All issues > Volume 55(5); 2012
Food allergy
- Corresponding author: Youngshin Han, PhD. Department of Pediatrics, Environmental Health Center for Atopic Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-3619, Fax: +82-2-3410-0805, snuhan@skku.edu
- Received March 16, 2012 Accepted March 27, 2012
- Abstract
-
Food allergy is an important public health problem affecting 5% of infants and children in Korea. Food allergy is defined as an immune response triggered by food proteins. Food allergy is highly associated with atopic dermatitis and is one of the most common triggers of potentially fatal anaphylaxis in the community. Sensitization to food allergens can occur in the gastrointestinal tract (class 1 food allergy) or as a consequence of cross reactivity to structurally homologous inhalant allergens (class 2 food allergy). Allergenicity of food is largely determined by structural aspects, including cross-reactivity and reduced or enhanced allergenicity with cooking that convey allergenic characteristics to food. Management of food allergy currently focuses on dietary avoidance of the offending foods, prompt recognition and treatment of allergic reactions, and nutritional support. This review includes definitions and examines the prevalence and management of food allergies and the characteristics of food allergens.
- Introduction
- Introduction
Food allergy (FA) is an important public health problem that affects children and adults worldwide. However, awareness of FA among health care professionals in Korea has been lacking. FA carries the risk of severe allergic reactions and even death, but there are no curative treatments and no effective prevention methods for FA yet. FA can only be managed by avoidance of the allergen or by treatment of symptoms as they arise. The National Institute of Allergy and Infectious Diseases (NIAID) in the United States (US) has recently published FA guidelines, including a consensus definition for FA, a discussion of comorbid conditions often associated with FA, and information on the epidemiology, natural history, diagnosis, and management of FA, including recommendations for treatment of severe symptoms and anaphylaxis1). The European Academy of Allergy and Clinical Immunology has created a task force to develop guidelines for the diagnosis and management of FA, and Japan has published a Japanese guideline for FA2).Food-induced anaphylaxis and accidental exposure to food allergens in day care centers are primary reasons for hospital emergency department visits by children in Korea3,4). In addition, nutritional problems caused by dietary avoidance of allergens have become an important public health issue5). Therefore, it is necessary to develop guideline for FA in Korea.
- Definitions
- Definitions
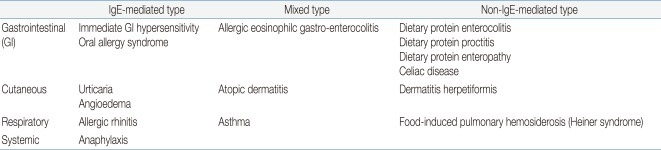
The following definitions comply with the guidelines of the NIAID in the US1).FA is an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food.Food intolerance is a non-immunologic adverse reaction to toxic contaminants or pharmacologic properties of the food; it may be due to characteristics of the host, including metabolic disorders (i.e., lactase deficiency) or idiosyncratic responses.Food is defined as any substance-whether processed, semi-processed, or raw-that is intended for human consumption, and includes drinks, chewing gum, food additives, and dietary supplements.Food allergens are defined as those specific components of food or ingredients within food (typically proteins, but sometimes also chemical haptens) that are recognized by allergen-specific immune cells and elicit specific immunologic reactions, resulting in characteristic symptoms.Cross-reactivity is the reaction between an antibody and an antigen that differs from the immunogen. Cross-reactivity may occur when an antibody reacts not only with the original allergen, but also with a similar allergen. In FA, cross-reactivity occurs when a food allergen shares structural or sequence similarity with a different food allergen or an aeroallergen, which may then trigger an adverse reaction similar to that triggered by the original food allergen.Allergic sensitization is the presence of allergen-specific immunoglobulin E (sIgE) to allergen. Because individuals can develop allergic sensitization to food allergens without having clinical symptoms on exposure to those foods, a sIgE mediated FA requires both the presence of sensitization and the development of specific signs and symptoms on exposure to the food. Sensitization alone is not sufficient to define FA.Food allergic disorders can be broadly divided into those that are mediated by IgE antibodies and those that are not (Table 1). Disorders with acute onset of symptoms after food ingestion are usually mediated by IgE. FA may coexist with asthma, atopic dermatitis, eosinophilic esophagitis, and exercise-induced anaphylaxis (Table 2). Atopic dermatitis is highly associated with FA. Children with moderate to severe persistent atopic dermatitis have a higher prevalence of IgE-mediated FA, estimated at about 35%6,7).
- Prevalence and natural history
- Prevalence and natural history
In Korea, the prevalence of FA is 5.3% in infants, and the mean lifetime prevalence of FA is 4.7% for children aged 6 to 12 and 5.1% for those aged 12 to 158,9). These rates are similar to those of westernized countries10,11). The reported prevalence of FA differs from the true prevalence. The true prevalence of FA is difficult to establish for several reasons. One of the reasons is that studies of FA incidence and prevalence are difficult to compare due to inconsistencies in study design and variations in the definition of FA. Furthermore, there are more people who believe they have FA that those who actually do, so that prevalence of self-reported FA is overestimated. A recently published meta-analysis regarding prevalence of FA included data from 51 publications and provided separate analyses of the prevalence of FA for 5 foods: milk, egg, peanut, fish, and crustacean shellfish12). The investigators reported an overall prevalence of self-reported FA of 12% for children and 13% for adults, while overall prevalence of self-reported symptoms plus sensitization by double blind placebo-controlled food challenge for any of these 5 foods was 3% for adults and children.Over 90% of FA results from exposure to egg, milk, peanut, tree nut, fish, shellfish, soy, and wheat13). The prevalence of FA is influenced by age, culture, and dietary habits. Age is one of the most important factors determining the type of FA. The most common offending foods are egg, milk, soy, and peanut in children, and wheat, and shellfish, tree nuts, and peanuts in adults. The order of importance of specific allergens varies by country, reflecting the interaction of culture and dietary habits. Peanut, buckwheat, mustard, and sesame are good examples of this. Despite similar levels of peanut consumption, there is a difference in the prevalence of peanut allergy between the US and China, and it is generally agreed that this discrepancy stems from the effects of different cooking methods on the allergenicity of peanuts; i.e., roasting using higher temperatures apparently increases the allergenic properties of peanut proteins14). Buckwheat is an important food causing anaphylaxis in Korea and Japan, but this is very rare in other countries15). Sesame seed allergy is more commonly observed in Israel than elsewhere, and mustard allergy is mainly observed in France16,17). The prevalence of shellfish allergy is 0.5% in Canada, but 4% in Singapore and the Philippines18).Patients with FA developed during infancy tend to become tolerant and able to eat causative foods with ageing, so children with FA should have periodic evaluation for remission. Remission rates vary according the offending foods, although the reason for this is still unknown. High remission rates are observed for eggs and cow's milk, while low remission rates are reported for peanut and tree nut. In a retrospective study from Korea including 106 children with atopic dermatitis and egg allergy diagnosed before 2 years of age, 60% became tolerant to egg by age 5, and the age at the diagnosis of the egg allergy was the significant prognostic factor for tolerance19). A study from Spain reported that 66% of children with early FA became tolerant by age 520). In a retrospective study from the US, the rate of egg allergy remission was 26% by age 6, and risk factors for persistence of egg allergy included a high initial level of egg sIgE, the presence of other atopic disease, and the presence of an allergy to other foods21). A study at a US university referral hospital reported that 80% of all infants who developed milk allergy in the first year of life became tolerant by their fifth birthday13), while a more recent US study has reported a much lower rate (5% at age 4 and 21% at age 8) of FA remission22). Bock and Atkins23) reported that most patients with peanut allergy did not develop tolerance. Until recently, peanut allergy was considered life-long, but a small percentage of children tolerated peanut several years after their initial diagnosis24,25).
- Food allergens
- Food allergens
- 1. General characteristics
- 1. General characteristics
Traditional or class 1 food allergens, such as egg, are heat-, enzyme-, and low pH-resistant water-soluble glycoproteins ranging in size from 10 to 70 kD. Class 1 food allergens induce allergic sensitization via the gastrointestinal tract and are responsible for systemic reactions (traditional or class 1 FA)13). Class 2 food allergens, such as apple and celery, are heat-labile, susceptible to digestion, and highly homologous with proteins in pollens. Class 2 FA (oral allergy syndrome, OAS) is typically the result of sensitization to labile proteins, such as pollens, encountered through the respiratory route. IgE antibodies to pollens recognize homologous epitopes on food proteins of plant origin26).- 2. Cross-reactivity
- 2. Cross-reactivity
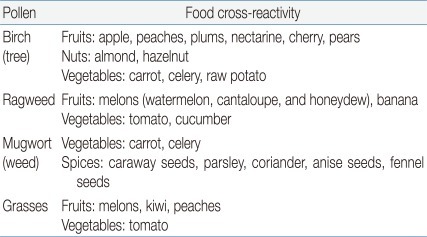
Cross-reactivity is largely determined by structure: 2 proteins are cross-reactive if they share structural features. Phylogenetically related mammals express similar milk proteins with significant amino acid sequence homology, resulting in clinical cross-reactivity among cow, sheep, and goat milk27,28). Clinical cross-reactivity between peanut and other legumes is extremely rare, despite the high degree of cross-sensitization based on IgE binding and results of skin prick tests29). OAS is a form of contact allergy to raw fruits and vegetables (Table 3). OAS affects approximately 50% of pollen allergic adults and represents the most common adult FA. It results from the cross-reactivity between the allergenic proteins in pollens and plant foods26,30).- 3. Cooking
- 3. Cooking
Thermal processes can reduce or increase the allergenicity of certain food proteins. Thermal denaturation of globular proteins disrupts the tertiary structure, leading to random-coiled aggregation and insolubility. In one study, heating for 15 minutes at 95℃ reduced IgE binding with ovalbumin and ovomucoid, and egg white proteins boiled for 5 or 60 minutes showed greatly decreased allergenicity, especially after prolonged boiling for 60 minutes31,32). Lemon-Mule et al.33) reported that 64 of 117 subjects tolerated egg baked in muffins (176℃ for 30 minutes) and waffles (260℃ for 30 minutes), but only 23 tolerated regular scrambled egg or French toast. However, other studies have shown that the IgE binding capacity of soy 2S-globulin is strengthened by heating, and, as noted above, roasting using higher temperatures apparently increases the allergenic properties of peanut proteins14). Chemical processing can also change allergenicity. The allergenicity of the major peanut allergens Ara h 1, Ara h 2, and Ara h 3 was decreased by vinegar treatment, suggesting that the extent of allergenicity varies with pH34).
- Management
- Management
Currently, the only way to manage FA is avoidance of the allergen and prompt treatment of symptoms when they arise. Accidental exposure to food allergens is inevitable and patient and family education regarding cross-contamination, label reading, and prompt recognition and treatment of food allergic reactions is a cornerstone of FA management. Because avoidance of specific allergens can limit the availability of nutritious food choices, nutritional counseling and regular growth monitoring are recommended for all children with FA, especially during infancy.Elimination of causative foods should be minimized to prevent nutritional disorders and improve the quality of dietary life. Even if a food is positive for sIgE antibodies and skin prick test, it should not be eliminated if an oral challenge test is negative. Tolerance to foods, especially egg and milk, should be checked periodically, because children tend to outgrow the allergies. Strict avoidance of offending allergen-containing food products has been the standard of care for children with FA. However, as noted, Lemon-Mule et al.33) have recently demonstrated that ingestion of extensively heated egg products was well-tolerated and safe for some patients with egg allergy, and they have suggested that strict dietary avoidance of heated egg might not be necessary for all patients with egg allergy.Because even trace amounts of food allergens may trigger severe reactions in highly sensitive food-allergic individuals, strict avoidance is very important in these individuals. It is essential to educate patients and families regarding cross-contamination and label reading, because many patients and their caregivers do not recognize the risk of exposure to trace amounts of food allergen. In Korea, food labeling regulations announced by the Korean Food & Drug Administration (effective June 17, 2006) require food manufacturers to indicate if a product contains any of 12 food allergens: milk, eggs, peanuts, soy, wheat, buckwheat, shrimp, crab, mackerel, pork, tomato, and peach. However, this regulation is not sufficient to prevent FA accident because it excludes tree nut and fish, which are also important allergens that can cause anaphylaxis. Therefore, food labeling regulations need to be revised. Another danger faced by food-allergic patients is occult contamination with trace amounts of food from shared of equipment. All equipment and utensils used to prepare allergenic food should be cleaned with hot soapy water before being used to prepare allergen-free food.The US NIAID guidelines recommend nutritional counseling and regular growth monitoring for all children with FA. Children with FA may be at risk for inadequate nutritional intake or poor growth without apparent nutritional problems35-37). Flammarion et al.37) assessed food intake and nutritional status of children (mean age, 4.7±2.5 years) with FA following an elimination diet and reported that children with food allergies were smaller for their age than controls, even when they received similar nutrients. Young children who are sensitive to multiple major food allergens are at risk for protein and calorie deficiency and may require a hypoallergenic formula to meet their needs. Hypoallergenic formulas available in Korea are based on extensively hydrolyzed casein derived from cow's milk (i.e., Babywell HA, Pregestimil, Neutramigen, Alimentum) or on a mixture of single amino acids (Neocate). Soy protein formula is not an appropriate substitute for patients with cow milk allergy because soy allergy is highly prevalent in patients with cow milk allergy38,39). Sheep and goat milk-based formulas are also discouraged because of cross-reactivity with cow milk28).Children may be at risk for accidental exposure to food allergens and FA reactions, including anaphylaxis, in schools and daycares, where they spend many of their days. In Korea, 99.6% of all schools provide school lunches, and 8 million students, representing approximately 20% of the Korean population, are offered school lunches during school days40). Presently, most students with FA must rely solely on self-care, because no emergent measures for FA provided by the schools are in place. Other developed countries, including the US and Japan, have introduced school-wide or government-supported plans for management FA in schools41,42). Government-supported management and treatment action plans for FA at schools should also be established in Korea.For families of patients in the US with FA who need practical information and a comprehensive curriculum regarding FA management, www.foodallergy.org provides advice on dietary avoidance, along with survival strategies for schools, restaurants, and camps, manufacturers' updates, and special support programs for teenagers and FA experts43). In Korea, www.foodallergy.or.kr supplies food allergic families and supervisors with similar information.In summary, FA is an emerging worldwide health problem requiring increased attention from primary care clinicians in Korea. Although FA has a relatively low mortality and an almost continual absence of physical symptoms, patients with FA face the possibility of potentially severe, and even fatal, reactions and must maintain dietary vigilance. It is essential to offer information and support to children with FA and their families. General practitioners should tailor this information to the specific needs of each FA patient and parents and pediatricians should be aware of psychosocial problems in children with allergic diseases.
- References
- 1. NIAID-Sponsored Expert Panel. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126(6 Suppl): S1–S58.
[Article] [PubMed] [PMC]2. Urisu A, Ebisawa M, Mukoyama T, Morikawa A, Kondo N. Japanese Society of Allergology. Japanese guideline for food allergy. Allergol Int 2011;60:221–236.
[Article] [PubMed]3. Lim DH. Epidemiology of anaphylaxis in Korean children. Korean J Pediatr 2008;51:351–354.
[Article]4. Seo WH, Jang EY, Han YS, Ahn KM, Jung JT. Management of food allergies in young children at a child care center and hospital in Korean. Pediatr Allergy Respir Dis 2011;21:32–38.
[Article]5. Chung SJ, Han YS, Chung SW, Ahn KM, Park HY, Lee SI, et al. Marasmus and Kwashiorkor by nutritional ignorance related to vegetarian diet and infants with atopic dermatitis in South Korea. Korean J Nutr 2004;37:540–549.6. Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998;101:E8
[Article]7. Burks AW, Mallory SB, Williams LW, Shirrell MA. Atopic dermatitis: clinical relevance of food hypersensitivity reactions. J Pediatr 1988;113:447–451.
[Article] [PubMed]8. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci 2004;19:716–723.
[Article] [PubMed] [PMC]9. Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatr Allergy Immunol 2011;22:715–719.
[Article] [PubMed]10. Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med 2009;60:261–277.
[Article] [PubMed]12. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol 2007;120:638–646.
[Article] [PubMed]13. Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol 1999;103(5 Pt 1): 717–728.
[Article] [PubMed]14. Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol 2001;107:1077–1081.
[Article] [PubMed]16. Dalal I, Binson I, Reifen R, Amitai Z, Shohat T, Rahmani S, et al. Food allergy is a matter of geography after all: sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy 2002;57:362–365.
[Article] [PubMed]18. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol 2010;126:324–331. 331.e1–331.e7.
[Article] [PubMed]19. Kim J, Chung Y, Han Y, Ahn K, Lee SI. The natural history and prognostic factors of egg allergy in Korean infants with atopic dermatitis. Asian Pac J Allergy Immunol 2009;27:107–114.
[PubMed]20. Boyano-Martinez T, Garcia-Ara C, Diaz-Pena JM, Martin-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol 2002;110:304–309.
[Article] [PubMed]21. Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol 2007;120:1413–1417.
[Article] [PubMed]22. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol 2007;120:1172–1177.
[Article] [PubMed]23. Bock SA, Atkins FM. The natural history of peanut allergy. J Allergy Clin Immunol 1989;83:900–904.
[Article] [PubMed]24. Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol 2001;107:367–374.
[Article] [PubMed]25. Spergel JM, Beausoleil JL, Pawlowski NA. Resolution of childhood peanut allergy. Ann Allergy Asthma Immunol 2000;85(6 Pt 1): 473–476.
[Article] [PubMed]26. Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy 2006;61:461–476.
[Article] [PubMed]27. D'Auria E, Agostoni C, Giovannini M, Riva E, Zetterstrom R, Fortin R, et al. Proteomic evaluation of milk from different mammalian species as a substitute for breast milk. Acta Paediatr 2005;94:1708–1713.
[Article] [PubMed]28. Jarvinen KM, Chatchatee P. Mammalian milk allergy: clinical suspicion, cross-reactivities and diagnosis. Curr Opin Allergy Clin Immunol 2009;9:251–258.
[Article] [PubMed]29. Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol 2001;108:881–890.
[Article] [PubMed]30. Valenta R, Kraft D. Type 1 allergic reactions to plant-derived food: a consequence of primary sensitization to pollen allergens. J Allergy Clin Immunol 1996;97:893–895.
[Article] [PubMed]31. Mine Y, Zhang JW. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J Agric Food Chem 2002;50:2679–2683.
[Article] [PubMed]32. Peng HJ, Chang ZN, Tsai LC, Su SN, Shen HD, Chang CH. Heat denaturation of egg-white proteins abrogates the induction of oral tolerance of specific Th2 immune responses in mice. Scand J Immunol 1998;48:491–496.
[Article] [PubMed]33. Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol 2008;122:977–983.e1.
[Article] [PubMed]34. Kim J, Lee J, Seo WH, Han Y, Ahn K, Lee SI. Changes in major peanut allergens under different pH conditions. Allergy Asthma Immunol Res 2012;4:157–160.
[Article] [PubMed]35. Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc 2002;102:1648–1651.
[Article] [PubMed]36. Tiainen JM, Nuutinen OM, Kalavainen MP. Diet and nutritional status in children with cow's milk allergy. Eur J Clin Nutr 1995;49:605–612.
[PubMed]37. Flammarion S, Santos C, Guimber D, Jouannic L, Thumerelle C, Gottrand F, et al. Diet and nutritional status of children with food allergies. Pediatr Allergy Immunol 2011;22:161–165.
[Article] [PubMed]38. Muraro MA, Giampietro PG, Galli E. Soy formulas and nonbovine milk. Ann Allergy Asthma Immunol 2002;89(6 Suppl 1): 97–101.
[Article] [PubMed]39. Ahn KM, Han YS, Nam SY, Park HY, Shin MY, Lee SI. Prevalence of soy protein hypersensitivity in cow's milk protein-sensitive children in Korea. J Korean Med Sci 2003;18:473–477.
[Article] [PubMed] [PMC]40. The Ministry of Education, Science and Technology. The 2010 status of school foodservice. 2011;Seoul: The Ministry of Education, Science and Technology.41. Accommodating children with special dietary needs in the school meal programs-guidance for school food service staff. United States Department of Agriculture, Food and Nutrition Service [Internet]. 2001;cited Feb 29 2012. Washington: United States Department of Agriculture, Available from: http://www.fns.usda.gov/cnd/guidance/special_dietary_ needs.pdf.42. Takanori I. National survey of food allergy symptoms by the school lunch. J Jpn Pediatr Soc 2006;110:1545–1549.
Table 2
Conditions that Coexist with Food Allergy (FA)

This table is made based on NIAID guideline summary1).
AD, atopic dermatitis; IgE, immunoglobulin E.

 About
About Browse articles
Browse articles For contributors
For contributors