All issues > Volume 54(1); 2011
A case of tacrolimus-induced encephalopathy after kidney transplantation
- Corresponding author: Yong Hoon Park, M.D. Department of Pediatrics, College of Medicine, Yeungnam University, Daemyeong 5-dong, Namgu, Daegu 705-717, Korea. Tel: +82.53-620-3532, Fax: +82.53-629-2292, yhpark@med.yu.ac.kr
- Received September 15, 2010 Revised October 05, 2010 Accepted October 20, 2010
- Abstract
-
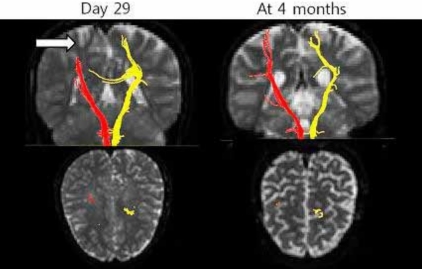
We present a case of tacrolimus-induced encephalopathy after successful kidney transplantation. An 11-year-old girl presented with sudden onset of neurologic symptoms, hypertension, and psychiatric symptoms, with normal kidney function, after kidney transplantation. The symptoms improved after cessation of tacrolimus. Magnetic resonance imaging (MRI) showed acute infarction of the middle cerebral artery (MCA) territory in the right frontal lobe. Three days later, she had normal mental function and maintained normal blood pressure with left hemiparesis. Follow-up MRI was performed on D19, showing new infarct lesions at both cerebral hemispheres. Ten days later, MRI showed further improvement, but brain single photon emission computed tomography (SPECT) showed mild reduction of uptake in both the anterior cingulate gyrus and the left thalamus. One month after onset of symptoms, angiography showed complete resolution of stenosis. However, presenting as a mild fine motor disability of both hands and mild dysarthria, what had been atrophy at both centrum semiovale at 4 months now showed progression to encephalomalacia. There are two points of interest in this case. First, encephalopathy occurred after administration of tacrolimus and improved after discontinuation of the drug. Second, the development of right-side hemiplegia could not be explained by conventional MRI; but through diffusion tensor imaging (DTI) and diffusion tensor tractography (DTT) of white matter tract, visualization was possible.
- Introduction
- Introduction
Cyclosporin A and tacrolimus are important immunosuppressant drugs used to prevent rejection of transplanted organs or bone marrow1). However, development of posterior reversible encephalophathy syndrome (PRES) is commonly seen in association with cyclosporin A and tacrolimus after transplantation1). PRES is a relatively new clinicoradiological entity first described as reversible posterior leukoencephalopathy syndrome in 1996, characterized by headaches, altered mental status, seizures, and visual loss. Clinical neuroimaging shows edema involving the white matter in the posterior portions of the cerebral hemispheres, especially bilaterally in the parieto-occipital regions2, 3). Prompt recognition is vital, as most patients will experience complete recovery by reduction of drug dosage or by withholding the immunosuppressive agent altogether4). Symptomatology and characteristic MRI features of this clinicoradiological entity have been reported, but it still is poorly understood by pediatricians5-11). Here, we present a case of tacrolimus-induced encephalopathy which was visualized through diffuson-tensor tractography.
- Case report
- Case report
An eleven-year old girl has had chronic renal insufficiency since her neonatal period. She was born premature at 32 weeks gestation, diagnosed with renal hypoplasia, hypocalcemia, neonatal sepsis, urinary tract infection, acute renal failure, and atrial septal defect during neonatal period. She has been under conservative treatment (erythropoietin, angiotensin-converting enzyme (ACE) inhibitors, and calcium) without dialysis since then. At the age of 11 years, she received kidney transplantation from her mother, a preemptive living related donor kidney. There were two HLA-B, and HLA-DR mismatches but ABO-identity (O positive). Immunosuppression included tacrolimus (0.1 mg/kg/day, bid), mycophenolate mofetil (600 mg/m2, bid), and prednisolone (0.5 mg/kg qd). Postoperatively, her renal function was good without acute rejection.On day 12 after transplantation, sudden onset of left side motor weakness of grade 0 developed. Blood pressure was 160/100 mmHg and consequently, antihypertensive drugs were administered.Brain magnetic resonance imaging (MRI) and angiography (MRA) showed acute infarction at right frontal lobe of middle cerebral artery territory (Fig. 1, 2). Other laboratory findings were normal, including serum creatinine of 0.69 mg/dL. Serum tacrolimus level was 9 ng/mL. On day 13, psychiatric symptoms such as irritability, mood instability, aggressive behavior, and dysarthria developed, diagnosed as adjustment disorder, antipsychotics medication started. Within few days, psychotic symptoms improved.On day 19, mentality and blood pressure were restored, with slight improvement of left hemiparesis of grade 1 to 2. Follow-up MRI was taken after some clinical improvement, showing new infarct lesions at both cerebral hemispheres. Tacrolimus dose was reduced. But with no recognizable clinical response even after decreased serum concentration, it was replaced by cyclosporine.On day 29, her renal function was normal, and her blood pressure was normotensive. Brain single photon emission computed tomography (SPECT) (Fig. 3) and tensor MRI for diffusion tensor tractography (DTT) (Fig. 4) were performed. The patient went under comprehensive rehabilitation. Tensor MRI showed improving status of multifocal infarcts at both cerebral hemispheres. SPECT showed reduction of perfusion in both cingulate gyrus and left thalamus.On day 42, improvement of left side hemiparesis was noted, and follow-up MRA showed improvement of cerebral artery stenosis.On day 56, the patient was discharged from hospital, with improvement of left hand function, and gait disturbance.At four months after transplantation, increased dysarthria, along with right hand tremor and clumsiness were noted. Tensor MRI showed atrophy at both centrum semiovale, with progression to encephalomalacia. There were no further aggravations during follow-up.
- Discussion
- Discussion
Causative agents of PRES include immunosuppressives such as tacrolimus, cyclosporine, cisplatin, and erythropoietin, with the condition manifesting in such presentations as preeclampsia and eclampsia, acute glomerulonephritis, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome4). Severe hypertension with autoregulatory failure and forced hyperperfusion remains to be widely accepted1).The key pathophysiological process of this syndrome was identified as vasogenic edema2, 12, 13). A breakdown in cerebral autoregulation results in the leakage of fluid into the interststium, which is detected as vasogenic edema. In patients with PRES, myogenic response is blunted by either passive overdistension of the vessels due to elevations in blood pressure or direct toxic effects on the endothelium4). The abnormalities primarily affect white matter but the cortex can also be involved4). The posterior cerebral hemispheres are susceptible to the neurotoxicity of calcineurin inhibitors, especially the occipital lobes7). The posterior circulation is most vulnerable because of poor sympathetic innervation. Diffusion-weighted imaging (DWI) has been shown to be reliable in distinguishing vasogenic edema from cytotoxic edema4, 14).The mechanism by which calcineurin inhibitors lead to neurotoxicity remains poorly understood. Both cyclosporine and tacrolimus decreased endothelial cell viability and increased permeability, which is consistent with impaired function of the blood-brain barrier. Both drugs inhibit P-glycoprotein, which could lead to enhanced brain distribution of theses drugs and neurotoxicity7). The encephalopathy associated with tacrolimus is multifactorial in origin. Known factors include changes in intracellular urea concentration in the brain, fluid overload, high blood levels of tacrolimus/cyclosporine, and inadequate blood pressure control7).PRES clinically presents with seizures, severe headaches, and mental and visual changes15). Risk factors include hypertension and calcineurin inhibitor administration, fluid retention, renal failure, and alteration in vascular permeability2). Discontinuation of tacrolimus may be considered as diagnostic tool, if symptoms persist or other complications warrant this approach16).Differential diagnosis of PRES has been reported as ischemic stroke, cerebral venous thrombosis, intracranial vasculitis, progressive multifocal leukoencephalopathy, X-linked adrenoleukodystrophy, acute disseminated encephalomyelitis, encephalitis caused by electrolyte disturbance, and encephalopathy of unknown origin2).Of the imaging modalities, MRI is most sensitive diagnostic study, and is preferred tool than CT. It is more accurate for diagnosis and gives better prediction of PRES outcomes. DWI and apparent diffusion coefficient (ADC) maps can reliably differentiate the changes from cytotoxic edema4). Typical findings show high signal intensity mainly in subcortical white matter in the posterior parietal and occipital lobes in T2-weighted and FLAIR sequences, whereas atypical findings show involvement of gray matter, frontal lobes, thalamus, brainstem, and cerebellum as well as restricted diffusion on DWI (cytotoxic edema). At MRA, moderate to severe vessel irregularity consistent with vasoconstriction and vasodilation has been described in most patients, with reversal of vasoconstriction and vasodilation in nearly all patients4).Catheter angiography, MRA and MR perfusion (MRP) demonstrate evidence of vasculopathy with focal and diffuse vasoconstriction, vasodilatation, and a string-of-bead pattern along with reduced relative cerebral blood volume in PRES patients1).Brain SPECT shows the underlying cerebral blood flow and consequently metabolic activity patterns of the brain.Diffusion tensor imaging (DTI) is a recently developed technique by virtue of its ability to visualize water diffusion characteristics and very useful to evaluate the extent of the fiber damage in diseases that affect the white matter. DTT, derived from DTI, allows for threedimensional visualization of the architecture and integrity of the white matter tract at the subcortical level. DTT is more sensitive to explain the character of white matter lesions which cannot be explained by conventional MRI22-24).Rigorous and meticulous control of blood pressure is mandatory for successful treatment. Using various antihypertensive drugs, nicardipine is effective to limit blood pressure promptly with less fluctuation2, 17). Cessation of calcineurin inhibitor use is a key component. Most were reversible by reducing the dosage or withholding the drugs, but some reports showed successful control without cessation2).Symptomatic treatment measures and antipsychotic medication for possible psychotic stages, as in this case, may be needed. Also, aggressive, comprehensive early rehabilitation improves prognostic outcomes.The prognosis of PRES generally accepted as benign, except with intracranial hemorrhage. Recurrence is rare, but prolonged seizures, hypertension, or both may result in permanent neurological deficit and cerebral infarction2, 8, 18). If the intervention is not done in a timely manner, the functional vasogenic brain edema may convert to cytotoxic brain edema, giving permanent sequelae15).This case, after tacrolimus adminstration, presenting with sudden onset of neurologic symptoms, hypertension, and psychiatric symptoms showed improvement after cessation of tacrolimus and blood pressure control. Conventional MRI showed ischemic infarction, which could not explain the development of right-sided hemiplegia. However, using DTI, visualization was possible through DTT of white matter tract.
- References
- 1. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. Am J Neuroradiol 2008;29:447–455.
[Article] [PubMed] [PMC]2. Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Shishido S, Asanuma H, et al. Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis 2006;48:231–238.
[Article] [PubMed]3. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500.
[Article] [PubMed]4. Hodnett P, Coyle J, O'Regan K, Maher MM, Fanning N. PRES (posterior reversible encephalopathy syndrome), a rare complication of tacrolimus therapy. Emerg Radiol 2009;16:493–496.
[Article] [PubMed]5. Ikeda M, Ito S, Hataya H, Honda M, Anbo K. Reversible posterior leukoencephalopathy in a patient with minimal-change nephrotic syndrome. Am J Kidney Dis 2001;37:E30
[PubMed]6. Ikeda M, Yata N, Kamei K, Mori K, Ishikura K, Hataya H, et al. Posterior leukoencephalopathy syndrome in pediatric patients with kidney disease. Pediatr Nephrol 2002;17:71
[Article] [PubMed]7. Parvex P, Pinsk M, Bell LE, O'Gorman AM, Patenaude YG, Gupta IR. Reversible encephalopathy associated with tacrolimus in pediatric renal transplants. Pediatr Nephrol 2001;16:537–542.
[Article] [PubMed]8. Prasad N, Gulati S, Gupta RK, Kumar R, Sharma K, Sharma RK. Is reversible posterior leukoencephalopathy with severe hypertension completely reversible in all patients? Pediatr Nephrol 2003;18:1161–1166.
[Article] [PubMed]9. Casey SO, Truwit CL. Pontine reversible edema: a newly recognized imaging variant of hypertensive encephalopathy? Am J Neuroradiol 2000;21:243–245.
[PubMed] [PMC]10. Singh N, Bonham A, Fukui M. Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation 2000;69:467–472.
[Article] [PubMed]11. Kwon S, Koo K, Lee S. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Pediatr Neurol 2001;24:361–364.
[Article] [PubMed]12. Lamy C, Oppenheim C, Meder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging 2004;14:89–96.
[Article] [PubMed]14. Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000;217:371–376.
[Article] [PubMed]15. Onder AM, Lopez R, Teomete U, Francoeur D, Bhatia R, Knowbi O, et al. Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol 2007;22:1921–1929.
[Article] [PubMed]16. Kemper MJ, Sparta G, Laube GF, Miozzari M, Neuhaus TJ. Neuropsychologic side-effects of tacrolimus in pediatric renal transplantation. Clin Transplant 2003;17:130–134.
[Article] [PubMed]17. Schwartz RB. Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions. Neurologist 2002;8:22–34.
[Article] [PubMed]18. Abe K. Reversible posterior leukoencephalopathy syndrome. Intern Med 2004;43:900–901.
[Article] [PubMed]19. Furukawa M, Terae S, Chu BC, Kaneko K, Kamada H, Miyasaka K. MRI in seven cases of tacrolimus (FK-506) encephalopathy: utility of FLAIR and diffusion-weighted imaging. Neuroradiology 2001;43:615–621.
[Article] [PubMed]20. Akutsu N, Iwashita C, Maruyama M, Ootsuki K, Ito T, Saigo K, et al. Two cases of calcineurin inhibitor-associated reversible posterior leukoencephalopathy syndrome in renal transplant recipients. Transplant Proc 2008;40:2416–2418.
[Article] [PubMed]21. Emiroglu R, Ayvaz I, Moray G, Karakayali H, Haberal M. Tacrolimus-related neurologic and renal complications in liver transplantation: a single-center experience. Transplant Proc 2006;38:619–621.
[Article] [PubMed]22. Nguyen TH, Yoshida M, Stievenart JL, Iba-Zizen MT, Bellinger L, Abanou A, et al. MR tractography with diffusion tensor imaging in clinical routine. Neuroradiology 2005;47:334–343.
[Article] [PubMed]
Fig. 1
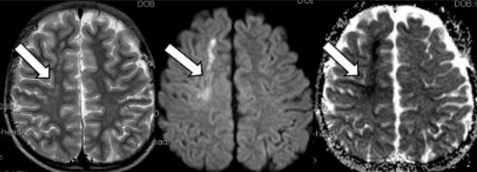
High-signal intensity at right frontal lobe affecting mainly white matter in T2-weighted images on day 12, was bright on diffusion-weighted images and low-intensity on ADC maps, consistent with cytotoxic edema, suggesting acute cerebral infarction (arrows).

 About
About Browse articles
Browse articles For contributors
For contributors