All issues > Volume 54(5); 2011
A sclerosing stromal tumor of the ovary with masculinization in a premenarchal girl
- Corresponding author: Chan Jong Kim, M.D. Department of Pediatrics, Chonnam National University Medical School, 5 Hak-dong, Dong-gu, Gwangju 501-746, Korea. Tel: +82-62-220-6645, Fax: +82-62-222-6103, cjkim@jnu.ac.kr
- Received September 02, 2010 Revised November 12, 2010 Accepted December 17, 2010
- Abstract
-
A sclerosing stromal tumor of the ovary is an extremely rare benign tumor; it usually is found during the second and third decades of life. Patients present with pelvic pain or a palpable abdominal mass. Hormonal effects such as masculinization are uncommon. Here, an 11-year old premenarchal girl presented with deepening of the voice. In addition, clitoromegaly and hirsutism with a male suprapubic hair pattern were observed. The laboratory findings showed that the testosterone level was elevated to 3.67 ng/mL, andostenedione to above 10 ng/mL, dehydroepiandrosterone-sulfate to 346 µg/dL and 17-hydroxy progesterone (17-OHP) to 11.28 ng/mL. The chromosome evaluation revealed a 46,XX female karyotype. An adrenocorticotropic hormone stimulation test was performed. The 17-OHP to cortisol ratio in 30 minutes was 0.045, which suggested a heterozygote for the 21-hydroxylase deficiency. However, the CYP21A2 gene encoding steroid 21-hydroxylase showed normal. The pelvic ultrasound showed a heterogeneous mass consisting of predominantly solid tissue in the pelvic cavity. The pelvic magnetic resonance imaging revealed an 8.9×6.2×6.6 cm mass of the left ovary. A left oophrectomy was performed and microscopic examination confirmed a sclerosing stromal tumor. Immunohistochemical studies showed that the tumor was positive for smooth muscle actin and vimentin, but negative for S-100 protein and cytokeratin. Following surgery, the hormone levels returned to the normal range and the hirsutism resolved.
- Introduction
- Introduction
A sclerosing stromal tumor (SST) of the ovary is a rare benign subtype of a sex cord stromal tumor. Since its first description in 1973 by Chalvardjian and Scully1), fewer than 100 cases have been reported2). The tumor occurs predominantly during the second and third decades of life with a mean age of 28 years3). Most of the reported cases have described women 14 years of age or older that have undergone menarche, except for three cases involving premenarchal girls2-4). The common presenting clinical symptoms are pelvic pain, hypermenorrhea, and menstrual irregularities. Although these tumors were initially believed to be non-functional1,5), there have been more recent reports suggesting the presence of hormone production both from a biochemical standpoint and the associated manifestations of infertility and irregular menses6). Hormonal effects such as masculinization are uncommon. Two cases of SSTs with virilization have been reported, both were pregnant women7,8). Here, an atypical case of an SST of the ovary with virilization is reported in a premenarchal girl.
- Case report
- Case report
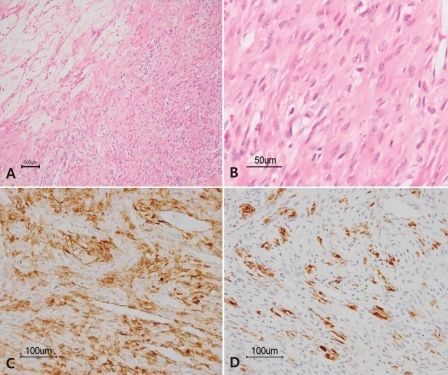
An 11-year-old premenarchal girl presented to the hospital with deepening of the voice and hirsutism, which was reported to have started about one year ago. Physical examination showed a male suprapubic hair pattern and 1 cm sized enlarged clitoris. The Tanner stage was II for breast and V for pubic hair development. The bone age was 13 years of age, advanced compared to the chronological age. The laboratory findings showed that the testosterone level was elevated to 3.67 ng/mL (normal range, 0.2 to 0.38 ng/mL), andostenedione to above 10 ng/mL (maximum level 1.7 ng/mL), dehydroepiandrosterone-sulfate (DHEA-S) to 346 µg/dL (normal range, 34 to 129 µg/dL) and 17-hydroxy progesterone (17-OHP) to 11.28 ng/mL (normal range, 0.2 to 2.65 ng/mL). The chromosome evaluation revealed a 46,XX female karyotype. The impression was non-classical congenital adrenal hyperplasia. An adrenocorticotropic hormone stimulation test (15 µg/kg bolus i.v.) was performed. The 17-OHP to cortisol ratio in 30 minutes was 0.045, which suggested a heterozygote for the 21-hydroxylase deficiency. However, the CYP21A2 genetic studies were normal.The pelvic ultrasound showed an 8.5×5.9 cm mass in the left pelvic cavity. For further evaluation of the mass and adnexa, magnetic resonance imaging (MRI) was carried out and revealed an 8.9×6.2×6.6 cm well-defined mass of the left ovary (Fig. 1). The patient was diagnosed as having an ovarian tumor with suspicion of a sex-cord stromal tumor. A left oophorectomy was performed. On gross inspection, the removed left ovarian mass measured 9×7.5×6 cm and weighed 182 g. The mass was yellow to pink in color and had a smooth and well-encapsulated surface. On cut sections, the mass was solid with a rubbery consistency. Neither definite necrosis nor hemorrhage was present. Microscopic examination showed cellular portions with fibroblasts, rounded vacuolated cells and prominent thin walled vessels, as well as edematous and collagenous hypocellular areas (Fig. 2A, B). Immunohistochemical staining was positive for vimentin, smooth muscle actin, and α-inhibin, but negative for S-100 protein and cytokeratin (Fig. 2C, D). Following surgery, the hormone levels recovered to the normal range, testosterone to 0.12 ng/mL, andostenedione to 1.3 ng/mL, DHEA-S to 85.7 µg/dL and 17-OHP to 0.54 ng/mL. Three months after the surgery, the patient had menarche. The development of the breasts was normal and the hirsutism with a male suprapubic hair pattern decreased on the six month follow-up.
- Discussion
- Discussion
Children and adolescents account for only 1% of all tumors in girls younger than 17 years, and sex cord stromal tumors constitute 10% of pediatric ovarian neoplasms3,9). SSTs of the ovary are extremely rare benign neoplasms that occur early in life (80% occurring in second and third decades), in contrast to other stromal tumors that commonly occur during the fifth and sixth decades1,2).Most SSTs are hormonally inactive. Chalvardjian and Scully1) originally considered ovarian SSTs to be nonfunctioning tumors. However, some investigators have reported endocrine changes associated with secretion of estrogen and progesterone10) as wells as induction of precocious puberty11). Other symptoms include: pelvic pain, a palpable pelvic mass, metrorrhagia and menstrual irregularities including postmenopausal bleeding in elderly patients. Hormonal effects such as masculinization are uncommon. Two cases of SST with virilization have been reported, and they were both pregnant women7,8). They were virilized at 10 to 14-16 weeks of gestation; the testosterone, DHEA-S, and androstenedione levels were elevated7,8).The case reported here is a unique case of an SST presenting with masculinization in an 11 year-old premenarchal girl. The patient had Tanner II breast and Tanner V male suprapubic hair development. In addition, there were elevated hormonal levels of testosterone, andostenedione, and DHEA-S. After resection of the tumor, the hormone levels returned to the normal range and the hirsutism resolved. Three months after surgery, the patient had spontaneous menarche. Ultrasonography is a useful initial tool for differentiating between cystic and solid masses and determining the organ of origin. However, computed tomography and MRI are both more sensitive for delineating the nature of the mass and tumor extension. On MRI, a diagnosis of SST can be strongly suggested, when typical signal patterns such as hypointense nodules, hyperintense stroma, lobulation, strong enhancement with gadolinium and a peripheral hypointense rim are present12).The differential diagnosis of SSTs of the ovary includes other sex cord stromal tumors such as fibromas and thecomas. These may be differentiated from the SST on the basis of histopathology and immunohistochemical findings2). The histopathology of the SST shows a pseudolobular pattern of cellular areas and hypocellular, edematous or collagenous areas; it has a prominent vasculature; and prominent sclerosis around clusters of individual cells; as well as cellular heterogeneity of the vacuolated luteinized theca-like cells and spindle-shaped fibroblast-like cells in the cellular areas1). Pseudolobulation and prominent vasculature are extremely rare in luteinized thecomas and fibromas1). Immunohistochemically, the cells of SSTs are positive for vimentin, smooth muscle actin, α-inhibin, and CD99; and are negative for S-100 protein and epithelial markers2). The excised tumor of the patient reported here showed cellular portions with fibroblasts, rounded vacuolated cells, prominent vessels, edematous and collageneous hypocellular areas. Immunohistochemical studies were positive for vimentin, smooth muscle actin, and α-inhibin, but negative for S-100 protein and cytokeratin.The SST presented here was atypical as it showed virilization in a premenarchal girl. Additional cases, especially those studied hormonally, may help detailed understanding for clinical manifestations of SST of the ovary.
- References
- 1. Chalvardjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer 1973;31:664–670.
[Article] [PubMed]2. Chang W, Oiseth SJ, Orentlicher R, Agarwal G, Yahr LJ, Cayten CG. Bilateral sclerosing stromal tumor of the ovaries in a premenarchal girl. Gynecol Oncol 2006;101:342–345.
[Article] [PubMed]3. Fefferman NR, Pinkney LP, Rivera R, Popiolek D, Hummel-Levine P, Cosme J. Sclerosing stromal tumor of the ovary in a premenarchal female. Pediatr Radiol 2003;33:56–58.
[Article] [PubMed]4. Chang YW, Hong SS, Jeen YM, Kim MK, Suh ES. Bilateral sclerosing stromal tumor of the ovary in a premenarchal girl. Pediatr Radiol 2009;39:731–734.
[Article] [PubMed]5. Marelli G, Carinelli S, Mariani A, Frigerio L, Ferrari A. Sclerosing stromal tumor of the ovary. Report of eight cases and review of the literature. Eur J Obstet Gynecol Reprod Biol 1998;76:85–89.
[Article] [PubMed]6. Suit PF, Hart WR. Sclerosing stromal tumor of the ovary. An ultra-structural study and review of the literature to evaluate hormonal function. Cleve Clin J Med 1988;55:189–194.
[Article] [PubMed]7. Cashell AW, Cohen ML. Masculinizing sclerosing stromal tumor of the ovary during pregnancy. Gynecol Oncol 1991;43:281–285.
[Article] [PubMed]8. Ismail SM, Walker SM. Bilateral virilizing sclerosing stromal tumours of the ovary in a pregnant woman with Gorlin's syndrome: implications for pathogenesis of ovarian stromal neoplasms. Histopathology 1990;17:159–163.
[Article] [PubMed]9. Torricelli P, Caruso Lombardi A, Boselli F, Rossi G. Sclerosing stromal tumor of the ovary: US, CT, and MRI findings. Abdom Imaging 2002;27:588–591.
[Article] [PubMed]10. Quinn MA, Oster AO, Fortune D, Hudson B. Sclerosing stromal tumour of the ovary case report with endocrine studies. Br J Obstet Gynaecol 1981;88:555–558.
[Article] [PubMed]11. Murthy DP, SenGupta SK, Mola G, Rageau O, Mathias A. Sclerosing stromal tumour of the ovary. P N G Med J 1996;39:48–55.
[PubMed]
Fig. 1
Magnetic resonance imaging of a large ovarian tumor (arrows). A) Axial T2-weighted image shows about a 9×6 cm well-circumscribed hypointense mass in the pelvic cavity, arised from left ovary. B) Axial Gd-enhanced T1-weighted image demonstrates strong contrast enhancement of the tumor.

Fig. 2
Histologic findings of sclerosing stromal tumor. A) The tumor shows an alternative pseudolobular pattern constiting of cellular and hypocellular areas (H&E, ×100). B) The cellular area is composed of vacuolated cells and spindled fibroblast-like cells (H&E, ×400). Immunohistochemically, the tumor cells are positive for smooth muscle actin (C, ×200) and α-inhibin (D, ×200).

 About
About Browse articles
Browse articles For contributors
For contributors
