All issues > Volume 54(1); 2011
Percutaneous endoscopic gastrostomy in children
- Corresponding author: Su Jin Jeong, M.D. Department of Pediatrics, Bundang CHA Medical center CHA University School of Medicine, 351, Yatap-dong, Bundang-gu, Seongnam-si, Gyeonggi-do 463-712, Korea. Tel: +82.031-780-5230, Fax:+82.31-780-5239, jinped@cah.ac.kr
- Received September 13, 2010 Revised October 14, 2010 Accepted November 9, 2010
- Abstract
-
- Purpose
- Purpose
- Percutaneous endoscopic gastrostomy (PEG) can improve nutritional status and reduce the amount of time needed to feed neurologically impaired children. We evaluated the characteristics, complications, and outcomes of neurologically impaired children treated with PEG.
- Methods
- Methods
- We retrospectively reviewed the records of 32 neurologically impaired children who underwent PEG between March 2002 and August 2008 at our medical center. Forty-two PEG procedures comprising 32 PEG insertions and 10 PEG exchanges, were performed. The mean follow-up time was 12.2 (6.6) months.
- Results
- Results
- Mean patient age was 9.4 (4.5) years. The main indications for PEG insertion were swallowing difficulty with GI bleeding due to nasogastric tube placement and/or the presence of gastroesophageal reflux disease (GERD). The overall rate of complications was 47%, with early complications evident in 25% of patients and late complications in 22%. The late complications included one gastro-colic fistula, two cases of aggravated GERD, and four instances of wound infection. Among the 15 patients with histological evidence of GERD before PEG, 13 (87%) had less severe GERD, experienced no new aspiration events, and showed increased body weight after PEG treatment.
- Conclusion
- Conclusion
- PEG is a safe, effective, and relatively simple technique affording long-term enteral nutritional support in neurologically impaired children. Following PEG treatment, the body weight of most patients increased and the levels of vomiting, GI bleeding, and aspiration fell. We suggest that PEG with post-procedural observation be considered for enteral nutritional support of neurologically impaired children.
- Introduction
- Introduction
There are two methods commonly used to feed patients with swallowing disabilities; these are nasogastric tube (NGT) placement and percutaneous endoscopic gastrostomy (PEG). NGT feeding is non-invasive, but is associated with complications such as aspiration pneumonia, reflux esophagitis, esophageal mucosal damage caused by mechanical stimulation, sinusitis, and problems attributable to the need to change the tube every 3-4 weeks1). PEG feeding is employed if NGT feeding is likely to be required for more than 3 months2). In the past, surgical gastrostomy was used, but this procedure is highly invasive and is associated with numerous potential complications3). However, previous studies have shown that PEG is safe, effective over the long-term and associated with a low risk of complications4). Thus, PEG is the preferred method for enteral nutritional support of patients who experience chronic problems with normal feeding4-6).PEG involves insertion of a feeding tube into the stomach via an endoscope. The procedure is associated with low mortality and is relatively easy to perform. Thus, the use of PEG has increased significantly since the time of introduction of the technique in 19817, 8). An estimated 61,000 PEG procedures were performed worldwide in 1988 and an estimated 216,000 are performed annually today. PEG placement is currently the second most common indication for endoscopy of the upper gastrointestinal tract8).PEG is frequently used in neurologically impaired children to improve nutritional status, to reduce the amount of time needed for feeding, and to lower the prevalence rate of diseases that may result from poor nutrition1, 9). Such patients may be diagnosed with hypoxic brain damage, leukomalacia, infantile spasms, epilepsy, or cerebral palsy.Neurologically impaired children of poor nutritional status and suffering from immune deficiencies are at high risk of various PEG-related complications even though they are form the group that has most to benefit from PEG. Thus, it is necessary to carefully follow-up such patients after PEG procedures10).In the present study, we evaluated the effectiveness of PEG by analyzing the characteristics, complications, and outcomes of a group of neurologically impaired children who were treated with PEG at our hospital.
- Materials and methods
- Materials and methods
A total of 32 pediatric patients underwent PEG at the Bundang CHA Hospital (Gyeonggi-do, Korea), from March 2002 to August 2008. We reviewed the medical records of all patients, including clinical presentations, details of operative procedures, and postoperative complications.NSAIDs and other oral medications were withheld from all patients for 1-7 days prior to the procedure, and oral food was withheld for 6-24 h before PEG tube feeding was initiated. Intravenous antibiotics, given either prophylactically or concurrently were given to reduce the incidence of peristomal wound infection after PEG placement.With the consent of parents, patients were intravenously sedated using midazolam and/or fentanyl, and pethidine was administered intravenously during the procedure. PEG was performed using a commercial PEG kit (Bard Endoscopic Technologies, Billerica, MA) employing the Ponsky-pull technique11). A video endoscope of appropriate size was used. After the stomach was insufflated, transillumination and finger indentation were applied to confirm the puncture site. An insertion wire was passed into the stomach via the cannula, grasped by a wire snare, and withdrawn through the mouth together with the endoscope. The PEG tube was next attached to the end of the wire and pulled in an ante-grade manner through the esophagus and out of the abdominal wall. The final position of the dome was confirmed by a second endoscopic examination. Tube feeding with liquid food began 6-24 h after placement.Early complications were defined as those occurring within 6 days after PEG insertion. Late complications were defined as those occurring after that time4). To evaluate clinical outcomes, body weight was recorded 1 day before PEG and at least 6 months after PEG. Weight change was compared with the age-adjusted Z-score of weight before and after PEG. The Z-score of weight was calculated as: (actual weigh-mean weight)/SD. A retrospective study of clinical outcomes was performed with checking new aspiration event, aggravated GER, aggravated or new GI bleeding and removal of PEG4).SPSS version 17.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis of data.
- Results
- Results
- 1. Patient characteristics
- 1. Patient characteristics
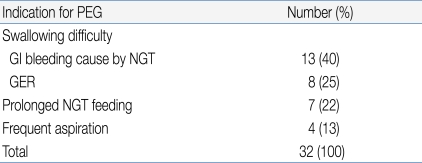
During the study period, 42 consecutive PEG procedures were performed in 32 patients (32 insertions and 10 tube exchanges). There were 14 males and 18 females, of age 2.4-17 years (mean age: 9.4±4.5 years), and mean patient weight at the time of PEG insertion was 14.9±4.42 kg.All patients were diagnosed with a neurological dysfunction (a neurodisability). Swallowing difficulties and associated complications were the main indicators for PEG treatment. Table 1 lists all specific indications. NGT feeding was the most common method of nutritional support before PEG procedures were performed.- 2. PEG procedure
- 2. PEG procedure
All 32 patients tolerated the procedure well, and no mortality was associated with PEG placement or exchange. The causes of exchange (n=10) were planned tube replacement (n=6), accidental self-removal (n=2), discharge (n=1), and rash or infection around the PEG insertion site (n=1).The location of puncture points varied greatly. These were situated over the right upper quadrant in 31% of patients, the left upper quadrant in 59%, the right lower quadrant in 5%, and the left lower quadrant in 5%.- 3. Complications
- 3. Complications
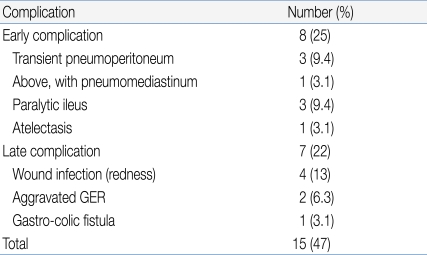
We observed 15 complications in our 32 patients (Table 2), corresponding to an overall complication rate of 46.9%. However, most complications were minor, transient, and self-limited; no special treatment was required. Early complications (within 48 h of tube placement) occurred in 25% of patients and late complications in 22%. There were no complications related to PEG exchange.Table 3 shows the characteristics of children with or without PEG-related complications. There were no between-group statistically significant differences in any of gender, age, past history of intractable seizure, or tracheotomy status. However, patients with complications were of significantly lower body weight (P=0.028).Wound infection was the most common late complication (n=4). Such patients were treated with topical and oral antibiotics during the active stage and trace element nutritional supplementation was given. In all four patients, infections resolved in 2-3 weeks.The two other late major complications were a gastro-colic fistula (n=1) and aggravated gastroesophageal reflux (GER, n=2). The patient with the gastro-colic fistula was 8 years old, showed poor weight gain, had watery yellowish diarrhea, and suffered from recurrent vomiting. A dirty fluid leak was apparent around the PEG tube and the diarrhea resembled formula. The gastro-colic fistula was identified after a contrast bowel study. During laparotomy, we removed the tube and allowed the fistula to close. An intravenous broad-spectrum antibiotic was administered for 10 days to guard against peritonitis. Her subsequent progress was good.In the two patients with aggravated GER, PEG feeding was difficult because of recurrent vomiting. One patient was a 42 month-old girl with cerebral palsy and severe laryngomalacia prior to the PEG procedure. We managed her by medical treatment of GER and use of less liquid in the feeding regimen. She subsequently improved and continued with PEG feeding. The other patient was a 9 year-old girl with severe scoliosis and cerebral palsy. Even though we administered several anti-GER medications and tried various feeding modes, a Nissen fundoplication and gastrostomy were necessary 10 months after the PEG procedure.- 4. Clinical outcomes
- 4. Clinical outcomes
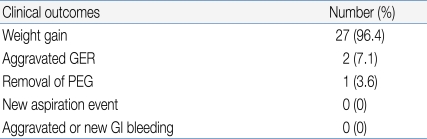
One patient died of underlying disease and three patients were lost to follow-up. The mean follow-up time was 12.2±6.6 months. Patients were encouraged to use individually prescribed diets, and to take about five meals per day. Six months after the PEG procedure, the average weight gain was 1.03±1.41 kg. The age-adjusted Z-score of weight before and 6 months after PEG was -1.34±1.82 and -1.01±1.44, respectively (paired t- test, P<0.0001). The 9-year old girl who had cerebral palsy with severe scoliosis (mentioned above) was the only patient to lose weight (Table 4).We performed the PEG procedure in four patients who suffered from frequent aspiration. No new aspiration events occurred after PEG. There were no aspiration-related problems in any other patient.During PEG, histology indicated the presence of GER (histologic reflux esophagitis in the absence of erosion while simultaneously eliminating allergic and infectious causes) in 15 patients, who were managed by appropriate medical treatment for 2-8 weeks. This relieved GI bleeding and vomiting events in 13 patients, and it was thus possible to reduce the medication dose. The other two patients with aggravated GER (the 42 month-old and 9 year-old girls) have been described above.
- Discussion
- Discussion
Gastrostomy feeding is preferred over NTG feeding if nutritional support is likely to be required for more than 3 months. PEG reduces morbidity, mortality, and costs associated with surgical gastrostomy. PEG also lessens the frequency of choking episodes, aspiration events, and chest infections; and improves nutritional status2, 12, 13). In our experience, neurodisability is the main indication for PEG. A previous study noted that cerebral palsy was the single most important cause of PEG9). Patient weight of less than 10 kg and a history of previous gastric surgery are no longer considered contraindications to PEG placement14). In the present study, two patients weighed less than 10 kg and one had a history of previous gastric surgery, but none of these patients experienced complications.Possible complications associated with PEG include aspiration, bleeding, perforation of the viscera, and prolonged ileus15); as well as major complications such as peritonitis and necrotizing fasciitis9, 16-17). In addition, PEG site infection, PEG site leakage, buried bumper syndrome, gastric ulceration, development of a gastro-colic fistula, tumor implantation at the PEG site, and GER, are all possible18). Previous studies have reported that the rate of major complications was 3-17.5% and that of minor complications 2-25%4, 10, 13, 19). Compared with other PEG studies, our complication rate (47%) was high. This may be attributable to our small sample size, among-study population differences (perhaps in body weight), and/or the use of different definitions of complications.In our study, one patient had a gastro-colic fistula; this is a major complication. In the literature, the incidence of gastro-colic fistula caused by PEG has been reported to be 0.3-6.7%5, 10, 13, 20). This complication is typically treated by laparotomy. Changes in normal anatomy, such as occur in those with scoliosis and kyphoscoliosis, as in our patient, can predispose to development of a gastro-colic fistula. This can make it difficult to identify an appropriate puncture site for PEG insertion, because anatomical changes can displace the colon, small bowel, and stomach, as a result of over-inflation21). In such patients, we suggest careful selection of a cutaneous puncture point using transillumination of the abdominal wall and clear visualization employing direct external palpation with digital pressure.We found the Z-score of weight after PEG was larger than that before PEG. It indicated accelerated weight gain after PEG with catch-up growth4). We also found a relationship between low body weight and the frequency of PEG complications, but no such association with any of gender, age, presence of underlying seizures, or tracheotomy. This suggests that the probability of complications associated to PEG rises if nutritional status is poor.It has been suggested that PEG may exacerbate GER9), and previous studies reported a GER rate after PEG of 14-47%22, 23). However, other workers found that GER frequency did not increase after PEG, so that concomitant anti-reflux procedures were not required24). No clear definitions of GER severity are available, and most GER complaints are subjectively reported. Thus, the reported association between PEG and GER has been debated23). In the present retrospective study, we did not assess GER severity before PEG, and we were thus unable to evaluate any association among GER, PEG, and nutritional status. However, in most children with histologically confirmed GER, vomiting and other symptoms seemed to resolve, and the need for anti-reflux medications fell. One patient had laryngomalacia and required anti-reflux medication, whereas another patient with severe scoliosis needed Nissen fundoplication. No new aspiration events occurred after PEG, and we thus recommend that fundoplication should not be routinely used25).Various guidelines have been suggested to avoid the complications associated with PEG tube placement1, 13, 26). Based on our own experience and the results of the present study, we suggest additional measures to avoid major complications associated with PEG. First, the puncture site must be appropriately identified and prepared, especially in patients with severe anatomical abnormalities. Second, an abdominal supine x-ray should be obtained after feeding to guard against overinflation during PEG20). Third, nutritional support should include trace elements (zinc and iron) and multi-vitamins, to prevent wound infection. Finally, a longer PEG tube should be used, to allow of clear liquid flushing before and after PEG feeding.Traditionally, enteral feeding was initiated 24 h after tube placement. However, it has been suggested that PEG tube feeding as early as 3 h after placement may be safe and effective. The maturation phase of wound healing is attained only after 20 days or more, so a 24 h wait would seem to allow of only minimal maturation of the gastrostomy tract. However, early feeding seems to be safe, and allows rapid administration of nutritional support, making the procedure less costly from a physiological viewpoint27).The PEG tube replacement process is relatively simple and usually does not require any additional endoscopic procedure. Typically, the tubing is merely pulled out through the stomach site and replaced with a new catheter. In the present study, no complications were associated with PEG tube exchange, and most patients simply required larger PEG tubes because of increased body weight and expansion of puncture diameter28).In conclusion, our results indicate that PEG is safe and effective when used to provide long-term nutritional support to severely handicapped children who require lifelong tube feeding. However PEG is associated with both minor and major complications. Therefore, we believe it essential to customize the PEG procedure for each patient, and we emphasize that post-procedural observation is imperative.
- References
- 1. Maitines G, Ugenti I, Memeo R, Clemente N, Iambrenghi OC. Endoscopic gastrostomy for enteral nutrition in neurogenic dysphagia: Application of a nasogastric tube or percutaneous endoscopic gastrostomy. Chir Ital 2009;61:33–38.
[PubMed]2. Behrens R, Lang T, Muschweck H. Percutaneous endoscopic gastrostomy in chilren and adolescents. J Pediatr Gastroenterol Nutr 1997;25:487–491.
[Article] [PubMed]3. Ho HS, Ngo H. Gastrostomy for enteral access: a comparison among placement by laparotomy, laparoscopy, and endoscopy. Surg Endosc 1999;13:991–994.
[Article] [PubMed]4. Chang SH, Kim DY, Kim SC, Kim IK, Kim KM. Complications of percutaneous endoscopic gastrostomy (PEG) in children. Korean J Pediatr Gastroenterol Nutr 2004;7:8–15.
[Article]5. Avitsland TL, Kristensen C, Emblem R, Veenstra M, Mala T, Bjornland K. Percutaneous endoscopic gastrostomy in children: a safe technique with major symptom relief and high parental satisfaction. J Pediatr Gastroenterol Nutr 2006;43:624–628.
[Article] [PubMed]6. Ponsky JL. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc 1981;27:9–11.
[Article] [PubMed]7. Eger R, Reif S, Yaron A, Bojanover Y. Percutaneous endoscopic gastrostomy (PEG) in children: indications, the procedure, outcomes, short and long-term complications. Harefuah 2008;147:21–24. 95
[PubMed]8. Srinivasan R, Irvine T, Dalzell M. Indications for percutaneous endoscopic gastrostomy and procedure-related outcome. J Pediatr Gastroenterol Nutr 2009;49:584–588.
[Article] [PubMed]9. Khattak IU, kiely EM, Spitz L. Percutaneous endoscopic gastrotomy in pediatric practices : Complication and outcome. J Pediatr Surg 1998;33:67–72.
[Article] [PubMed]10. Campoli PM, Cardoso DM, Turchi MD, Ejima FH, Mota OM. Assessment of safety and feasibility of a new technical variant of gastropexy for percutaneous endoscopic gastrostomy: an experience with 435 cases. BMC Gastroenterol 2009;9:48
[Article] [PubMed] [PMC]11. Grant JP. Comparison of percutaneous endoscopic gastrostomy with Stamm -gastrostomy. Ann Surg 1988;207:598–603.
[Article] [PubMed] [PMC]12. Marin OE, Glassman MS, Schoen BT, Caplan DB. Safety and efficacy of percutaneous endoscopic gastrostomy in children. Am J Gastroenterol 1994;89:357–360.
[PubMed]13. Gauderer MWL. Percutaneous endoscopic gastrostomy: A 10 year experience with 220 children. J Pediatr Surg 1991;26:288–294.
[Article] [PubMed]14. Guvenc BH, Rasa K, Guvenc S. The presence of percutaneous endoscopic gastrostomy (PEG)-related postprocedural pneumoperitoneum. Endoscopy 2009;41(Suppl 2): E269–E270.
[Article] [PubMed]15. Vervloessem D, van Leersum F, Boer D, Hop WC, Escher JC, Madern GC, et al. Percutaneous endoscopic gastrostomy (PEG) in children is not a minor procedure: risk factors for major complications. Semin Pediatr Surg 2009;18:93–97.
[Article] [PubMed]16. Segal D, Michaud L, Guimber D, Ganga-Zandzou PS, Turck D, Gottrand F. Late-onset complications of percutaneous endoscopic gastrostomy in children. J Pediatr Gastroenterol Nutr 2001;33:495–500.
[Article] [PubMed]17. Srinivasan R, Irvine T, Dalzell M. Indications for percutaneous endoscopic gastrostomy and procedure-related outcome. J Pediatr Gastroenterol Nutr 2009;49:584–588.
[Article] [PubMed]18. Kim SJ, Shin WC, Lee JH. The proper patient selection for percutaneous endoscopic gastrostomy according to the complications and tube replacement. Korean J Gastrointest Endosc 2005;30:60–65.19. Beasley SW, Catto-Smith AG, Davidson PM. How to avoid complications during percutaneous endoscopic gastrostomy. J Pediatr Surg 1995;30:671–673.
[Article] [PubMed]20. Chang WK, McClave SA, Yu CY, Huang HH, Chao YC. Positioning a safe gastric puncture point before percutaneous endoscopic gastrostomy. Int J Clin Pract 2007;61:1121–1125.
[Article] [PubMed]21. Samuel M, Holmes K. Quantitative and qualitative analysis of gastroesophageal reflux after percutaneous endoscopic gastrostomy. J Pediatr Surg 2002;37:256–261.
[Article] [PubMed]22. Saitua F, Acuña R, Harrera P. Percutaneous endoscopic gastrostomy: The technique of choice? J Pediatr Surg 2003;38:1512–1515.
[Article] [PubMed]23. Wilson GJ, van der Zee DC, Bax NM. Endoscopic gastrostomy placement in the child with gastroesophageal reflux: is concomitant antireflux surgery indicated? J Pediatr Surg 2006;41:1441–1445.
[Article] [PubMed]24. Michaud L. Percutaneous endoscopic gastrostomy in children. Arch Pediatr 2009;16:814–815.
[Article] [PubMed]25. Schurink CA, Tuynman H, Scholten P, Arjaans W, Klinkenberg-Knol EC, Meuwissen SG, et al. Percutaneous endoscopic gastrostomy: complications and suggestions to avoid them. Eur J Gastroenterol Hepatol 2001;13:819–823.
[Article] [PubMed]26. Srinivasan R, Fishev RS. Early initiation of post-PEG feeding. Dig Dis Sci 2000;45:2065–2068.
[Article] [PubMed]27. Hahn SJ, Lim YJ, Yang CH, Lee JH. Satisfaction survey of care providers for enteral feeding by the use of percutaneous endoscopic gastrostomy. Korean J Gastrointest Endosc 2009;39:8–13.

 About
About Browse articles
Browse articles For contributors
For contributors