All issues > Volume 59(1); 2016
Factors associated with mumps meningitis and the possible impact of vaccination
- Corresponding author: Jung Sook Yeom, MD. Department of Pediatrics, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 52727, Korea. Tel: +82-55-750-8161, Fax: +82-55-752-9339, polo96@daum.net
- Received December 18, 2014 Revised May 12, 2014 Accepted June 13, 2015
- Abstract
-
- Purpose
- Purpose
- Mumps meningitis is a common complication of mumps infection; however, information on mumps meningitis in the postvaccine era is limited. The purpose of the present study was to determine factors associated with mumps meningitis and to discuss the effect of vaccination on this disease.
- Methods
- Methods
- We retrospectively reviewed patients younger than 19 years with mumps, diagnosed at a university hospital in Korea between 2003 and 2013. Patients were divided into groups with and without meningitis, and the clinical features of the 2 groups were compared.
- Results
- Results
- The study enrolled 119 patients: 19 patients with meningitis and 100 patients without. Univariate analysis showed that older age (median: 15 years vs. 9.5 years, respectively), a longer interval from last vaccination (median: 10.2 years vs. 4.8 years, respectively), and febrile presentation (94.7% vs. 31.0%, respectively) were significantly associated with mumps meningitis. Sex, number of vaccination doses, bilateral parotitis, and the presence of complications other than meningitis did not differ between the 2 groups. In multivariate logistic regression analysis, age (odds ratio, 1.38; 95% confidence interval, 1.01–1.89; P=0.04) and fever (odds ratio, 30.46; 95% confidence interval, 3.27–283.61; P<0.01) remained independent factors for mumps meningitis.
- Conclusion
- Conclusion
- Clinicians in the postvaccine era should be aware of the possibility of mumps meningitis in febrile cases of mumps in adolescents, regardless of the number of vaccination doses. To establish the role of vaccination in mumps meningitis, further studies will be necessary.
- Introduction
- Introduction
Mumps is an acute viral infection characterized by unilateral or bilateral swelling of the parotid gland. Since the introduction of universal vaccination, the worldwide incidence of mumps infection has sharply declined1). In addition to the reduction in the burden of mumps infection, changes in the susceptible population and the disease severity have been observed2,3). The burden of disease has shifted to a higher age group and entails lower complication rates4). Vaccination significantly reduced the risk of hospitalization or complications2,5). However, in recent years, major outbreaks have been reported in highly vaccinated populations5,6). With the incidence of mumps rising, the numbers of cases with complications have also increased during such outbreaks2). Although the epidemiological impacts of the mumps vaccine may also influence the clinical features of mumps complications, clinical studies on mumps complications in the postvaccine era have been limited, especially with regard to mumps meningitis. Neurological manifestations and sequelae are generally more common in older patients7,8). Therefore, considering the recent increase in average age at the time of infection, understanding the clinical aspects of mumps meningitis is essential in the postvaccine era.Here, we examined the clinical factors associated with mumps meningitis in the postvaccine era. We also analyzed the role of vaccination in mumps meningitis. We compared the clinical features as well as vaccine history between pediatric mumps cases with and without meningitis over a recent 11-year period in a single center in Korea. The measles-mumps-rubella (MMR) vaccine has been included in the National Immunization program in Korea since 1985, and a booster dose has been given since 1997. The Korean vaccination program recommends administration of the first MMR vaccine at 12–15 months and the second at 4–6 years. The second-dose MMR vaccine coverage rate was maintained at over 95% during the study period9,10).
- Methods
- Methods
A retrospective chart review was performed on pediatric cases (under 19 years old) of mumps infection at the Gyeongsang National University of Hospital between January 2003 and December 2013. Cases meeting the criteria for confirmed or probable cases were included in the analysis. Patient with confirmed disease had positive laboratory findings or fulfilled the clinical case definition (i.e., acute onset of unilateral or bilateral tender, self-limited swelling of the parotid or other salivary glands lasting for ≥2 days and occurring in the absence of other apparent causes11)) and were epidemiologically linked to a confirmed or probable case. Cases that met the clinical case definition that were not laboratory confirmed were classified as probable cases. Laboratory confirmation of mumps infection was based on detection of antimumps IgM antibodies in serum by enzyme-linked immunosorbent assay. Viral tests are performed routinely on clinical specimens collected from pediatric patients with parotitis at the hospital. The tests include serology for Ebstein-Barr virus and cytomegalovirus, and polymerase chain reaction (PCR) tests for influenza virus, parainfluenza virus, and adenovirus. A diagnosis of mumps meningitis was based on aseptic meningitis in a confirmed or probable case of mumps. A diagnosis of aseptic meningitis was based on cerebrospinal fluid (CSF) pleocytosis and negative bacteriological studies, including CSF culture and latex agglutination test. Pleocytosis was defined as CSF white blood cell (WBC) count>5 cells/mm3.We excluded patients without laboratory confirmation of mumps infection if routine tests for parotitis were not performed, other viruses were detected, or underlying diseases were finally diagnosed. We also excluded patients with suspected vaccine-related mumps or mumps meningitis. Vaccine-related mumps infection was defined as symptom onset 15–35 days after vaccination12). The patients were divided into 2 groups: those with meningitis (the meningitis group) and those without meningitis (the nonmeningitis group). The clinical data obtained were onset age, gender, year of hospital visit, fever (≥38.0℃), bilaterality of parotitis, number of vaccination doses, interval since last vaccination, and accompanying complications of mumps. In the meningitis group, additional data were obtained, including headache, nausea/vomiting, meningeal irritation signs, and CSF profiles. The vaccination status of patients was verified by medical records or immunization registries. The status of unverified cases was classified as "unknown." In verified cases, vaccination status was categorized as none, one, or two-dose coverage. The interval from last vaccination was calculated as the number of years between the time of the last vaccination and mumps infection.Data are presented as the median (range) or mean (±standard deviation). Categorical data were analyzed by Fisher exact test, and median values from the 2 groups were compared using the Mann-Whitney U test. P<0.05 was taken to indicate statistical significance. Variables that were statistically significant (P<0.05) were entered into multivariate logistic regression analysis. All analyses were performed using SPSS ver. 12.0 (SPSS, Chicago, IL, USA).
- Results
- Results
- 1. Patient demographics and characteristics
- 1. Patient demographics and characteristics
During the study period, 132 patients age 1–19 years were diagnosed with mumps. Of these, 13 patients were excluded from the analysis: routine tests for parotitis were not performed in nine patients without laboratory confirmation of mumps infection, other viruses were confirmed in 2 patients (influenza virus in a respiratory specimen in one patient, positive serology for Epstein-Barr virus in one patient), recurrent sialadenitis was ultimately diagnosed in one patient, and vaccine-related mumps infection was suspected in 1 patient. Consequently, 119 patients were ultimately included in the study and the clinical characteristics and demographics of these patients are shown in Table 1. Sixty-five patients were confirmed cases and 54 were probable cases. The median age was 11.5 years. Of the 119 mumps patients, 87 (73.1%) were male. Parotitis was noted in all included patients. Fever was present in 49 patients (41.2%), with median duration of 3 days (range, 1–6 days). The number of mumps cases had clearly increased since 2011, and the majority (72.5%) occurred during this period. Of the 117 cases for which patient vaccination status was verified, 75 patients (63.0%) had received two doses of vaccine, 37 (31.1%) had received one dose, and 5 (4.2%) were unvaccinated. Complications were notified in 26 cases (21.8%), including meningitis (n=19), orchitis (n=7), pancreatitis (n=1), and oophoritis (n=1). Two patients with meningitis had orchitis. Thus, 19 patients (15.9%) were included in the meningitis group, and 100 patients (84.0%) were included in the nonmeningitis group.No PCR test for mumps with CSF was performed in the meningitis group; however, no other viruses (including enterovirus, herpes simplex virus 1 and 2, cytomegalovirus, varicella-zoster virus, Epstein-Barr virus, and human herpesvirus 6) were detected in any patient in this group. Patients <12 years of age accounted for 57% of the nonmeningitis group, whereas there were no patients in this age category in the meningitis group (Fig. 1). The male/female ratios in the meningitis and nonmeningitis groups were 3.6 and 2.6, respectively. Considerable patients in the 2 groups had been vaccinated twice (73.7% vs. 61.0%, meningitis and nonmeningitis group, respectively). Meningitis was diagnosed before parotid gland swelling in three patients. Except these patients, meningitis was diagnosed a median of 5 days (range, 1–9 days) after parotid gland swelling. All patients in the meningitis group had symptoms and signs consistent with meningitis. Other clinical features and CSF profiles are summarized in Table 2.- 2. Comparison of clinical parameters in the meningitis and nonmeningitis groups
- 2. Comparison of clinical parameters in the meningitis and nonmeningitis groups
Compared with the nonmeningitis group, the meningitis group had an older onset age (median: 15.0 years vs. 9.5 years, P<0.01), longer interval from last vaccination (median: 10.2 years vs. 4.8 years, P<0.01), and a higher rate of febrile presentation (94.7% vs. 31.0%, P<0.01) in univariate analysis (Table 1). After multivariate analysis, age (odds ratio, 1.38; 95% confidence interval [CI], 1.01–1.89, P=0.04) and presence of fever (odds ratio, 30.46; 95% CI, 3.27–283.61; P<0.01) remained independent risk factors for mumps meningitis (Table 3). Gender, number of vaccine doses, bilaterality of parotitis, and accompanying complications other than meningitis did not differ between the 2 groups.
- Discussion
- Discussion
Most data for mumps meningitis in the postvaccine era have described simple epidemiological features at one specific point in time, as just one of the complications of mumps. However, we focused on mumps meningitis cases that occurred over a decade, and analyzed the clinical factors associated with this particular disease entity. A recent trend toward an increase in the incidence of mumps meningitis in our study also shows why pediatricians should consider this disease, even in highly vaccinated regions. In the present study, an older age at onset (P<0.01), longer interval from last vaccination (P<0.01), and more frequent febrile presentation (P<0.01) were observed in the meningitis group compared with the nonmeningitis group (Table 1). Among these, age (P=0.04) and presence of fever (P<0.01) were identified as independent risk factors for mumps meningitis (Table 3). Gender, number of vaccination doses, bilaterality of parotitis, and accompanying complications other than meningitis did not differ between the two groups.In the nonmeningitis group, fever was present in 31% of patients and resolved within a few days, consistent with population-based surveillance data in the postvaccine era6,13). On the other hand, fever occurred in most of the patients in the meningitis group (94.7%) and resolved within 3–5 days, which is also consistent with studies from the prevaccine era14,15,16). Thus, the febrile presentation of the meningitis group seemed not to have changed since the introduction of vaccination programs. Local viral invasion of the parotid gland accounts for mumps infection without complications. However, viremia can be documented and is the likely cause of mumps meningitis17). Viremia or viral loads in the blood were shown to be correlated with disease severity, including fever, and to be associated with neurological complications for several other viruses18,19). Thus, the differences in febrile presentation between the 2 groups seem to reflect differences in pathophysiology. Other clinical and laboratory features of mumps meningitis observed here also did not differ from those of the prevaccine era: male predominance, meningitis presentation approximately 5 days after onset of parotitis, and slightly increased CSF protein level17). All patients in the meningitis group had received at least one dose of vaccine. Therefore, with the exception of onset age, clinical features of vaccinated mumps meningitis patients were not different from those in the prevaccine era.The frequency of complication follows closely the prevalence of general mumps infection20). In the prevaccine era, the onset age of mumps complications did not differ from that of overall mumps infection, ranged from 6 to 8 years14,21). In postvaccine era, mumps meningitis as well as overall mumps infection was most common in adolescents and young adults2,5). Our results also showed that overall mumps infection was most prevalent in around the age of 15 years, which was consistent with the peak prevalence of mumps meningitis. Thus, after vaccine introduction, the age of mumps complications has increased since the introduction of vaccination, as the burden of disease shifts to older age groups. On the other hand, the conventional wisdom, the rates of mumps complications including meningitis increased with age7,8,20), has not been changed. An epidemiological study from prevaccine era22) showed that the peak incidence of mumps meningitis was at the age of 7 years, while the highest rate of mumps meningitis was in those age 15 or more. The recent epidemiological studies of mumps infection in the postvaccine era2) revealed that the rate of mumps meningitis was higher in late adolescent to young adulthood and lower in younger cohorts. Our data also showed that older onset age was independently associated with mumps meningitis, and the median age of this patients group was 15 years. Therefore, regardless of vaccination program introduction, adolescents and young adults seem to be strongly associated with mumps meningitis.However, our findings raise questions regarding why no patients younger than 12 years of age had mumps meningitis, although half of the patients with mumps infection were in this age group. Our findings probably suggest that vaccination effectively prevents mumps meningitis, at least in children under a certain age or within a certain vaccination interval. However, mumps meningitis of infants and young children may be underestimated in the present study due to nonspecific or mild illness of mumps meningitis in this age23). It has been also reported that the hospitalization rate for mumps cases without complications was highest in younger patients, but the rate of mumps meningitis cases was highest in the older cohort2). Thus, the disproportionate hospitalization rates between mumps cases with and without meningitis may have been responsible for the observed differences in age distribution in the present study. To verify the discrepancy between mumps infection and mumps meningitis in young children, further studies with large numbers will be necessary.Although the median interval from last vaccination was significantly longer in mumps meningitis group (10.2 years vs. 4.8 years) in univariate analysis, the association did not reach statistical significance (P=0.19) in the multivariate analysis. Interval from last vaccination strongly correlated with onset age (Pearson correlation coefficient 0.91, P<0.01). These findings suggest that the association between mumps meningitis and interval from last vaccination in univariate analysis was due to the confounding effect of age. No studies have documented a direct relationship between the vaccination interval and mumps meningitis. However, some studies have suggested that vaccine effectiveness24) and neutralizing antibody levels25) against mumps can decline with time since vaccination. The effectiveness of the vaccine seems to decline markedly approximately 10 years after 2 doses24). Significantly lower levels of mumps-neutralizing antibodies have been reported in students who had been vaccinated 15 or more years previously than in those who had been vaccinated 1 to 5 years previously26). Although interval from last vaccination was different between the 2 groups, only a few patients (6.7%) had the interval over more than 10 years in the present study. Thus, to identify association between interval from last vaccination (or role of circulating mumps antibodies) and mumps meningitis, a further study including a lot of patients with a longer interval more than 10 or 15 years will be necessary.There is a great deal of controversy regarding the number of vaccination doses and gender in terms of mumps complications. A study of an outbreak in the United States in 2006 found that older age and male gender were associated with complications, but the number of vaccination doses was not6). Other studies showed that vaccination reduced the risk for meningitis; the risk of meningitis was significantly lower in the vaccinated than unvaccinated patients in a study performed in the United Kingdom from 2002 to 20062), and 2 doses of vaccine tended to reduce the risk of meningitis in a study performed in the United States between 2009 and 201027). Male gender was reported to be a risk factor for mumps meningitis in the former study, but not in the latter. We found that the number of vaccination doses and male gender were not associated with mumps meningitis. However, the small sample size, inclusion of few unvaccinated patients, or the possibility of inaccuracies in the vaccine status of our analysis might not allow us to detect differences in vaccine-related factors between the 2 groups, which might be major weaknesses of the present study. The coverage rate of second-dose MMR vaccine of patients over 7 years in the present study (82%) was considerably lower, compared with documented coverage rate at 7-year-old children from Korea Center for Disease Control and Prevention (over 95%)9,10). This difference in coverage rate may suggest the possibility of inaccuracy in the vaccine status of the present study. To minimize the limitations of the retrospective study, we verified the vaccine status based on Immunization Information System. Registration rate to Immunization Information System tended to increase, but remained 81.7% in 201110). Thus, we might have some patients of missing vaccine record due to relatively low registration rate of vaccination.Our study was also limited by the fact that considerable patients were underestimated. The proportion of meningitis (15.9%) observed here was high compared with the results of population-based surveillance from the postvaccine era (<1%)2,5,6). The much higher proportion observed in this study likely reflects the fact that the denominator was derived from tertiary hospital-based studies, which might exclude mild cases of mumps infection. The number of meningitis cases might also have been underestimated in our study because we included patients with parotitis only. It has been reported that half of all mumps meningitis cases do not have parotitis17). Considering the increasing trends of mumps meningitis, routine tests for mumps should be considered in aseptic meningitis cases, particularly in adolescents. Although we included parotitis patients with no apparent causes other than mumps infection, our study was limited by the fact that not all cases were laboratory confirmed. In addition, most of complications of included patients in the present study were meningitis. For these reasons, we could not verify whether age and presence of fever are uniquely associated with mumps meningitis or not. The role of vaccination in mumps meningitis could be established by prospective, population-based studies of laboratory-confirmed cases of all ages, with large numbers including all complications.Despite these limitations, our results suggest that pediatricians should consider the possibility of mumps meningitis in febrile mumps cases in adolescents, regardless of the number of vaccination doses. To identify mumps meningitis without parotitis, routine tests for mumps should be considered in aseptic meningitis cases, particularly in adolescents.
- Conflicts of interest
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Peltola H, Davidkin I, Paunio M, Valle M, Leinikki P, Heinonen OP. Mumps and rubella eliminated from Finland. JAMA 2000;284:2643–2647.
[Article] [PubMed]2. Yung CF, Andrews N, Bukasa A, Brown KE, Ramsay M. Mumps complications and effects of mumps vaccination, England and Wales, 2002-2006. Emerg Infect Dis 2011;17:661–667.
[Article] [PubMed] [PMC]3. Barskey AE, Glasser JW, LeBaron CW. Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine 2009;27:6186–6195.
[Article] [PubMed]4. Plotkin SA, Rubin SA. Mumps vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: WB Saunders Co, 2008:435–465.5. Sane J, Gouma S, Koopmans M, de Melker H, Swaan C, van Binnendijk R, et al. Epidemic of mumps among vaccinated persons, The Netherlands, 2009-2012. Emerg Infect Dis 2014;20:643–648.
[Article] [PubMed] [PMC]6. Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, et al. Recent resurgence of mumps in the United States. N Engl J Med 2008;358:1580–1589.
[Article] [PubMed]7. Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ 2005;330:1132–1135.
[Article] [PubMed] [PMC]8. Yeom JS, Moon YJ, Kim ES, Choi YH, Song YG, Kim E, et al. Clinical characteristics of mumps in postpubertal age. Korean J Infect Dis 1997;29:305–311.9. Status of measles after declaration on measles elimination in Korea, 2006-2011 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention, c2013;cited 2015 Jan 10. Available from: http://www.cdc.go.kr/CDC/contents/CdcKrContentLink.jsp?fid=31&cid=20060&ctype=6.10. Results of 2nd MMR entry requirement, 2006-2011 [Internet]. Cheongju: Korea Centers for Disease Control and Prevention, c2013;cited 2015 Mar 10. Available from: http://www.cdc.go.kr/CDC/contents/CdcKrContentLink.jsp?fid=31&cid=12684&ctype=6.12. Farrington P, Pugh S, Colville A, Flower A, Nash J, Morgan-Capner P, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 1995;345:567–569.
[Article] [PubMed]13. Marin M, Quinlisk P, Shimabukuro T, Sawhney C, Brown C, Lebaron CW. Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students--Iowa, 2006. Vaccine 2008;26:3601–3607.
[Article] [PubMed]14. Kanra G, Isik P, Kara A, Cengiz AB, Seçmeer G, Ceyhan M. Complementary findings in clinical and epidemiologic features of mumps and mumps meningoencephalitis in children without mumps vaccination. Pediatr Int 2004;46:663–668.
[Article] [PubMed]15. Azimi PH, Cramblett HG, Haynes RE. Mumps meningoencephalitis in children. JAMA 1969;207:509–512.
[Article] [PubMed]16. Guitierrez K. Mumps virus. In: Long SS, Pickering LK, Prober CG, editors. Principles and practice of pediatric infectious diseases. 4th ed. Philadelphia: Elsevier Saunders, 2012:1125–1130.17. Gnann JWJ. Meningitis and encephalitis caused by mumps virus. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the central nervous system. 3rd ed. Philladelphia: Lippincott Williams & Wilkins, 2004:231–242.18. Sugata K, Taniguchi K, Yui A, Miyake F, Suga S, Asano Y, et al. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 2008;122:392–397.
[Article] [PubMed]19. Berrington WR, Jerome KR, Cook L, Wald A, Corey L, Casper C. Clinical correlates of herpes simplex virus viremia among hospitalized adults. Clin Infect Dis 2009;49:1295–1301.
[Article] [PubMed] [PMC]20. Leinikki P. Mumps. In: Zuckerman AJ, Banatvala JE, Schoub BD, Griffiths PD, Mortimer P, editors. Principles and practice of clinical virology. 6th ed. West Sussex: Wiley-Blackwell Publishing, 2009:593–600.21. Sul IJ, Lee WG, Rhee YS, Park CM. A clinical study of mumps menigitis. J Korean Pediatr Soc 1979;22:709–715.22. Bjorvatn B, Skoldenberg B. Mumps and its complications in Stockholm. Br Med J 1978;1:788
[Article]23. Mason WH. Mumps. In: Kliegman RM, Stanton B, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders, 2011:1078–1081.24. Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis 2007;13:12–17.
[PubMed] [PMC]25. Dayan GH, Rubin S. Mumps outbreaks in vaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Clin Infect Dis 2008;47:1458–1467.
[Article] [PubMed]26. Date AA, Kyaw MH, Rue AM, Klahn J, Obrecht L, Krohn T, et al. Long-term persistence of mumps antibody after receipt of 2 measles-mumps-rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J Infect Dis 2008;197:1662–1668.
[Article] [PubMed]
Fig. 1
Age distributions of groups with and without meningitis. Overall mumps infection was most prevalent around the age of 15 years, which was consistent with the peak prevalence of mumps meningitis. Patients <12 years of age accounted for 57% of the nonmeningitis group, whereas there were no patients in this age category in the meningitis group.
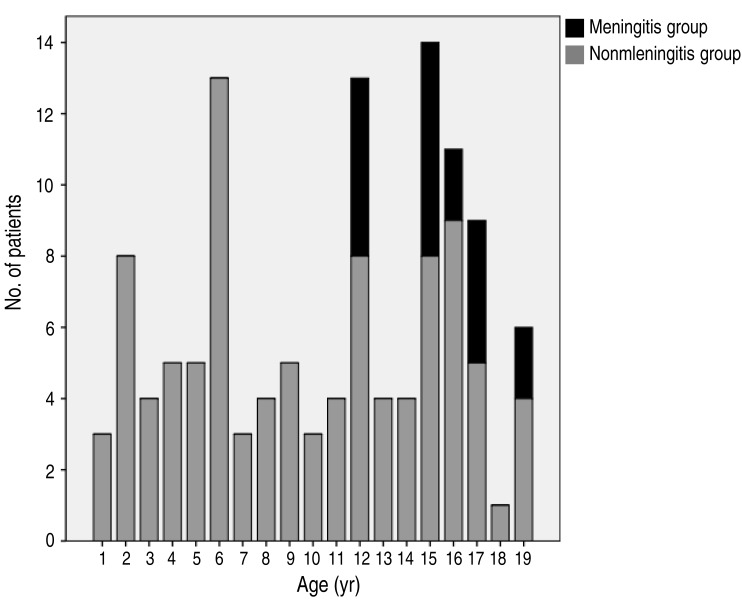
Table 1
Patient characteristics and comparison between groups with and without meningitis
Table 2
Clinical features and cerebrospinal fluid profiles of mumps meningitis patients
Table 3
Risk factors for meningitis complications in mumps patients, derived using multivariate regression analysis
| Variable | Adjusted odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Age | 1.38 | 1.01-1.89 | 0.041 |
| Interval from last vaccination | 0.79 | 0.55-1.13 | 0.194 |
| Fever | |||
| Absent | 1.00 | - | - |
| Present | 30.46 | 3.27-283.61 | 0.003 |

 About
About Browse articles
Browse articles For contributors
For contributors
