All issues > Volume 54(2); 2011
A study on the measurement of the nucleated red blood cell (nRBC) count based on birth weight and its correlation with perinatal prognosis in infants with very low birth weights
- Corresponding author: Jae Woo Lim, M.D. Department of Pediatrics, Konyang University Hospital, 685 Gasuwon-dong, Seo-gu, Daejon Metropolitan City 302-718, Korea. Tel: +82.42-600-9230, Fax: +82.42-600-9090, limsoa@hanmail.net
- Received August 08, 2010 Revised October 28, 2010 Accepted December 19, 2010
- Abstract
-
- Purpose
- Purpose
- The aim of this study was conducted to investigate the mean nRBC count in very low births weight infants (VLBWIs) and to determine the usefulness of the nRBC as an independent prognostic factors of perinatal complications in VLBWIs.
- Methods
- Methods
- This study was conducted on 112 VLBWIs who were hospitalized in the neonatal intensive care unit (NICU) of the author's hospital within the period from March 2003 to and May 2008. Based on the infants' nucleated red blood cells (nRBC) counts at birth, on the third day after birth, on the seventh day after birth, in the second week after birth, and in the fourth week after birth in the medical records, the correlation between nRBC or absolute nRBC counts with birth weight, gestational age, and other perinatal outcomes were retrospectively investigated.
- Results
- Results
- In VLBWIs, their mean nRBC and absolute nRBC counts were showing a gradual decrease after birth, and they were consisteantly kept at low values since one week after and inversely proportional to the birth weights. The mean nRBC counts based on the stage after birth showed a significant correlation with perinatal death, necrotizing enterocolitis, and severe intraventricular hemorrhage.
- Conclusion
- Conclusion
- The increase in the nRBC count showed a significant correlation with having a severe intraventricular hemorrhage, necrotizing enterocolitis, and perinatal death in VLBWIs. If an increase or no decrease in the nRBC count after birth is observed, newborn-infant care precautions should be required.
- Introduction
- Introduction
Factors predicting harmful perinatal complications have been an issue along with the improved prematurity survival. Nucleated red blood cell (nRBC), a premature red blood cell, is an indicator of hematopoiesis in a newborn infant and has been known to be associated with intrauterine hypoxia1, 2). An increase in nRBC count at birth has been known to be attributable to fetus hemorrhage3), early pregnancy4), intrauterine growth retardation, or fetal stress of an infant born from a mother with pregnancy-induced hypertension (PIH)5-9). In addition, as a prognostic factor of perinatal complications, nRBC count has been known to be closely associated with bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis, and death10-12).As a method that describes nRBC, the nRBC count is measured per 100 white blood cells (WBC). The maximal 30/100 WBC can be observed at a gestational age of less than 30 weeks with a physiologically high extramedullary hematosis. After that, the count returns to the normal 5-10/100 WBC13). The mean nRBC count of the donated cord blood in South Korea was found to be 3.3/100 WBC14), which is the same as that previously reported in other countries15, 16). However, the normal value based on gestational age and birth weight and that based on the perinatal period, have not been established. Furthermore, it is difficult for the nRBC count to reflect hematopoiesis due to the broad range of normal WBC counts in newborn infants. Therefore, this study was conducted to investigate the mean nRBC count in VLBWIs and the usefulness of the nRBC as independent prognostic factors of perinatal complications in VLBWIs.
- Materials and methods
- Materials and methods
- 1. Subjects
- 1. Subjects
This study was conducted in a total of 126 very low birth weight infants (VLBWIs) at birth and who were hospitalized in the neonatal intensive care unit (NICU) of Konyang University Hospital within the period from March 2003 to May 2008. Infants with congenital anomalies, chromosomal abnormalities, twin-to-twin transfusion, expected death within 48 hours after birth, and severe anemia were excluded. Based on their nRBC counts at birth, on the third day after birth, on the seventh day after birth, in the second week after birth, and in the fourth week after birth reflected in the medical records, the correlation between the nRBC or absolute nRBC counts with birth weight, gestational age, 5-min Apgar score, small for gestational age (SGA), hyaline membrane disease (HMD), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), pulmonary hemorrhage, and death after birth were retrospectively investigated.The gestational age was calculated starting from the first day of the mother's last menstruation, and was confirmed through the physical and neurological examinations conducted by Dubowitz et al.18). In the case of the evaluation of neonatal asphyxia, as the gestational age affects the Apgar score in premature infants, it is more difficult to evaluate neonatal asphyxia in premature infants than in full-term infants19, 20). As such, the subjects were divided into two groups based on their 5-min Apgar scores, which are clinically more important than the 1-min Apgar scores: one group consisting of those with Apgar scores of 6 points or less, and the other consisting of those with Apgar scores of 7 points or more21, 22). SGA was defined as less than 10 percentile on the Lubchenco curve23). HMD and BPD were evaluated based on the chest X-ray findings. Patent ductus arteriosus (PDA) was diagnosed based on characteristic heart murmur and transthoracic echocardiography. IVH was categorized into four grades, according to the classification of Papile et al.24), and the subjects were further classified into one group with grade 3 or higher IVH and another with less than grade 3 IVH. As for ROP, funduscopy was conducted by an ophthalmologist starting from four to six weeks after birth, according to International Classification of Retinopathy of Prematurity (ICROP, 1987)25). The subjects were classified into one group with grade 3 or higher ROP and another with less than grade 3.- 2. Methods
- 2. Methods
Umbilical or peripheral venous blood was collected from all the subject infants within two hours after their birth, after which the blood was transferred into an EDTA-treated sample bottle. In the hospital, according to the evaluation guideline on infants with very low birth weights, peripheral venous blood was collected on the third day, seventh day, second week, and fourth week after birth, using the aforementioned method, after which the hemoglobin, hematocrit, WBC count, and platelet count were measured. The nRBC count was described per 100 WBC through the Wright stain of the blood smear, and was then converted into the absolute count over the corrected WBC count to calculate the absolute nRBC count. The corrected WBC count and the absolute nRBC count were calculated as follows17):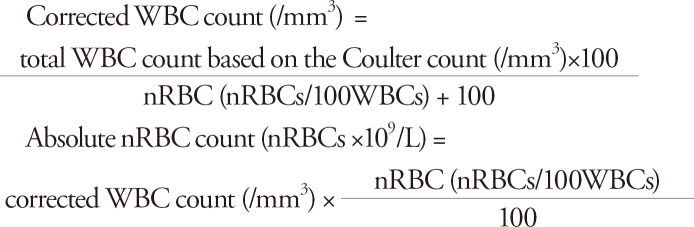
- 3. Statistics
- 3. Statistics
Student's t-test was conducted using the SPSS software (version 12.0, Chicago, IL, USA) for statistical analysis. The mean and continuous variables were expressed as mean±standard deviation. To determine whether the nRBC count has a correlation with birth weight and gestational age, one-way ANOVA was used for the comparison of the three groups. Receiver-operating characteristics (ROC) curve analysis was performed to evaluate the predictive accuracy of nRBC for severe IVH and death. It was considered statistically significant if the P value was less than 0.05. Multiple linear-regression analysis was conducted on the univariates.
- Results
- Results
- 1. Characteristics of the subject infants
- 1. Characteristics of the subject infants
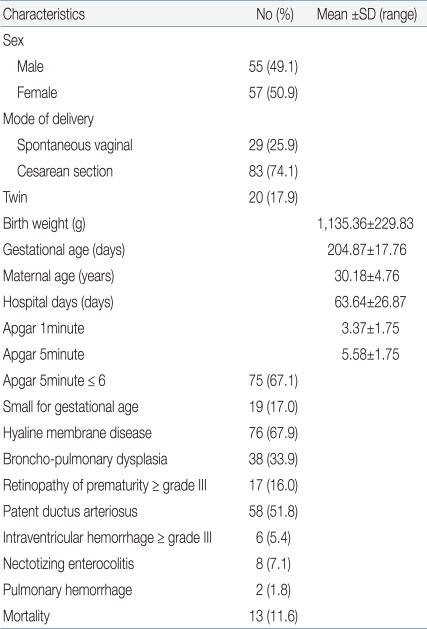
This study was conducted on a total of 126 VLBWIs and who were hospitalized in the NICU. Of these infants, 14 infants with congenital anomalies, chromosomal abnormalities, twin-to-twin transfusion, expected death within 48 hours after birth, and severe anemia were excluded from the study. The study population consisted of 55 males (49.1%) and 57 females (50.9%). As for the delivery mode, 29 cases (25.9%) had spontaneous vaginal delivery, and 83 cases (74.1%) had Cesarean section. The mean birth weight was 1,135±229.83 g, and the mean gestational age was 204.87±17.76 days. The mean maternal age was 30.18±4.76 years, and the mean hospitalization duration was 63.64±26.87 days. The mean 1-min Apgar score was 3.75±1.75 points whereas the mean 5-min Apgar score was 5.58±1.75 points. 5-min Apgar scores of 6 points or less were shown in 75 cases (67.1%); SGA was shown in 19 cases (17%); HMD was shown in 76 cases (67.9%); BPD was shown in 38 cases (33.9%); grade 3 or higher ROP was shown in 17 cases (16%), of which 14 infants had grade 3 and three had grade 4; PDA was shown in 58 cases (51.8%); grade 3 or higher IVH was shown in six cases (5.4%), of which one infant had grade 3 and five had grade 4; necrotizing enterocolitis (NEC) was shown in eight cases (7.1%); pulmonary hemorrhage was shown in two cases (1.8%); and death was shown in 13 cases (11.6%) (Table 1).- 2. Correlation of a change in the nRBC count with the birth weight and gestational age after birth
- 2. Correlation of a change in the nRBC count with the birth weight and gestational age after birth
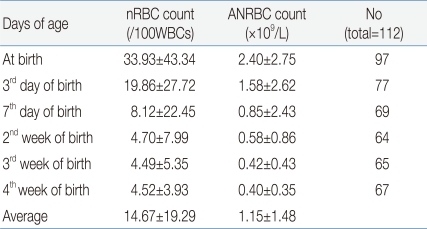
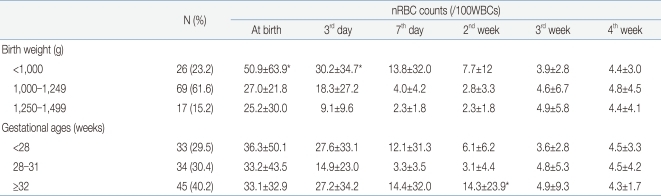
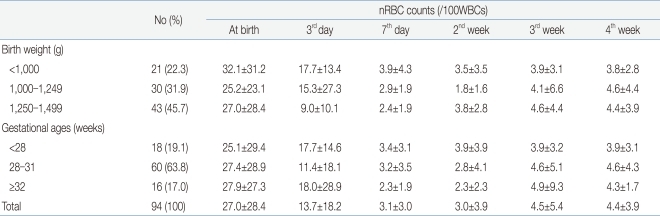
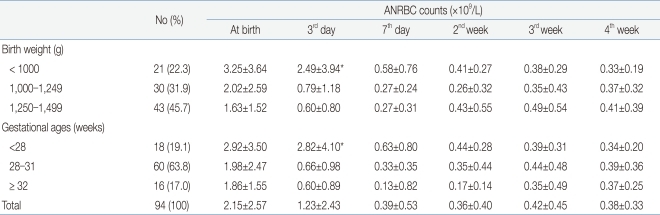
The mean nRBC and mean absolute nRBC counts in 112 VLBWIs were 33.93±43.34/100 WBC and 2.4±2.75×109/L, respectively, at birth; 19.86±27.72/100 WBC and 1.58±2.62×109/L, on the third day after birth; 8.12±22.45/100 WBC and 0.85±2.43×109/L, on the seventh day after birth; 4.7±7.99/100 WBC and 0.58±0.86×109/L, in the second week after birth; 4.49±5.35/100 WBC and 0.42±0.43×109/L, in the third week after birth; and 4.52±3.93/100 WBC and 0.4±0.36×109/L, in the fourth week after birth. These results showed that the nRBC and absolute nRBC counts gradually decreased with the passage of time after birth, and had constantly low values since one week after birth (Table 2). A difference between the subject group and the group with actual blood collection was observed due to blood collection on weekend, inaccurate blood collection time, and death before blood collection.When the infants were classified into three groups based on their birth weights (less than 1,000 g, 1,000 g or more to less than 1,250 g, and 1,250 g or more), and were then compared, the nRBC counts at birth (P=0.04) and on the third day after birth (P=0.02) were significantly higher in the group with birth weights of less than 1,000 g, corresponding to the extremely low birth weight infants (ELBWIs) compared to the other groups. No significant difference, however, was found after that. When the subject infants were classified into three groups based on their gestational ages (less than 28 weeks, 28 weeks or more to less than 32 weeks, and 32 weeks or more), the nRBC count on the second week after birth was significantly higher in the group with gestational ages of 32 weeks or more (P=0.02) compared to the others (Table 3). As the mean birth weight in the group with gestational ages of 32 weeks or more was higher than that in the group with gestational ages of less than 32 weeks, the nRBC count in the group with gestational ages of 32 weeks or more was expected to be lower than those in the other groups. The higher mean nRBC count in the second week after birth in the group with gestational ages of 32 weeks or more, however, is likely to be attributable to the fact that one infant in that group died on the 20th day after birth due to IVH, and that the infant's nRBC count of 130 in the second week after birth contributed to the increased group mean. The absolute nRBC count was shown to have the same statistical significance as the nRBC count in the same group.- 3. Correlation of the nRBC count with the perinatal complications
- 3. Correlation of the nRBC count with the perinatal complications
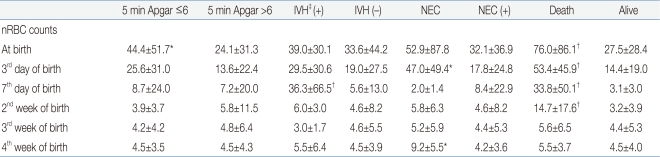
In determining the correlation of the nRBC count with the perinatal complications, when the 5-min Apgar score was 6 points or below, the mean nRBC count at birth was 44.4±51.7/100 WBC, which was significantly higher compared to the control group (P<0.05). As for grade 3 or higher IVH, the mean nRBC count on the seventh day after birth was 36.3±66.5/100 WBC, which was significantly higher compared to that of the control group (P<0.01). As for NEC, the mean nRBC counts on the third and 28th days after birth were 47.0±49.4 and 9.2±5.5/100 WBC, respectively, which were significantly higher compared to those of the control group (P<0.05). As for death, the mean nRBC counts at birth, on the third day, on the seventh day, and on the 14th day after birth were 76.0±86.1, 53.4±45.9, 33.8±50.1, and 14.7±17.6/100 WBC, respectively, which were significantly higher compared to those of the control group (P<0.01) (Table 4). For SGA, HMD, BPD, grade 3 or higher ROP, and PDA, however, no significant difference in nRBC count was found between the subject and control groups.In addition, no significant difference in nRBC count was found in the cases of sex and delivery mode. The absolute nRBC count showed the same statistical significance as that of the nRBC count in the same group, except for a difference at birth in the case of death.- 4. Correlation of the increase in the nRBC count with the perinatal complications and cutoff reference
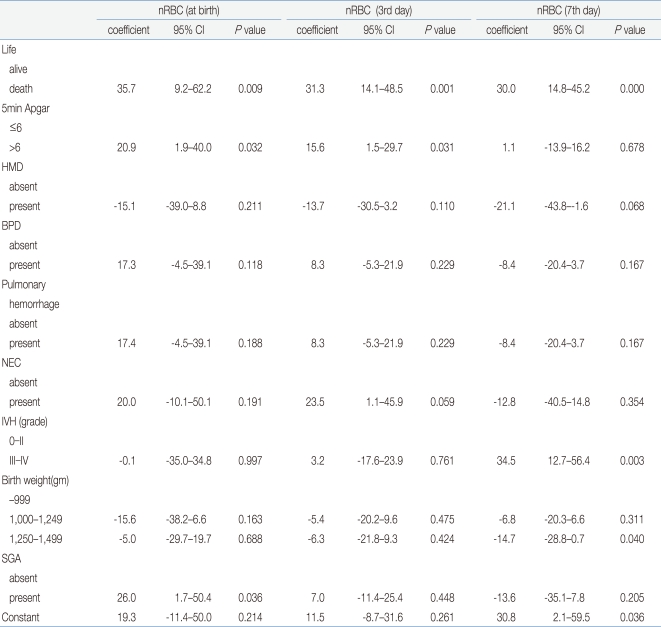
- 4. Correlation of the increase in the nRBC count with the perinatal complications and cutoff reference
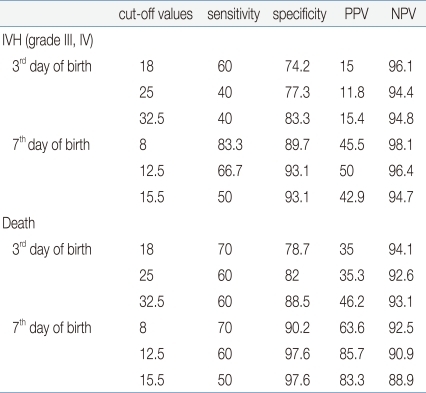
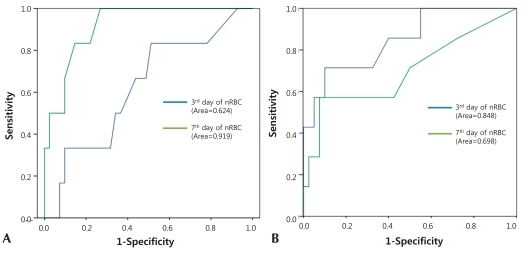
Among the factors that showed the correlation of the nRBC count with the perinatal complications in VLBWIs, multiple linear-regression analysis was conducted on death, neonatal asphyxia, IVH, NEC, HMD, BPD, pulmonary hemorrhage, birth weight, and SGA to identify the expected risk factors. Death showed a close correlation with the mean nRBC count at birth (coefficient: 35.7; 95% CI: 9.2-62.2), on the third day after birth (coefficient: 31.3; 95% CI: 14.1-48.5), on the seventh day after birth (coefficient: 30.0; 95% CI: 14.8-45.2), and on the 14th day after birth (coefficient: 12.2; 95% CI: 5.6-18.8) (P<0.05). Grade 3 or higher IVH showed a high correlation on the seventh day after birth (coefficient: 34.5; 95% CI: 12.7-56.4). In addition, the 5-min Apgar score of less than 6 points showed a significant correlation with the mean nRBC counts at birth and on the third day after birth; NEC showed a significant correlation on the third day after birth; and SGA showed a significant correlation with the mean nRBC at birth (P<0.05) (Table 5).In the cutoff evaluation of grade 3 or higher IVH and perinatal death by ROC curve analysis, which showed a higher correlation with the nRBC count. The area under the ROC curve (AUC) was 0.919±0.046 on the nRBC count on the seventh day after birth with grade 3 or higher IVH. And it was 0.848±0.082 on the nRBC count on the third day after birth with perinatal death (Fig. 1). The optimal cutoff value of the nRBC count on the third day after birth was 32.5/100 WBC, 60% sensitivity and 88.5% specificity were shown in the case of perinatal death. The optimal cutoff value of the nRBC count on the seventh day after birth was 12.5/100 WBC, 66.7% sensitivity and 93.1% specificity were shown in the case of grade 3 or higher IVH (Table 6). Except for the positive predictive value on the seventh day after birth in the case of death, however, the positive predictive value was less than 50% in the cases of all the other factors.- 5. Reference counts of the nRBC and absolute nRBC counts in infants with very low birth weights
- 5. Reference counts of the nRBC and absolute nRBC counts in infants with very low birth weights
Excluding the 18 infants with perinatal death and grade 3 or higher IVH, where a higher correlation was shown among the factors that showed statistical significance in the multiple linear-regression analysis, the nRBC and absolute nRBC counts based on the birth weight and gestational age were reviewed in 94 infants to determine the reference count. The reference count of the nRBC count was 27.04±28.37/100 WBC at birth, 13.7±18.2/100 WBC on the third day after birth, and 3.1±3.0/100 WBC on the seventh day after birth, showing a continuous decrease after birth. No significant change in the mean nRBC count was found, however, since the seventh day after birth. When classified based on birth weight, the reference count of the nRBC count was 32.1±31.2/100 WBC in the case of those with a birth weight of less than 1,000 g, 25.2±23.1/100 WBC in the case of those with a birth weight of 1,000-1,249 g, and 27.0±28.4/100 WBC in the case of those with a birth weight of 1,250 g or more. When classified based on gestational age, the reference count of the nRBC count at birth was 25.1±29.4/100 WBC in the case of those with a gestational age of less than 28 weeks, 27.4±28.9/100 WBC in the case of those with a gestational age of 28-31 weeks, and 27.9±27.3/100 WBC in the case of those with a gestational age of 32 weeks or more. Since then, decrease in the nRBC count showed the same pattern (Table 7). The reference count of the absolute nRBC count was 2.15±2.57×109/L at birth, 1.23±2.43×109/L on the third day after birth, and 0.39±0.53×109/L on the seventh day after birth, showing the same continuous decrease as that of the nRBC count. The nRBC count was inversely proportional to both the birth weight and gestational age, but no statistical significance was found. The absolute nRBC count on the third day after birth was significantly higher in the group with birth weights of less than 1,000 g than in the other groups (P=0.03) and was also significantly higher in the group with gestational ages of less than 28 weeks than in the other groups. The mean nRBC count until seven days after birth was inversely proportional to the birth weight and gestational age (Table 8).
- Discussion
- Discussion
Several studies have been reported on nRBC and absolute nRBC in full-term infants. However, no study has been conducted on VLBWIs, and on the reference count based on gestational age and birth weight as well as the normal count based on the stage after birth in premature infants. The mean nRBC and mean absolute nRBC counts at birth in a full-term infant's cord blood were 3.3±3.9/100 WBC14) and 0.4±1.3×109/L12), respectively, and decreased gradually since then. The mean nRBC and mean absolute nRBC counts have been known to be 21/100 WBC and 1.0-1.5×109/L3), respectively, in premature infants, and have been known to decrease gradually since then3, 24). In this study, the mean nRBC and mean absolute nRBC counts at birth were 33.9±43.4/100 WBC and 2.40±2.75×109/L, respectively, in VLBWIs. In addition except for perinatal death and severe IVH, the mean nRBC and mean absolute nRBC counts at birth were 27.0±28.4/100 WBC and 2.15±2.57×109/L, respectively. These results showed higher counts than those in the other studies conducted on low birth weight infants12, 27, 28). Excluding the 18 infants with perinatal death and grade 3 or higher IVH, where a higher correlation was shown among the factors that showed statistical significance in the multiple linear-regression analysis, the nRBC and absolute nRBC counts based on the birth weight and gestational age were reviewed in 94 infants to determine the reference count. The mean nRBC and mean absolute nRBC counts on the third day after birth were 13.7±18.2/100 WBC and 1.6±2.6×109/L, respectively, and were 3.1±3.0/100 WBC and 0.8±2.4×109/L, respectively, after the seventh day after birth, a gradual decrease, was maintained after wards. When the infants were classified into three groups based on their birth weights and were then compared, their mean nRBC count at birth was 32.1±31.2/100 WBC in the case of those who weighed less than 1,000 g, 25.2±27.0/100 WBC in the case of those who weighed 1,000-1,249 g, and 27.0±28.4/100 WBC in the case of those who weighed 1,250 g or more. When classified based on gestational age, the mean nRBC count at birth was 25.1±29.4/100 WBC in the case of those with gestational ages of less than 28 weeks, 27.4±28.9/100 WBC in the case of those with gestational ages of 28-31 weeks, and 27.9±27.3/100 WBC in the case of those with gestational ages of 32 weeks or more. All the results showed a similar pattern; that is, a gradual decrease in the counts was observed with the passage of time. The higher mean nRBC count in the second week after birth in the group with gestational ages of 32 weeks or more, however, is likely to be attributable to the fact that one infant in that group died on the 20th day after birth due to IVH, and that the infant's nRBC count of 130 in the second week after birth contributed to the mean of group. By eliminating the two factors that showed a high correlation with the increased nRBC counts in VLBWIs, the mean nRBC count at each stage based on birth weight and gestational age can be used as a reference count, and the mean nRBC counts at birth and on the third day after birth in all the infants were higher in the group with birth weights of less than 1,000 g compared to those in the other groups (P<0.05). No significant difference was found, however, among the three groups after eliminating the two factors. The nRBC and absolute nRBC counts were inversely proportional to the birth weight and gestational age, but no statistical significance was found, except for the clearer aforementioned pattern of the absolute nRBC count.Previous studies have reported the correlation of neonatal asphyxia and nRBC count4, 27, 28), and the correlation is likely to be attributable to the mechanism in which asphyxia, upon delivery, transfers blood from the intrauterine placenta to a fetus29), and the circulatory blood increases due to the increased blood influx into the placenta30), leading to an increase in the nRBC count due to the influx of premature red blood cells into the circulation along with the increased blood volume17). It is more difficult, however, to evaluate neonatal asphyxia in premature infants than in full-term infants20), and the gestational age affects the Apgar score in premature infants19). Thus, based on studies that reported increased perinatal death and poor prognosis in the group with 5-min Apgar scores of 6 points or below21, 22), the infants were classified into two groups: one group with 5-min Apgar scores of 6 points or below, and another with 5-min Apgar scores of 7 points or higher. As a result, the mean nRBC count at birth was significantly higher in the group with 5-min Apgar scores of 6 points or below than in the control group. The multiple linear-regression analysis also showed a close correlation of the increased mean nRBC counts at birth and on the third day after birth with 5-min Apgar scores of 6 points or below.Philip et al.37) reported that the nRBC count was significantly higher in the infants who were SGA than in those who had appropriate for gestational age, due to chronic intrauterine hypoxia. In this study, multiple linear-regression analysis showed a correlation between having a SGA and increased nRBC count despite the fact that there was no difference in the nRBC count based on the stage after birth between the two groups.Although the nRBC count increased significantly in the infants with HMD in domestic studies27, 28), no significant difference in the nRBC count in VLBWIs accompanying HMD was found compared to the control group. The frequency of HMD increased in newborn infants with gestational ages of less than 32 weeks30), and that phenomenon was maintained even when the factors correlated with birth weight and gestational age were adjusted. Based on these results, it is likely that no correlation can be found between HMD and nRBC count in VLBWIs. In addition, in the case of BPD, which is most persistent in premature infants due to the damage caused by continuous mechanical ventilation along with the treatment for HMD, pulmonary fibrosis or pulmonary prematurity caused by oxygen poisoning, perinatal infection, and inflammatory reaction33), the mean nRBC count was reported to increase in premature infants with growth retardation for one week after birth10). In this study, the nRBC count increased in the group with BPD for three days after birth compared to the control group, but no statistical significance was found.Ahmet et al.10) reported that the nRBC count increased in infants with grade 3 or higher IVH and NEC after birth. In this study, the mean nRBC count on the seventh day after birth in the infants with grade 3 or higher IVH was 36.3±66.5/100 WBC, which was significantly higher than the 5.6±13.0/100 WBC mean nRBC count in the control group (P<0.01). The multiple linear-regression analysis also showed a close correlation (P<0.01). The mean nRBC counts on the third and 28th days after birth were significantly higher in the group with NEC than in the control group. In six infants with grade 3 or higher IVH, the mean diagnosis date was the ninth day after birth (three cases on the fifth day after birth, one case on the sixth day after birth, one case on the ninth day after birth, and one infant who showed grade 2 IVH on the fifth day after birth and who died of grade 4 IVH and disseminated intravascular coagulation on the 24th day after birth), and the diagnosis time was similar to the time of nRBC count increase. The mean date of the diagnosis of NEC in eight infants was on the 24th day after birth, which was similar to the time of nRBC count increase. As the IVH frequency is highest in premature infants within four days after birth34) and NEC frequency is highest at two to three weeks after birth35), newborn infant care precautions should be required if no nRBC count decrease or increase is observed.The mean nRBC count increased significantly in the group with perinatal death at birth, on the third day, on the seventh day, and on the 14th day after birth compared to the control group. These results were consistent with those of the other studies that showed an increased mean nRBC count in the group with death12, 27). The multiple linear-regression analysis showed that perinatal death had the closest correlation with increased mean nRBC count (P<0.01). No difference in the mean nRBC count based on the stage after birth was found in ROP and PDA, among the perinatal complications. A previous study reported that the nRBC count increased in infants with early sepsis due to inflammatory reaction36). However, no correlation between newborn infants with sepsis and nRBC count was found in this study.There are two ways of describing the nRBC count. One way is per 100 WBC, and the other by the absolute nRBC count, which is corrected based on the corrected WBC count. Under the condition where leukocytosis such as neonatal asphyxia or sepsis can occur due to the variation in the WBC count, the absolute nRBC count was reported to be more accurate3). No statistical significance was found, however, between the two ways in this study. Considering that the nRBC count can be more easily used in a clinical study and that almost no difference was found between the two groups, it is likely that the use of nRBC count is comparable to the use of absolute nRBC count.In the cutoff evaluation of perinatal death and grade 3 or higher IVH, which showed a higher correlation with nRBC count, when the cutoff value was set at 32.5/100 WBC on the third day after birth and at 12.5/100 WBC on the seventh day after birth based on the mean nRBC count of 19.9/100 WBC on the third day after birth and of 8.1/100 WBC on the seventh day after birth, the highest sensitivity and specificity over perinatal death and severe IVH were shown. These results could be useful in that they point out that specificity is more important than sensitivity when it comes to disease severity.This study is a retrospective one with a few restrictions, such as the fact that nRBC counting could be on weekdays, so no evaluation of the nRBC count in a test was conducted on weekend, only evaluation of late sepsis as there are no infants diagnosed with early sepsis, no evaluation of various factors, such as the measurement of the fetus blood flow volume and cord blood, and evaluation of multivariate analysis was not conducted. The results of this study, however, present the nRBC count for reference purposes based on birth weight and gestational age in VLBWIs. They also show that the mean perinatal nRBC count in VLBWIs is inversely proportional to the birth weight and gestational age, and exhibit the highest peak at birth and continuous decrease after birth. If no decrease or increase in the nRBC count is observed, precautions on newborn infant care should be required as perinatal poor prognoses such as newborn infant death, severe IVH, and NEC could be associated. A further study on the correlation of the nRBC count with perinatal complications will be required.
- References
- 1. Buonocore G, Perrone S, Gioia D. Nucleated red blood cell count at birth as an index of perinatal brain damage. Am J Obstet Gynecol 1999;181:1500–1505.
[Article] [PubMed]2. Thilaganathan B, Athanasiou S, Ozmen S, Creighton S, Watson NR, Nicolaides KH. Umbilical cord blood erythroblast count as an index of intrauterine hypoxia. Arch Dis Child Fetal Neonatal Ed 1994;70:F192–F194.
[Article] [PubMed] [PMC]3. Oski FA, Naiman JL. Hematologic Problems in the Newborn. 1982;Philadelphia: WB Saunders Co, :1–31.4. Anderson GW. Studies on the nucleated red cell count in the chorionic capillaries and the cord blood of various ages of pregnancy. Am J Obstet Gynecol 1941;42:1–14.
[Article]5. Ferber A, Fridel Z, Weissmann-Brenner A, Minior VK, Divon MY. Are elevated fetal nucleated red blood cell counts an indirect reflection of enhanced erythropoietin activity. Am J Obstet Gynecol 2004;190:1473–1475.
[Article] [PubMed]6. Huch R, Huch A. Maternal and fetal erythropoietin: physiological aspects and clinical significance. Ann Intern Med 1993;25:289–293.7. Teramo K, Vilho K, Hiilesmaa VK. Amniotic fluid and cord plasma erythropoietin levels in pregnancies complicated by preeclampsia, pregnancy-induced hypertension and chronic hypertension. J Perinat Med 2004;32:240–247.
[PubMed]8. Doi S, Osada H, Seki K, Sekiya S. Relationship of amniotic fluid index and cord blood erythropoietin levels in small for and appropriate for gestational age fetuses. Obstet Gynecol 1999;94:768–772.
[Article] [PubMed]9. Baschat AA, Gembruch U, Reiss I, Gortner L, Harman CR. Neonatal nucleated red blood cell count and postpartum complications in growth restricted fetuses. J Perinat Med 2003;31:323–329.
[PubMed]10. Baschat AA, Gungor S, Kush ML, Berg C, Gembruch U, Harman CR. Nucleated red blood cell counts in the first week of life: a critical appraisal of relationships with perinatal outcome in preterm growth-restricted neonates. Am J Obstet Gynecol 2007;197:286.e1–286.e8.
[Article] [PubMed]11. Minior VK, Bernstein PS, Divon MY. Nucleated red blood cells in growth-restricted fetuses: associations with short-term neonatal outcome. Fetal Diagn Ther 2000;15:165–169.
[Article] [PubMed]12. Green DW, Hendon B, Mimouni FB. Nucleated erythrocytes and intraventricular hemorrhage in preterm neonates. Pediatrics 1995;96:475–478.
[Article] [PubMed]13. Baschat AA, Gembruch U, Reiss I, Gortner L, Harman CR. Neonatal nucleated red blood cell count and postpartum complications in growth restricted fetuses. J Perinat Med 2003;31:323–329.
[PubMed]14. Lee HR, Shin S, Yoon JH, Kim BJ, Hwang KR, Kim JJ, et al. Complete blood count reference values of donated cord blood from Korean neonates. Korean J Lab Med 2009;29:179–184.
[Article] [PubMed]15. Pranke P, Failace RR, Allebrandt WF, Steibel G, Schmidt F, Nardi NB. Hematologic and immunophenotypic characterization of human umbilical cord blood. Acta Haematol 2001;105:71–76.
[Article] [PubMed]16. Rolfo A, Maconi M, Cardaropoli S, Biolcati M, Danise P, Todros T. Nucleated red blood cells in term fetuses: reference values using an automated analyzer. Neonatology 2007;92:205–208.
[Article] [PubMed]17. D'Souza SW, Black P, MacFarlane T, Jennison RF. Haematological values in cord blood in relation to fetal hypoxia. Br J Obstet Gynaecol 1981;88:129–132.
[Article] [PubMed]18. Dubowitz KMS, Dubowitz V, Goldbergh CG. Clinical assessment of gestational age of the newbonr infant. J Pediatr 1970;77:1–14.
[Article] [PubMed]19. Catlin EA, Carpenter MW, Brann BS, Mayfield SR, Shaul PW, Goldstein M, et al. The Apgar score revisited: influence of gestational age. J Pediatr 1986;109:865–868.
[Article] [PubMed]20. Low JA, Wood SL, Killen HL, Pater EA, Karchmar EJ. Intrapartum asphyxia in the preterm fetus 2000 grams. Am J Obstet Gynecol 1990;162:378–382.
[Article] [PubMed]21. Thorngren-Jerneck K, Herbst A. Low 5-minute apgar score: a population-based register study of 1 million term births. Obstet Gynecol 2001;98:65–70.
[Article] [PubMed]22. Casey BM, McIntire DD, Leveno KJ. The continuing value of the apgar score for the assessment of newborn infants. New Engl J Med 2001;344:467–471.
[Article] [PubMed]23. Lubchenco LO, Hansman C, Dressler M, Body E. Intrauterine growth as estimated form livebone birth weight data at 24 to 42 weeks of gestation. Pediatrics 1963;32:793–801.
[PubMed]24. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr 1978;92:529–532.
[Article] [PubMed]25. Flynn JT. An international classification of retinopathy of prematurity: clinical experience. Trans Am Ophthalmol Soc 1984;82:218–238.
[PubMed] [PMC]26. Lippman HS. A morphologic and quantitative study of the blood corpuscles in the new-born period. Am J Dis Child 1924;27:473–526.
[Article]27. Song IH, Lee WK, Jung HL, Keum DH. The Significance of nucleated red blood cell counts in low birth weight neonates. J Korean Pediatr Soc 1993;36:1526–1533.28. Hur SK, Park MS, Namgoong MK, Kim HM, Yang JS, Lim BK, et al. The significance of nucleated red blood cell counts in various conditions associated with acute or chronic perinatal asphyxia. J Korean Pediatr Soc 1992;35:1514–1519.29. Flod NE, Ackerman BD. Perinatal asphyxia and residual placental blood volume. Acta Paediatr Scand 1971;60:433–436.
[Article] [PubMed]30. Yao AC, Wist A, Lind J. The blood volume of the newborn delivered by cesarean section. Acta Paediatr Scand 1967;56:585–592.
[Article] [PubMed]31. Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol 1992;262:R485–R491.
[Article] [PubMed]32. Usher RH, Allen AC, McKean FH. Risk of respiratory distress syndrome related to gestational age, route of delivery, and mternal diabetes. Am J obstet Gynecol 1971;111:826–832.
[Article] [PubMed]33. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 2003;8:63–71.
[Article] [PubMed]34. Ment LR, Oh W, Ehrenkranz RA, Philip AG, Duncan CC, Makuch RW. Antenatal steroids, delivery mode, and intraventricular haemorrhage in preterm infants. Am J Obstet Gynecol 1995;172:795–800.
[Article] [PubMed]35. Hsueh W, Caplan MS, Tan X, MacKendrick W, Gonzalez-Crussi F. Necrotizing enterocolitis of the newborn: pathogenetic concepts in perspective. Pediatr Dev Pathol 1998;1:2–16.
[Article] [PubMed] [PMC]
Fig. 1
Receiver operating characteristic (ROC) curves of nucleated red blood cell counts on 3rd and 7th days afterof birth and 7th day of birth in patients with grade III or higher intraventricular hemorrhage (A) and death (B). Area under ROC curves (95% confidence interval) were 0.624, 0.919 (A), and 0.848, 0.698 (B).

 About
About Browse articles
Browse articles For contributors
For contributors