654–659.
[Article] [PubMed]22. Levy E, Xanthou G, Petrakou E, Zacharioudaki V, Tsatsanis C, Fotopoulos S, et al. Distinct roles of TLR4 and CD14 in LPS-induced inflammatory responses of neonates. Pediatr Res 2009;66:179–184.
[Article] [PubMed]23. Harju K, Ojaniemi M, Rounioja S, Glumoff V, Paananen R, Vuolteenaho R, et al. Expression of Toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr Res 2005;57:644–648.
[Article] [PubMed]24. Krediet TG, Wiertsema SP, Vossers MJ. Toll-like receptor 2 polymorphism is associated with preterm birth. Pediatr Res 2007;62:474–476.
[Article] [PubMed]25. Abu-Maziad A, Schaa K, Bell EF, Dagle JM, Cooper M, Marazita ML, et al. Role of polymorphic variants as genetic modulators of infection in neonatal sepsis. Pediatr Res 2010;68:323–329.
[Article] [PubMed] [PMC]26. Mallard C, Wang X, Hagberg H. The role of Toll-like receptors in perinatal brain injury. Clin Perinatol 2009;36:763–772.
[Article] [PubMed]27. Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112(Suppl 1): 16–18.
[Article] [PubMed]28. Hagberg H, Peebles D, Mallard C. Models of white matter injury; comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev 2002;8:30–38.
[Article] [PubMed]29. Le Mandat Schultz A, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, et al. Expression of TLR-2, TLR-4, NOD2 and pNFkB in a neonatal rat model of necrotizing enterocolitis. PLoS One 2007;2:e1102
[Article] [PubMed] [PMC]30. Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotizing enterocolitis. Semin Fetal Neonatal Med 2006;11:369–377.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors