All issues > Volume 58(11); 2015
Severe vitamin D deficiency in preterm infants: maternal and neonatal clinical features
- Corresponding author: Heng-Mi Kim, MD, PhD. Department of Pediatrics, Kyungpook National University Hospital, Kyungpook National University School of Medicine, 130 Dongdeok-ro, Jung-gu, Daegu 41944, Korea. Tel: +82-53-200-5704, Fax: +82-53-425-6683, hmkim@knu.ac.kr
- Received August 21, 2015 Revised October 01, 2015 Accepted October 07, 2015
- Abstract
-
- Purpose
- Purpose
- We investigated the vitamin D status of preterm infants to determine the incidence of vitamin D deficiency.
- Methods
- Methods
- A total of 278 preterm infants delivered at Kyungpook National University Hospital between January 2013 and May 2015 were enrolled. The serum concentrations of calcium, phosphorous, alkaline phosphatase, and 25-hydroxyvitamin D (25-OHD) were measured at birth. We collected maternal and neonatal data such as maternal gestational diabetes, premature rupture of membranes, maternal preeclampsia, birth date, gestational age, and birth weight.
- Results
- Results
- Mean gestational age was 33+5±2+2 weeks of gestation and mean 25-OHD concentrations were 10.7±6.4 ng/mL. The incidence of vitamin D deficiency was 91.7%, and 51.1% of preterm infants were classified as having severe vitamin D deficiency (25-OHD<10 ng/mL). The serum 25-OHD concentrations did not correlate with gestational age. There were no significant differences in serum 25-OHD concentrations or incidence of severe vitamin D deficiency among early, moderate, and late preterm infants. The risk of severe vitamin D deficiency in twin preterm infants was significantly higher than that in singletons (odds ratio, 1.993; 95% confidence interval [CI], 1.137-3.494, P=0.016). In the fall, the incidence of severe vitamin D deficiency decreased 0.46 times compared to that in winter (95% CI, 0.227-0.901; P=0.024).
- Conclusion
- Conclusion
- Most of preterm infants (98.9%) had vitamin D insufficiency and half of them were severely vitamin D deficient. Younger gestational age did not increase the risk of vitamin D deficiency, but gestational number was associated with severe vitamin D deficiency.
- Introduction
- Introduction
The importance of vitamin D as a modulator of calcium and bone metabolism is well known, and it also is involved in innate immune system and neuromuscular functions1). During infancy, low concentrations of cord blood vitamin D have been associated with increased incidence of neonatal sepsis and respiratory tract infections in the first year of life2,3). Vitamin D deficiency in pregnant women increased the risk of preeclampsia, gestational diabetes, preterm labor, and fetal intrauterine growth restriction4,5).In recent studies, vitamin D concentrations of Korean women were the lowest among studied international groups6,7). In a Korean study by Choi et al.8), three-fourths of Korean pregnant women had a risk of vitamin D deficiency and one-fourth had severe vitamin D deficiency (25-hydroxyvitamin D [25-OHD]<10 ng/mL). The prevalence of vitamin D deficiency was significantly higher during the first and second trimesters than during the third trimester in pregnant women8,9). The major determining factor for vitamin D concentrations during the fetal and newborn periods is the maternal vitamin D concentration10,11). Low maternal vitamin D status predisposed to low neonatal vitamin D status at birth, but infants born to mother with sufficient vitamin D status had a risk for vitamin D insufficiency or deficiency10,12). These observations suggest that Korean newborns, particularly preterm infants, might have a significant risk of vitamin D deficiency.We investigated the vitamin D status of preterm infants to determine the incidence of insufficiency or deficiency.
- Materials and methods
- Materials and methods
- 1. Subjects
- 1. Subjects
A total of 278 preterm infants (<37 weeks of gestation) who were delivered at Kyungpook National University Hospital (latitude 35.87°N) between January 2013 and May 2015 were enrolled. Infants with major congenital anomaly or chromosomal abnormality were excluded. Blood samples obtained from infants more than 1 hour after birth also were excluded. The study was approved by the institutional review board of Kyungpook National University Hospital and was given a waiver of informed consent.- 2. Assessment of vitamin D status
- 2. Assessment of vitamin D status
The concentrations of serum calcium, phosphorous, alkaline phosphatase (ALP) and 25-OHD were measured from cord blood or blood samples obtained within 1 hour after birth. The serum concentrations of 25-OHD were measured using radioimmunoassay (DIAsource 25OH-Vit.D3-Ria-CT kit, DIAsource ImmunoAssays S.A., Louvain-la-Neuve, Belgium).In the present study vitamin D deficiency was defined as 25-OHD concentration<20 ng/mL; vitamin D insufficiency, 20 ng/mL<25-OHD concentration<30 ng/mL; and vitamin D sufficiency, 25-OHD concentration≥30 ng/mL. We categorized severe vitamin D deficiency as 25-OHD concentrations below 10 ng/mL.- 3. Maternal and neonatal demographic and clinical data
- 3. Maternal and neonatal demographic and clinical data
Maternal and neonatal demographic and clinical characteristics were collected through medical record reviews. Maternal clinical data included were maternal age, delivery mode, and pregnancy complications such as gestational diabetes, premature rupture of membranes (PROM), and preeclampsia. Neonatal clinical data included were sex, gestational age, birth weight, length, head circumference and gestational number.Seasons were divided as spring (March to May), summer (June to August), fall (September to November), and winter (December to February). We categorized groups by gestational age as early preterm (<32 weeks of gestation), moderate preterm (32-33 weeks of gestation) and late preterm (34-36 weeks of gestation).- 4. Statistical analysis
- 4. Statistical analysis
Statistical analysis was performed by IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Partial correlation models were used to analyze the correlation between gestational age and 25-OHD concentrations while controlling for other factors. Comparisons were made using one-way analysis of variance and chi-square test according to continuous or categorical variables. The analysis of potential confounding factors affecting 25-OHD concentrations was performed by logistic regression. Statistical significance was defined as P value <0.05.
- Results
- Results
- 1. Population characteristics and vitamin D status
- 1. Population characteristics and vitamin D status
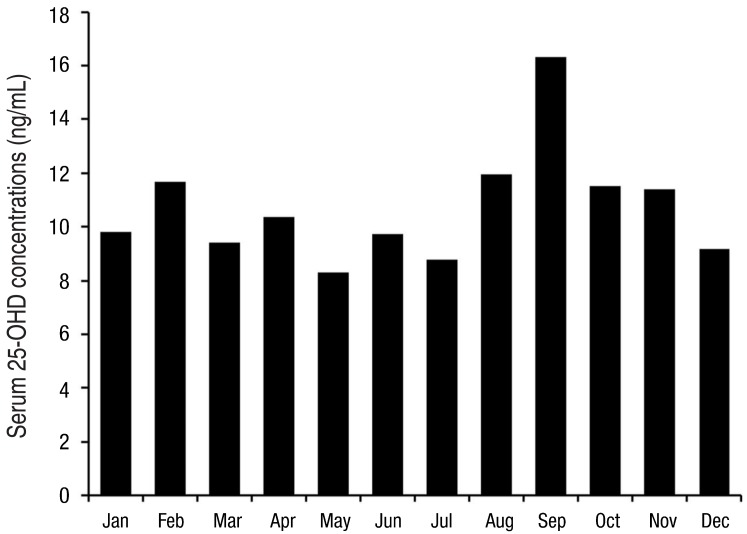
The clinical and demographic characteristics of 278 preterm infants are presented in Table 1. Mean gestational age was 33+5±2+2 weeks of gestation and mean birth weight was 2,044.4±493.9 g. Mean 25-OHD concentrations were 10.7±6.4 ng/mL (range, 1.9-41.4 ng/mL). The incidence of vitamin D deficiency or insufficiency was 91.7% and 7.2%, respectively, and 51.1% of preterm infants were classified as having severe vitamin D deficiency. Only 3 preterm infants were on sufficient vitamin D status. Seasonal variation of 25-OHD concentrations was significant (P=0.010); it was statistically higher in the fall compared to other seasons (P=0.013). Monthly 25-OHD concentrations of preterm infants are shown in Fig. 1. The serum 25-OHD concentrations in September (16.5±10.4 ng/mL) were significantly higher compared to other months (P=0.006).The serum 25-OHD concentrations were correlated to serum calcium (r=0.150, P=0.012) and ALP concentrations (r=-0.165, P=0.006) but not with serum phosphorous concentrations (r=0.079, P=0.192).- 2. Vitamin D status according to gestational age
- 2. Vitamin D status according to gestational age
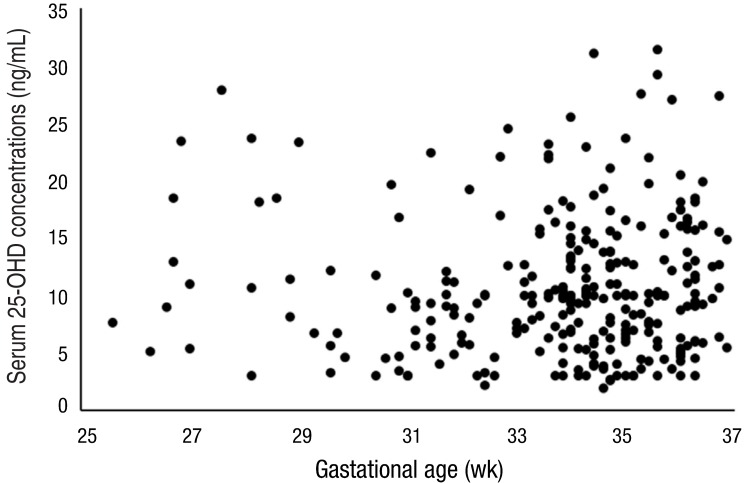
The serum 25-OHD concentrations were not significantly correlated to gestational age (r=-0.034, P=0.573) after controlling for potential confounding factors including season, gestational number, delivery mode, small for gestational age (SGA), and maternal PROM, diabetes and preeclampsia (Fig. 2).There were no significant differences in serum 25-OHD concentrations and incidence of severe vitamin D deficiency among early, moderate, and late preterm infants (Table 2). The serum calcium concentrations were lower in early preterm infants (9.1±1.1 mg/dL, P<0.001) than in moderate (9.7±0.7 mg/dL) and late preterm infants (9.6±0.7 mg/dL). The incidence of PROM (43.4%) and maternal diabetes (18.9%) was significantly higher in moderate preterm infants than in early and late preterm infants (P=0.004 and P=0.009, respectively).- 3. Comparison of demographic and clinical characteristics between infants with and without severe vitamin D deficiency
- 3. Comparison of demographic and clinical characteristics between infants with and without severe vitamin D deficiency
There were not significant differences in gestational age, birth weight, head circumference and length of infants with severe vitamin D deficiency compared to infants without severe vitamin D deficiency (Table 3). The severity of vitamin D deficiency was not different by the incidence of SGA, cesarean section, maternal diabetes, PROM or preeclampsia. Season and gestational number were associated with severe vitamin D deficiency. In preterm infants with severe vitamin D deficiency, the ratio of twin to singleton (31.0% vs. 69.0%) was higher than that in preterm infants with serum 25-OHD concentrations greater than 10 ng/mL (18.4% vs. 81.6%, P=0.014). In spring and winter, severe vitamin D deficiency was more frequent than in summer and fall (P<0.001). Serum ALP concentrations (203.0±77.5 mg/dL vs. 177.8±50.7 mg/dL, P=0.001) were significantly higher in preterm infants with severe vitamin D deficiency compared to infants without severe vitamin D deficiency, but serum phosphorous (5.4±0.8 mg/dL vs. 5.6±0.9 mg/dL, P=0.091) and calcium concentrations (9.4±0.8 vs. 9.6±0.8 mg/dL, P=0.110) were not different in both groups.- 4. Factors associated with severe vitamin D deficiency in preterm infants
- 4. Factors associated with severe vitamin D deficiency in preterm infants
We analyzed maternal and neonatal potential confounding factors associated with severity of vitamin D deficiency (Table 4). The risk of severe vitamin D deficiency in twin preterm infants was significantly higher than that in singletons (odds ratio [OR], 1.993; 95% confidence interval (CI), 1.137-3.494; P=0.016). In fall, the incidence of severe vitamin D deficiency decreased 0.46 times compared to that in winter (95% CI, 0.227-0.901; P=0.024). After adjustment for potential confounding factors, gestational number and season of birth were associated with severe vitamin D deficiency. Neonatal factors including gestational age and birth weight, and maternal clinical factors such as PROM, preeclampsia, and diabetes were not significantly associated with severe vitamin D deficiency.
- Discussion
- Discussion
In the present study, 98.9% of preterm infants had vitamin D insufficiency or deficiency, and 51.1% of preterm infants were severely vitamin D deficient. These results showed much lower 25-OHD concentrations compared to those reported in previous studies of preterm infants13,14,15,16,17,18), and also were lower than those in previous reports on Korean newborns3,19,20). Although almost all infants had vitamin D insufficiency or deficiency, the serum concentrations of calcium, phosphorous and ALP at birth were maintained within the normal range. Growth parameters including height, birth weight and head circumference were not significantly different in infants with severe vitamin D deficiency after controlling for gestational age.Previous studies have suggested that vitamin D status was affected by factors associated with vitamin D metabolism, including race, latitude, genetic traits, and season7,9,11,17,21,22). The present study was conducted on an ethnically homogenous group living in the same environment, providing more valuable data to investigate other factors affected vitamin D status. As in previous study reported by Yu et al.21), the seasonal variation of 25-OHD concentrations was significant. In spring and winter, severe vitamin D deficiency was more frequent than in summer and fall. The 25-OHD concentrations in September were statistically higher compared to other months. However, among other seasons and months, there were not significant differences in the incidence of severe vitamin D deficiency and 25-OHD concentrations. Only twin infants were enrolled to represent multiple pregnancies, and twin infants had tendency toward severe vitamin D deficiency compared to single infants. It was not known previously whether the gestational number was correlated to vitamin D deficiency, but gestational number was an important factor contributing to severe vitamin D deficiency in the present study.Very preterm infants had risks of developing vitamin D deficiency compared with full-term infants13,14,18), but the association between gestational age and vitamin D status remained unclear. In Korean pregnant women, the incidence of vitamin D deficiency was significantly higher in the first and second trimesters compared to that in the third trimester8). Considering that maternal vitamin D status is the most important factor determining vitamin D status at birth10,11), it was predicted that the incidence of vitamin D deficiency would be higher in early preterm infants. However, we did not find a significant linear correlation between 25-OHD concentrations and gestational age. Rather, the 25-OHD concentrations were not significantly different according to gestational age. The incidence of severe vitamin D deficiency also was not different in early preterm infants after control for other confounding factors.Our study reports the lowest 25-OHD concentrations of preterm infants published to date, as far as we have been able to determine. The previous studies involved preterm infants with younger gestational age compared to that in the present study13,14,15,16,17,18), but the 25-OHD concentrations were much higher than those of preterm infants in the present study. The 25-OHD concentrations were even lower than those of infants in Northern European countries including Norway (12.2±5.5 ng/mL)23), and Finland (11.7±4.7 ng/mL)24). The serum 25-OHD concentrations of infants at birth positively correlated with maternal 25-OHD concentrations during pregnancy10,18). Poor maternal vitamin D status and inadequate transplacental transfer at the time of birth are the most important factors for neonatal vitamin D deficiency18,25,26). Jang et al.6) and Lips et al.7) included international groups in their study, which showed that Korean women had the lowest vitamin D status. Choi et al.8) reported that a majority of Korean pregnant women had vitamin D deficiency. These conditions might influence the low vitamin D status of preterm infants in the present study, and also suggested strongly that maternal vitamin D status during pregnancy was too low to attain adequate fetal vitamin D status for preterm infants.Low maternal concentrations of 25-OHD during pregnancy are known to increase risks of prenatal death, gestational diabetes, preeclampsia, and preterm labor4,5). Although we did not include a comparable number of preterm infants with sufficient vitamin D status, there were no significant differences in 25-OHD concentrations between infants with and without maternal history of diabetes and preeclampsia. Maternal diabetes, preeclampsia, and younger gestational age were not correlated to an increased risk of severe vitamin D deficiency after controlling for other confounding factors.Our study is the first research into the subject of vitamin D status in Korean preterm infants to best of our knowledge, and the present study showed that almost all preterm infants had vitamin D insufficiency or deficiency. The present study did not include information regarding maternal vitamin D supplementation and maternal vitamin D status during pregnancy, so we could not assess the correlation among neonatal vitamin D status at birth, maternal vitamin D status during pregnancy and maternal vitamin D supplementation. However, based on our results, maternal vitamin D status during pregnancy was expected to be very low. Guidelines for vitamin D supplementation in Korean pregnant women to make the fetus and newborn maintain sufficient vitamin D status have not been established yet. Further study is needed to find the optimal maternal vitamin D status during pregnancy to obtain sufficient vitamin D status during the fetal period, especially in the Korean population.A limitation of our study was small numbers of early and moderate preterm infants compared to late preterm infants. The serum 25-OHD concentrations of newborns were generally very low, therefore it has limited ability to show the relationship between gestational age and the risk of vitamin D deficiency. Almost all preterm infants were vitamin D insufficient or deficient in the present study and it did not include a comparable group of preterm infants with vitamin D sufficiency, therefore it has limited ability to show the role of vitamin D contributed to neonatal and maternal complications. Although preterm infants with vitamin D insufficiency and deficiency at birth did not develop short-term complications such intrauterine growth restriction and hypocalcemic seizures, the association between vitamin D deficiency at birth and any long-term complications remains to be determined.In conclusion, 98.9% of preterm infants had vitamin D insufficiency and more than half of them were severely vitamin D deficient. Younger gestational age did not increase the risk of vitamin D deficiency, but gestational number was associated with severe vitamin D deficiency.
- Conflicts of interest
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 2. Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 2011;127:e1513–e1520.
[Article] [PubMed]3. Shin YH, Yu J, Kim KW, Ahn K, Hong SA, Lee E, et al. Association between cord blood 25-hydroxyvitamin D concentrations and respiratory tract infections in the first 6 months of age in a Korean population: a birth cohort study (COCOA). Korean J Pediatr 2013;56:439–445.
[Article] [PubMed] [PMC]4. Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2013;26:889–899.
[Article] [PubMed]5. Wei SQ. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol 2014;26:438–447.
[Article] [PubMed]6. Jang H, Koo FK, Ke L, Clemson L, Cant R, Fraser DR, et al. Culture and sun exposure in immigrant East Asian women living in Australia. Women Health 2013;53:504–518.
[Article] [PubMed]7. Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 2006;260:245–254.
[Article] [PubMed]8. Choi R, Kim S, Yoo H, Cho YY, Kim SW, Chung JH, et al. High prevalence of vitamin D deficiency in pregnant Korean women: the first trimester and the winter season as risk factors for vitamin D deficiency. Nutrients 2015;7:3427–3448.
[Article] [PubMed] [PMC]9. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA Jr. Vitamin D insufficiency in pregnant and nonpregnant women of child-bearing age in the United States. Am J Obstet Gynecol 2010;202:436.e1–436.e8.
[Article] [PubMed]10. Vio Streym S, Kristine Moller U, Rejnmark L, Heickendorff L, Mosekilde L, Vestergaard P. Maternal and infant vitamin D status during the first 9 months of infant life-a cohort study. Eur J Clin Nutr 2013;67:1022–1028.
[Article] [PubMed]11. Cadario F, Savastio S, Pozzi E, Capelli A, Dondi E, Gatto M, et al. Vitamin D status in cord blood and newborns: ethnic differences. Ital J Pediatr 2013;39:35
[Article] [PubMed] [PMC]12. Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res 2009;65(5 Pt 2): 106R–113R.
[Article] [PubMed] [PMC]13. Burris HH, Van Marter LJ, McElrath TF, Tabatabai P, Litonjua AA, Weiss ST, et al. Vitamin D status among preterm and full-term infants at birth. Pediatr Res 2014;75:75–80.
[Article] [PubMed]14. McCarthy RA, McKenna MJ, Oyefeso O, Uduma O, Murray BF, Brady JJ, et al. Vitamin D nutritional status in preterm infants and response to supplementation. Br J Nutr 2013;110:156–163.
[Article] [PubMed]15. Tergestina M, Jose A, Sridhar S, Job V, Rebekah G, Kuruvilla KA, et al. Vitamin D status and adequacy of standard supplementation in preterm neonates from South India. J Pediatr Gastroenterol Nutr 2014;58:661–665.
[Article] [PubMed]16. Dawodu A, Nath R. High prevalence of moderately severe vitamin D deficiency in preterm infants. Pediatr Int 2011;53:207–210.
[Article] [PubMed]17. Zhu T, Liu TJ, Ge X, Kong J, Zhang LJ, Zhao Q. High prevalence of maternal vitamin D deficiency in preterm births in northeast China, Shenyang. Int J Clin Exp Pathol 2015;8:1459–1465.
[PubMed] [PMC]18. Monangi N, Slaughter JL, Dawodu A, Smith C, Akinbi HT. Vitamin D status of early preterm infants and the effects of vitamin D intake during hospital stay. Arch Dis Child Fetal Neonatal Ed 2014;99:F166–F168.
[Article] [PubMed]19. Choi YJ, Kim MK, Jeong SJ. Vitamin D deficiency in infants aged 1 to 6 months. Korean J Pediatr 2013;56:205–210.
[Article] [PubMed] [PMC]20. Kim MJ, Na B, No SJ, Han HS, Jeong EH, Lee W, et al. Nutritional status of vitamin D and the effect of vitamin D supplementation in Korean breast-fed infants. J Korean Med Sci 2010;25:83–89.
[Article] [PubMed]21. Yu X, Wang W, Wei Z, Ouyang F, Huang L, Wang X, et al. Vitamin D status and related factors in newborns in Shanghai, China. Nutrients 2014;6:5600–5610.
[Article] [PubMed] [PMC]22. Godang K, Froslie KF, Henriksen T, Qvigstad E, Bollerslev J. Seasonal variation in maternal and umbilical cord 25(OH) vitamin D and their associations with neonatal adiposity. Eur J Endocrinol 2014;170:609–617.
[Article] [PubMed]23. Markestad T, Aksnes L, Finne PH, Aarskog D. Vitamin D nutritional status of premature infants supplemented with 500 IU vitamin D2 per day. Acta Paediatr Scand 1983;72:517–520.
[Article] [PubMed]24. Backstrom MC, Maki R, Kuusela AL, Sievanen H, Koivisto AM, Ikonen RS, et al. Randomised controlled trial of vitamin D supplementation on bone density and biochemical indices in preterm infants. Arch Dis Child Fetal Neonatal Ed 1999;80:F161–F166.
[Article] [PubMed] [PMC]
Table 1
Demographics and clinical characteristics of preterm infants (n=278)
Table 2
The differences of maternal and neonatal clinical characteristics and vitamin D status by gestational age
Table 3
Comparison of demographic and clinical characteristics between infants with and without severe vitamin D deficiency
Table 4
Factors associated with severe vitamin D deficiency in preterm infants

 About
About Browse articles
Browse articles For contributors
For contributors