All issues > Volume 55(5); 2012
Successful pleurodesis with OK-432 in preterm infants with persistent pleural effusion
- Corresponding author: Ran Namgung, MD. Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-2050, Fax: +82-2-393-9118, ranng@yuhs.ac
- Received September 06, 2011 Revised October 28, 2011 Accepted November 28, 2011
- Abstract
-
OK-432 (picibanil) is an inactivated preparation of Streptococcus pyogenes that causes pleurodesis by inducing a strong inflammatory response. Intrapleural instillation of OK-432 has recently been used to successfully treat neonatal and fetal chylothorax. Here we report a trial of intrapleural instillation of OK-432 in two preterm infants who were born with hydrops fetalis and massive bilateral pleural effusion. Both cases showed persistent pleural effusion, refractory to conservative treatment, up to postnatal days 26 and 46, respectively. An average of 80 to 140 mL of pleural fluid was drained daily. In case 1, the infant was treated with OK-432 during the fetal period at gestation 28 weeks and 4 days of gestation, but showed recurrence of pleural effusion and progressed into hydrops. Within two to three days after OK-432 injection, the amount of pleural fluid drainage was dramatically decreased and there was no reaccumulation. We did not observe any side effects related to OK-432 injection. We suggest that OK-432 should be considered as a therapeutic option in infants who have persistent pleural effusion for more than four weeks, with the expectation of the early removal of the chest tube and a good outcome.
- Introduction
- Introduction
Congenital chylothorax, although rare, is the most common form of pleural effusion in neonates. Its etiology is not fully understood, but may be related to maldevelopment of the lymphatic system. Overall mortality rates for chylothorax range from 18 to 44%, depending on associated conditions, gestational age and the duration and severity1-3).With conservative treatment, complete resolution of pleural fluid can be observed within 30 days for 80% of congenital chylothorax patients. Poorer prognosis is associated with hydrops fetalis, large pleural effusions leading to pulmonary hypoplasia, and preterm birth (<34 weeks). However, the point at which conservative treatment is considered to have failed has not been well established4).Recent reports have suggested pleurodesis with OK-432 (picibanil, Chugai Pharmaceutical Co., Tokyo, Japan), inactivated preparation of Streptococcus pyogenes, as an alternative therapeutic option. It has been used to treat malignant pleural effusion in adults, and both chylothorax after chest surgery and cystic lymphatic malformations in the pediatric population5-7). In fetal chylothorax, OK-432 has recently been reported to have a favorable outcome8-11). For its use in neonates, Matsukuma et al.12) lately reported the successful use of OK-432 for the treatment of prolonged congenital chylothorax in 2 newborn infants who failed to respond to conservative medical therapy, including octreotide infusion. Here we report a first trial of intrapleural instillation of OK-432 in two preterm infants with persistent congenital chylothorax in Korea.
- Case reports
- Case reports
- 1. Case 1
- 1. Case 1
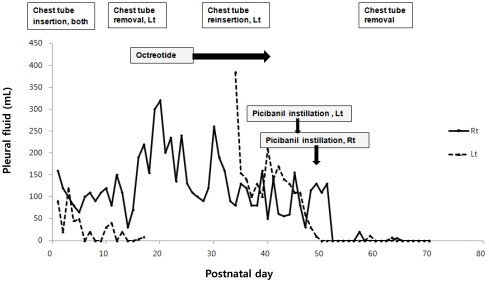
A male preterm infant (gestation 33 weeks, birth weight 2,720 g) was born by C-section with hydrops fetalis. A fetal sonogram showed generalized edema, bilateral pleural effusion (right, 2.3 cm; left, 1.3 cm) and pericardial effusion at 32 weeks gestation. No specific antenatal management was performed, except for one cycle of antenatal dexamethasone. APGAR scores were 3 at 1 minute and 4 at 5 minutes. The infant showed respiratory difficulty and required immediate mechanical ventilation. Congenital chylothorax was confirmed by analysis of pleural fluid: elevated triglyceride (202 mg/dL) after premature milk feeding, and predominant lymphocyte count (>90%). Other tests were all negative. The infant was initially treated with chest tube drainage and total parenteral nutrition combined with balanced replacement of fluid and albumin losses. Left pleural drainage had resolved after two week. However, amounts of right pleural fluid remained high, fluctuating between 30 to 320 mL/day until postnatal day 25. Octreotide infusion started on postnatal day 26, and lasted for two weeks. Initial dose was 0.5 µg/kg/hr and was increased up to a maximum dose of 10 µg/kg/hr. However, no significant decrease in right pleural drainage was noted and reaccumulation of left pleural effusion showed up. We decided to use intrapleural instillation of OK-432 after obtaining informed consent. 0.1 Klinische Einheit (KE) OK-432 (at a concentration of 1 KE in 10 mL normal saline) was injected into the left pleural cavity on postnatal day 46 and into the right pleural cavity on postnatal day 48. The amount of pleural fluid drainage dramatically decreased right after OK-432 injection, and did not reaccumulate, resulting in the chest tube finally being removed 14 days after the OK-432 instillation (Fig. 1). No immediate side effects were detected related to OK-432 instillation. Unfortunately, the infant was already suffering from significant oliguria, worsening edema and recurrent hypotension. He died from renal failure, shock, and candida peritonitis related to peritoneal dialysis on postnatal day 72, even though chylothorax had resolved completely after OK-432 pleurodesis.- 2. Case 2
- 2. Case 2
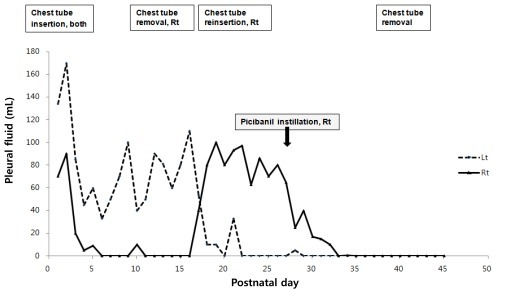
A female preterm infant (case 2, gestation 29 weeks and 6 days weeks, birth weight 1,790 g) was born by C-section with bilateral pleural effusion, diagnosed at 27 weeks of gestation. APGAR score was 2 at 1 minute, 6 at 5 minutes. The infant had been treated with thoracentesis and intrapleural instillation of OK-432 during the fetal period at 28 weeks and 4 days of gestation, but showed recurrence of pleural effusion after seven days of instillation of OK-432 and progressed into hydrops fetalis at 29 weeks and 6 days of gestation. Analysis of fetal pleural fluid demonstrated the presence of more than 99% lymphocytes suggesting congenital chylothorax, while culture of the fluid was sterile. Fetal karyotype and viral studies were all negative. Left pleural effusion was continued at an average rate of 80 mL/day during the first two week, and it was then decreased gradually. Right pleural drainage had resolved after three days, and the right chest tube was removed on postnatal day 14. However, the right pleural fluid reaccumulated three days after tube removal. The amount of right pleural fluid drained was 80 to 100 mL/day until postnatal day 25. We performed pleurodesis with OK-432 on the right pleural spaces on postnatal day 26 as a bedside procedure. The amount of pleural fluid drainage promptly decreased after OK-432 instillation and there was no reaccumulation (Fig. 2). We noticed no immediate side effects related to OK-432 instillation. Medium chain triglycerides (MCT)-rich formula feeding was successfully switched to regular formula before discharge. The infant was discharged in good condition with improvement at five months of age.
- Discussion
- Discussion
Congenital chylothorax is the accumulation of lymph fluid or chyle in the pleural space. The clinical course of chylothorax is highly variable and ranges from spontaneous resolution to progression into hydrops fetalis and fetal death. If congenital chylothorax is severe or develops in the second trimester, it may lead to pulmonary hypoplasia as a result of impaired lung growth by compression13). The prognosis mostly depends on the underlying etiology and associated conditions.Standard treatment algorithms are based on adequate drainage of the pleural fluid, in combination with parenteral nutrition or feeding with MCT-rich formula. With conservative treatment, complete resolution of pleural fluid can be observed within 30 days for 80% of congenital chylothorax patients14). However, there are no definite guidelines about which treatment is the most useful for persistent congenital chylothorax. Some authors recommend that surgical intervention may be necessary in cases with massive (50 mL/kg/day) or persistent (>4 weeks after onset of the chylothorax) pleural drainage4,15).Recent reports have suggested octreotide or pleurodesis as an alternative medical therapy for infants with persistent chylothorax. Octreotide, a somatostatin analogue, has been used effectively in postoperative cases of chylothorax in both adult and pediatric patients. There have been several previous reports of the use of octreotide as a second-line treatment of congenital chylothorax that did not resolve with conservative treatment16,17). Its usefulness and relative freedom from side effects have been documented. However, caution is advised for patients prone to necrotizing enterocolitis because of the detrimental effects of somatostatin on splanchnic blood flow, especially for premature babies. In our first case study, octreotide infusion was initially started up to a maximum dose of 10 µg/kg/hr for about two weeks, but no significant decrease in chest tube drainage was noted.Another treatment option is pleurodesis, which obliterates chylous leaks to obtain pleural adhesion. OK-432 (picibanil) is a lyophilized preparation of a low-virulence strain of group A S. pyogenes of human origin, inactivated by heating with penicillin G, and has been proposed as a sclerosing agent. It causes pleurodesis by inducing a strong cellular and cytokine-mediated inflammatory response. OK-432 has been widely used to treat postoperative chylothorax and cystic lymphangioma in children5-7). Recently, in fetal chylothorax, intrapleural administration of OK-432 has had favorable outcomes; 22 of 25 patients showed remission of pleural effusion after intrapleural injection since 20018-11). For its first use in neonates, Matsukuma et al.12) reported the successful use of OK-432 for the treatment of persistent congenital chylothorax which is resistant to octreotide infusion as second line therapeutic option.Long-lasting chyle drainage could put patients at increased risk for malnutrition, nosocomial infections, and fluid and electrolyte imbalances1,18). Ergaz et al.3) reported that two of 11 neonates died after resolution of chylothorax from deteriorating renal failure and a high rate of nosocomial infections that occurred in six out of 11 neonates with congenital chylothorax. Therefore, based on our first case experience, we decided to apply pleurodesis earlier for the second case. As a result, earlier removal of the chest tube was possible and the duration of hospitalization was reduced without any complications.Few complications related to OK-432 have been reported in adults, with the exception of signs of inflammatory reactions such as fever, pain, and local erythema. There have been no adverse effects of fetal therapy with OK-432, with the exception of one report of transient fetal tachycardia8,9). However, Cowie et al.19) recently described that intrapleural infusion of OK-432 was associated with transient suppression of fetal electroencephalographic activity, breathing and body movements in fetal sheep. In addition, Bennet et al.20) reported that a single injection of OK-432 can be associated with a risk of focal cerebral injury in the preterm fetus. Since the case numbers for treatment with OK-432 are small and limited, a prospective randomized study in newborns will be required to prove its effectiveness, and ascertain side effects and safety of use. Long term postnatal follow-up of neonates treated with OK-432 is also needed.In conclusion, we report that intrapleural injection of OK-432 was successful in two preterm infants with persistent chylothorax that was refractory to conservative treatment. If used appropriately, this option has the potential to reduce the morbidity related to prolonged pleural effusion and hospital stay, as well as early removal of the chest tube and a reduction in surgical intervention. In addition, this treatment involves simple and minimally invasive drug delivery with a chest tube in place, and can be carried out as a bedside procedure without the requirement of anesthesia.
- References
- 1. Al-Tawil K, Ahmed G, Al-Hathal M, Al-Jarallah Y, Campbell N. Congenital chylothorax. Am J Perinatol 2000;17:121–126.
[Article] [PubMed]2. Caserio S, Gallego C, Martin P, Moral MT, Pallas CR, Galindo A. Congenital chylothorax: from foetal life to adolescence. Acta Paediatr 2010;99:1571–1577.
[Article] [PubMed]3. Ergaz Z, Bar-Oz B, Yatsiv I, Arad I. Congenital chylothorax: clinical course and prognostic significance. Pediatr Pulmonol 2009;44:806–811.
[Article] [PubMed]4. Cleveland K, Zook D, Harvey K, Woods RK. Massive chylothorax in small babies. J Pediatr Surg 2009;44:546–550.
[Article] [PubMed]5. Katanyuwong P, Dearani J, Driscoll D. The role of pleurodesis in the management of chylous pleural effusion after surgery for congenital heart disease. Pediatr Cardiol 2009;30:1112–1116.
[Article] [PubMed]6. Okazaki T, Iwatani S, Yanai T, Kobayashi H, Kato Y, Marusasa T, et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg 2007;42:386–389.
[Article] [PubMed]7. Park JS, Kim JK, Kim CB. OK-432 (picibanil) chemical pleurodesis for the treatment of persistent chylothorax after esophagectomy. J Korean Surg Soc 2001;61:105–109.8. Nygaard U, Sundberg K, Nielsen HS, Hertel S, Jorgensen C. New treatment of early fetal chylothorax. Obstet Gynecol 2007;109:1088–1092.
[Article] [PubMed]9. Chen M, Shih JC, Wang BT, Chen CP, Yu CL. Fetal OK-432 pleurodesis: complete or incomplete? Ultrasound Obstet Gynecol 2005;26:791–793.
[Article]10. Yim GW, Kwon JY, Park YW, Kim YH. Successful antenatal treatment of early fetal chylothorax with OK-432 pleurodesis. Korean J Obstet Gynecol 2009;52:752–758.11. Kim GJ, Lee ES. Cesarean delivery with postnatal high-frequency ventilation immediately after thoracocentesis for a fetus with intractable congenital chylothorax. Korean J Obstet Gynecol 2011;54:99–102.
[Article]12. Matsukuma E, Aoki Y, Sakai M, Kawamoto N, Watanabe H, Iwagaki S, et al. Treatment with OK-432 for persistent congenital chylothorax in newborn infants resistant to octreotide. J Pediatr Surg 2009;44:e37–e39.
[Article]13. Rocha G. Pleural effusions in the neonate. Curr Opin Pulm Med 2007;13:305–311.
[Article] [PubMed]14. Beghetti M, La Scala G, Belli D, Bugmann P, Kalangos A, Le Coultre C. Etiology and management of pediatric chylothorax. J Pediatr 2000;136:653–658.
[Article] [PubMed]15. Buttiker V, Fanconi S, Burger R. Chylothorax in children: guidelines for diagnosis and management. Chest 1999;116:682–687.
[Article] [PubMed]16. Das A, Shah PS. Octreotide for the treatment of chylothorax in neonates. Cochrane Database Syst Rev 2010;(9): CD006388
[PubMed]17. Sahin Y, Aydin D. Congenital chylothorax treated with octreotide. Indian J Pediatr 2005;72:885–888.
[Article] [PubMed]18. Wasmuth-Pietzuch A, Hansmann M, Bartmann P, Heep A. Congenital chylothorax: lymphopenia and high risk of neonatal infections. Acta Paediatr 2004;93:220–224.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors