All issues > Volume 54(3); 2011
Mycoplasma pneumoniae infection in patients with Kawasaki disease
- Corresponding author: Young Mi Hong, M.D. Department of Pediatrics, Ewha Womans University, School of Medicine, 911-1 Mokdong, Yangcheon-gu, Seoul 158-710, Korea. Tel: +82.2-2650-2841, Fax: +82.2-2653-3718, hongym@chollian.net
- Received February 14, 2010 Revised October 09, 2010 Accepted March 07, 2011
- Abstract
-
- Purpose
- Purpose
- Kawasaki disease (KD) is the main cause of acquired heart disease in children. In addition to cardiovascular involvement, many complications have been recognized in KD. However, respiratory complications have been rarely reported. We investigated the differences in clinical characteristics, laboratory findings, radiography findings, and echocardiography findings of Mycoplasma pneumoniae infection and other types of pneumonia in KD patients.
- Methods
- Methods
- Among 358 patients with KD, 54 developed concurrent pneumonia. Among the 54 patients, 12 (22.2%) with high titers of anti-M. pneumoniae antibody (AMA) (>1:640) were grouped in the M. pneumoniae group and 42 were included in the control group. Serum AMA was measured in each patient. Clinical laboratory findings and total duration of fever were analyzed.
- Results
- Results
- The duration of fever, serum hemoglobin, white blood cell count, platelet count, erythrocyte sedimentation rate, C-reactive protein level, albumin level, and the incidence of coronary arterial lesions showed no statistical difference in the 2 groups. Neutrophil count was significantly higher in the M. pneumoniae group than in the control group. Among various radiography findings observed in pneumonia, consolidation and pleural effusion were more frequent in the M. pneumoniae group than in the control group. On the other hand, parahilar peribronchial opacification, diffuse interstitial lesion, and normal findings prevailed in the control group.
- Conclusion
- Conclusion
- KD patients can have concurrent infections, especially pulmonary symptoms. The cause of KD is likely to be associated with M. pneumoniae infection. Thus, immediate treatment of M. pneumoniae infection in KD patients is very important.
- Introduction
- Introduction
Kawasaki disease (KD) is an acute, self-limited vasculitis that affects mainly infants and young children1, 2). The major clinical features are fever lasting at least 5 days, bilateral conjunctival injection, changes of the oral mucosa, erythematous changes of the hands and feet, polymorphous skin rashes, and enlarged cervical lymphadenopathy. Of the patients with untreated KD, 10-15% develope coronary artery lesions and a greater proportion develop coronary arterial dilation3-5). KD is a major cause of acquired heart disease in children.The etiology of KD is unclear but superantigens may play a part. Despite multiple investigations and many candidates, no unique infectious agent has been identified as the sole etiologic agent responsible for KD. There is no definite diagnostic test for KD and therefore accurate and timely diagnosis of KD is challenging for clinicians. Clinicians must rely on the presence of specific clinical criteria and laboratory data, excluding other illnesses that can mimic the disease6). Many studies support the existence of an infectious trigger for KD including the epidemiology of the disease by seasonal fluctuations and the reported temporal relationship to various infectious agents7-9). Previous reports have frequently used a screening approach to determine the prevalence of an infectious agent in patients with KD.Many complications other than cardiovascular involvement have been recognized in KD. However, there have been few reports published concerning the involvement of the lungs in this disease10-17). In this study, we investigated differences in clinical characteristics, laboratory findings, X-ray findings and echocardiographic findings between Mycoplasma pneumoniae and other types of pneumonia developed in KD.
- Materials and methods
- Materials and methods
- Statistical analysis
- Statistical analysis
We performed all statistical analyses using SPSS (version 11.0, SPSS Inc, Chicago, IL, USA). Descriptive statistics were presented as means and standard deviations. The comparison of continuous variables was done using the Student t-test or one-way analysis of variance. A P-value less than 0.05 was considered as statistically significant.
This study was conducted at Ewha Womans University Hospital, Department of Pediatrics, from December 2003 to July 2007. A total of 358 patients with KD were admitted to Ewha Womans University Hospital and 54 patients of them were found to have pneumonia, concurrently. We estimated serum anti-Mycoplasma pneumoniae antibody (AMA) titer in patients with KD who had abnormal chest X-ray findings. The diagnosis of M. pneumoniae infection was confirmed by serologic tests with elevated single titers (>1:640) or a fourfold rise in titer. Among the enrolled 54 patients, 12 who had high titers of AMA were grouped as M. pneumoniae group, and the other 42 as control group. All of the patients received treatment with intravenous immunoglobulin (IVIG) (2 g/kg/day for 1 day) and oral aspirin (50 mg/kg/day).Echocardiography was obtained by pediatric cardiologists to detect the presence of any coronary artery lesions prior to IVIG administration. Coronary aneurysm was diagnosed from echocardiogram using the criteria proposed by the Japanese Kawasaki Disease Research Committee. Coronary arteries were classified as abnormal in the following cases: an internal lumen diameter greater than 3 mm in children at the age of 4 or younger and greater than 4 mm in children at the age of 5 or older; the internal diameter of a segment measured 1.5 times larger than that of the adjacent segment; or a coronary lumen that is clearly irregular18). Laboratory data were obtained from each child including Hb, white blood cell (WBC ) count, platelet count, serum albumin, erythrocyte sedimentation rate (ESR) and C-reactive protein level (CRP). The study was carried out with the approval of the Ethics Committee of the Ewha Womans University Hospital Institutional Review Board, and written informed consents were obtained from the parents of all the subjects. Serum AMA was measured using particle agglutination test according to the manufacturer's instructions. Clinical characteristics including Hb, WBC count, platelet count, serum albumin, ESR, CRP and total duration of fever were analyzed each as a quantitative trait.
- Results
- Results
Clinical characteristics of the study groups are shown in Table 1. The mean age of the M. pneumoniae group was 5.5±3.5 years and that of the control group was 2.8±2.2 years. The M. pneumoniae group was significantly older than the control group. There was no statistical difference in the date of intravenous immunoglobulin (IVIG) infusion or the duration of fever.We also analyzed echocardiographic findings of each group, which are shown in Table 2. The diameter of the right coronary artery was 4.9±2.1 mm in the M. pneumoniae group and 3.6±1.2 mm in the control group. The diameter of the left coronary artery was 2.8±0.8 mm and 2.7±0.8 mm, respectively, in the M. pneumoniae group and the control group. The echocardiographic findings showed no significant difference between these two groups.Clinical parameters were compared between the M. pneumoniae group and the control group. Laboratory findings of each group were shown in Table 3. Hb, WBC count, platelet count, ESR, CRP and serum albumin were not significantly different between the two groups. However, PMN was significantly higher and the lymphocyte count was significantly lower in the M. pneumoniae group than in the control group.We compared chest X-ray findings of each group to find any distinguished characteristics. The most common radiographic findings were listed in Table 4. Among the chest X-ray findings, focal reticulonodular lesion, atelectasis and hilar lymphadenopathy were not significantly different between the M. pneumoniae group and the control group. The relatively frequent chest X-ray image findings in the M. pneumoniae group were consolidation (58.3%, Fig. 1), parahilar peribronchial opacification (50%, Fig. 2) and focal reticulonodular lesion (41.7%, Fig. 3). In the X-ray findings of the M. pneumoniae group, consolidation and pleural effusion were prevalent. In contrast, parahilar peribronchial opacification, diffuse interstitial and normal findings were prevalent in the control group.
- Discussion
- Discussion
In a recent multicenter study, Leung et al.19) screened 45 patients with KD and 37 febrile controls for superantigen-producing bacteria; they identified superantigen-producing organisms in 56% of the patients with KD and 35% of the controls. Also, Baker et al.20) found that nonspecific symptoms such as vomiting, diarrhea, abdominal pain and cough occur commonly in 10 days before the diagnosis of KD. These nonspecific symptoms may reflect diffuse vasculitis or be the sequelae of an infectious trigger of KD. Although less likely, we cannot exclude the possibility that associated symptoms result from concurrent infections unrelated to KD. Within the study of Benseler et al.21), 64% of the patients with KD had been diagnosed with a presumptive infection before KD; most commonly tonsillitis, viral illness, pneumonia, urinary tract infection, gastroenteritis and/or sepsis.As mentioned above, the etiology of KD is unknown. However, many findings support the notion that infections may play a part. Also, nonspecific symptoms occur commonly in KD, which make it difficult for clinicians to diagnose KD. Recently specific infections are being reported in KD patients such as Chlamydia pneumoniae 22), M. pneumonia 23, 24), Staphylococcus aureus 25), Streptococcus 26), Yersinia pseudotuberculosis 8), and measles virus9). Based on these reported cases, Abe et al.27) suggested that the process of superantigens activating T cell may be important in the pathophysiology of KD. By interacting directly with class two major histocompatability complex molecules, superantigens can stimulate polyclonal T cell activation with subsequent massive cytokine release.Mycoplasma arthritidis is known to produce superantigen28) and it is therefore possible that other Mycoplasma organisms may do likewise. Therefore, it is crucial to closely observe nonspecific symptoms in KD and look for concurrent infections. M. pneumoniae is a major cause of respiratory infections in school-aged children and young adults. The most common manifestations include sore throat, hoarseness, fever, cough, headache, chills, coryza and myalgia23). Chest auscultation may detect rales, scattered or localized rhonchi and expiratory wheezes. Radiographic findings in M. pneumoniae group can be extremely variable and mimic a wide variety of lung diseases. The diagnosis of Mycoplasma pneumonia is made based on clinical manifestations, culture of sputum, cold agglutinins and antibody titer of M. pneumoniae specific IgM. In children, a single positive IgM assay may be considered diagnostic in most cases, as IgM titer typically rises within 7-10 days of infection and appears approximately 2 weeks before IgG24, 29, 30).Several manifestations of broncho-pulmonary involvement in KD have been described in patients who initially showed prolonged fever, lung parenchymal consolidation and laboratory findings of pneumonia, presented a clinical picture ascribed to KD10-17).Our previous study reported that abnormal chest X-ray findings were noted in 51.2%. Peribronchial cuffing was the most frequent abnormality (22.4%)17).In our present study, 15% of KD patients had pneumonia concurrently. The incidence of pulmonary involvement in KD was similar to that in other studies16). Among the patients, 22.2% were found to be infected by M. pneumoniae , as confirmed by high AMA titer (>1:640). The M. pneumoniae group was not statistically different from the control group in the duration of fever, Hb, WBC, platelet, ESR, CRP, albumin levels and the incidence of coronary arterial lesions. The M. pneumoniae group showed significantly higher PMNs and lower lymphocyte count than the control group. Among various X-ray findings in the pneumonia patients, consolidation and pleural effusion were more frequent in the M. pneumoniae group. On the other hand, parahilar peribronchial opacification, diffuse interstitial lesion and normal findings were prevalent in the control group.However, there was no significant difference in the incidence of coronary aneurysm in our study. Umezawa et al.16) reported that chest radiographic abnormalities were present in about 15% of Kawasaki patients as reticulo-granular opacities (90%), peribronchial cuffing (21%), atelectasis (10%) and air trapping (5%), all of which were observed within 10 days after the onset of KD; no case of lung parenchymal consolidation was mentioned . Kim et al.17) published that peribronchial cuffing (22.4%), reticular pattern (17.7%) and hyperaeration (8.2%) were noted in KD patients.Lung involvement in KD might be a consequence of vessel inflammation with increased vascular permeability and perivascular edematous changes. Histologic and immunohistochemical studies might suggest an underlying association between KD and primary respiratory involvement10).A delay in KD diagnosis is more likely to occur in case of incomplete KD. It is imperative to make efforts to reduce the lag between the onset of symptoms and the initiation of treatment.The limitation of this study was that the size of the study population was relatively small. Therefore, further large-scaled studies will be needed in order to firmly establish the relationship between simple M. pneumoniae infection and KD.In conclusion, we found that KD patients may have concurrent infections especially with pulmonary involvement, and a higher incidence of M. pneumoniae infection among the pulmonary infection. It is important not to delay treatment of M. pneumoniae infection in KD patients and vice versa.
- References
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967;16:178–222.
[PubMed]2. Melish ME, Hicks RM, Larson EJ. Mucocutaneous lymph node syndrome in the United States. Am J Dis Child 1976;130:599–607.
[Article] [PubMed]3. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymphnode syndrome (MLNS) prevailing in Japan. Pediatrics 1974;54:271–276.
[Article] [PubMed]4. Fulton DR, Newberger JW. Long-term cardiac sequelae of Kawasaki disease. Curr Rheumatol Rep 2000;2:324–329.
[Article] [PubMed]5. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379–1385.
[Article] [PubMed]6. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110:2747–2771.
[Article] [PubMed]7. Barton M, Melbourne R, Morais P, Christie C. Kawasaki Syndrome associated with gruop A streptococcal and Epstein-Barr virus co-infections. Ann Trop Paediatr 2002;22:257–260.
[Article] [PubMed]8. Konishi N, Baba K, Abe J, Maruko T, Waki K, Takeda N, et al. A case of Kawasaki disease with coronary artery aneurysms documenting Yersinia pseudotuberculosis infection. Acta Paediatr 1997;86:661–664.
[Article] [PubMed]9. Kuijpers TW, Herweijer TJ, Schölvinck L, Wertheim-Van Dillen PM, Van De Veer EM. Kawasaki disease associated with measles virus infection in a monozygotic twin. Pediatr Infect Dis J 2000;19:350–353.
[Article] [PubMed]10. de Maddi F, Cinelli R, Rigante D, Mazzarella G, Siani P. Lung parenchymal consolidation as an uncommon presentation and cause of delayed diagnosis in atypical Kawasaki syndrome. Rheumatol Int 2009;29:1373–1376.
[Article] [PubMed]11. Uziel Y, Hashkes PJ, Kassem E, Gottesman G, Wolach B. "Unresolving pneumonia" as the main manifestation of atypical Kawasaki disease. Arch Dis Child 2003;88:940–942.
[Article] [PubMed] [PMC]12. Voynow JA, Schanberg L, Sporn T, Kredich D. Pulmonary complications associated with Kawasaki disease. J Pediatr 2002;140:786–787.
[Article] [PubMed]13. Freeman AF, Crawford SE, Finn LS, López-Andreu JA, Ferrando-Monleón S, Pérez-Tamarit D, et al. Inflammatory pulmonary nodules in Kawasaki disease. Pediatr Pulmonol 2003;36:102–106.
[Article] [PubMed]14. Kobayashi Y, Koike Y, Tokutomi T, Kuroki Y, Todoroki I. Case 2: fever, rash and pulmonary involvement. diagnosis: Kawasaki disease. Acta Paediatr 2006;95:1145–1148.
[PubMed]15. Sengler C, Gaedicke G, Wahn U, Keitzer R. Pulmonary symptoms in Kawasaki disease. Pediatr Infect Dis J 2004;23:782–784.
[Article] [PubMed]16. Umezawa T, Saji T, Matsuo N, Odagiri K. Chest X-ray findings in the acute phase of Kawasaki disease. Pediatr Radiol 1989;20:48–51.
[Article] [PubMed]17. Kim JY, Kwon JH, Kim KH, Yu JH, Hong YM. Chest X-ray findings and serum tumor necrosis factor-α levels in patients with Kawasaki disease. Korean J Pediatr 2005;48:534–538.18. Research Committee on Kawasaki Disease. Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. 1984;Tokyo, Japan: Japanese Ministry of Health and Welfare.19. Leung DY, Meissner HC, Shulman ST, Mason WH, Gerber MA, Glode MP, et al. Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J Pediatr 2002;140:742–746.
[Article] [PubMed]20. Baker AL, Lu M, Minich LL, Atz AM, Klein GL, Korsin R, et al. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J Pediatr 2009;154:592–595.
[Article] [PubMed]21. Benseler SM, McCrindle BW, Silverman ED, Tyrrell PN, Wong J, Yeung RS. Infections and Kawasaki disease: implications for coronary artery outcome. Pediatrics 2005;116:e760–e766.
[Article] [PubMed]22. Chua PK, Nerurkar VR, Yu Q, Woodward CL, Melish ME, Yanagihara R, et al. Lack of association between Kawasaki syndrome and infection with parvovirus B19, human herpesvirus 8, TT virus, GB virus C/hepatitis G virus or Chlamydia pneumoniae. Pediatr Infect Dis J 2000;19:477–479.
[Article] [PubMed]23. Talkington DF, Waites KB, Schwartz SB, Besser RE. In: Scheld WM, Craig WA, Hughes JM,Emerging from obscurity: understanding pulmonary and extrapulmonary syndromes, pathogenesis and epidemiology of human Mycoplasma pneumoniae infections. editors. Emerging infections 5. 2001Washington, DC: American Society for Microbiology, :57–84.24. Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by Mycoplasma pneumoniae in children: a review of diagnostic and therapeutic measures. Eur J Pediatr 2001;160:483–491.
[Article] [PubMed]25. Hall M, Hoyt L, Ferrieri P, Schlievert PM, Jenson HB. Kawasaki syndrome-like illness associated with infection caused by enterotoxin B-secreting Staphylococcus aureus. Clin Infect Dis 1999;29:586–589.
[Article] [PubMed]26. Morita A, Imada Y, Igarashi H, Yutsudo T. Serologic evidence that streptococcal superantigens are not involved in the pathogenesis of Kawasaki disease. Microbiol Immunol 1997;41:895–900.
[Article] [PubMed]27. Abe J, Kotzin BL, Meissner C, Melish ME, Takahashi M, Fulton D, et al. Characterization of T cell repertoire changes in acute Kawasaki disease. J Exp Med 1993;177:791–796.
[Article] [PubMed] [PMC]28. Cole BC. The immunobiology of mycoplasma arthritidis and its superantigen MAM. Curr Top Microbiol Immunol 1991;174:107–119.
[PubMed]29. Waites KB, Rikihisa Y, Taylor-Robinson D. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken YH,Mycoplasma and Ureaplasma. editors. Manual of clinical microbiology. 19997th ed. Washington, DC: American Society for Microbiology, :782–794.
Fig. 1
Posteroanterior chest radiograph shows dense consolidation of the lower right lung zone accompanied by pleural effusion.

Fig. 2
Posteroanterior chest radiograph shows bilateral parahilar peribronchial opacifications, which are more prominent in the right than the left side.
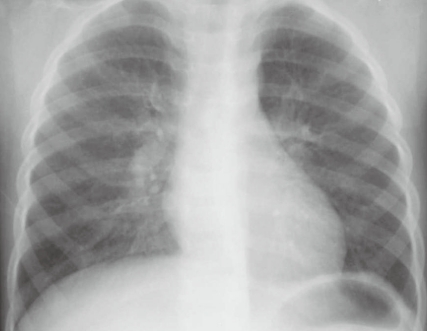
Fig. 3
Posteroanterior chest radiograph shows focal consolidation of the right upper lung zone and bilateral reticular densities throughout the lung fields.
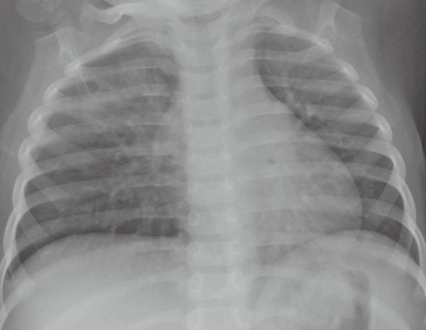
Table 3
Laboratory findings of Study Groups
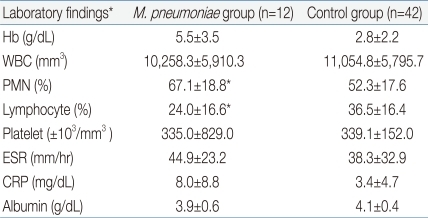
*Data for each group are expressed as mean±standard deviation.
The significance of clinical parameters according to groups was analyzed using the Mann-Whitney test.
Abbrevations: CBC, complete blood cell count; ESR, erythrocyte sendimentation rate; CRP, C-reactive protein; PMN, polymorphonuclear cell.
*P <0.05, significantly different from control group

 About
About Browse articles
Browse articles For contributors
For contributors