All issues > Volume 59(4); 2016
Deficiency of antidiuretic hormone: a rare cause of massive polyuria after kidney transplantation
- Corresponding author: Min Hyun Cho, MD. Department of Pediatrics, Kyungpook National University School of Medicine, 680 Gukchaebosang-ro, Jung-gu, Daegu 41944, Korea. Tel: +82-53-200-2741, Fax: +82-53-200-2029, chomh@knu.ac.kr
- Received April 09, 2014 Revised May 28, 2014 Accepted July 19, 2014
- Abstract
-
A 15-year-old boy, who was diagnosed with Alport syndrome and end-stage renal disease, received a renal transplant from a living-related donor. On postoperative day 1, his daily urine output was 10,000 mL despite normal graft function. His laboratory findings including urine, serum osmolality, and antidiuretic hormone levels showed signs similar to central diabetes insipidus, so he was administered desmopressin acetate nasal spray. After administering the desmopressin, urine specific gravity and osmolality increased abruptly, and daily urine output declined to the normal range. The desmopressin acetate was tapered gradually and discontinued 3 months later. Graft function was good, and urine output was maintained within the normal range without desmopressin 20 months after the transplantation. We present a case of a massive polyuria due to transient deficiency of antidiuretic hormone with the necessity of desmopressin therapy immediately after kidney transplantation in a pediatric patient.
- Introduction
- Introduction
The development of transient polyuria occasionally occurs in the recipients of kidney transplantation (KT) due to delayed adaptation of renal tubular function. This is the first sign of progressive recovery of kidney function preceding the decrease in serum creatinine or blood urea nitrogen (BUN). This clinical manifestation tends to recover fully within several days or weeks without specific treatments1). Central diabetes insipidus (DI) is caused by a deficiency in antidiuretic hormone (ADH) and is characterized by polyuria in response to exogenous ADH analog2). However, this condition is clinically masked by oliguric renal failure and may be unrecognized until successful KT3). We present a case of the transient deficiency of ADH, which is similar to central DI, with the necessity for desmopressin acetate therapy in a pediatric patient immediately after KT.
- Case report
- Case report
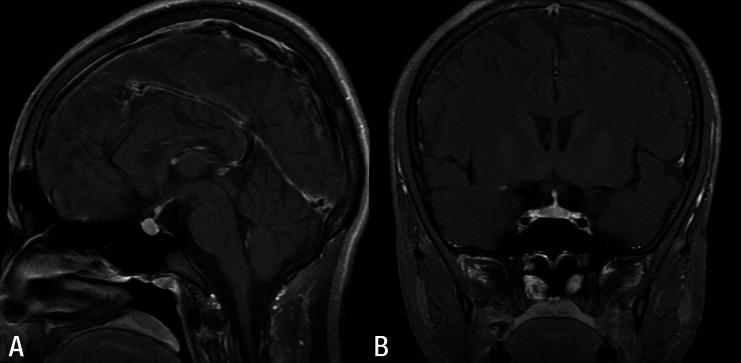
The patient was diagnosed with Alport syndrome at the age of 7 years by percutaneous renal biopsy performed due to recurrent gross hematuria and proteinuria. The patient's condition progressed to end-stage renal disease (ESRD), and peritoneal dialysis was started at the age of 13 years. Since then, his urine output had decreased gradually to the anuric state during the 32 months before KT.The patient received a KT from a living-related donor (his father) at the age of 15 years. His blood pressure was 146/92 mmHg, and height was 161 cm. On the day of admission, BUN and serum creatinine were 62 and 15.3 mg/dL, respectively. The operation was successful, and initial immunosuppressive medications were prescribed, including tacrolimus, mycophenolate mofetil, and corticosteroid. His urine flow started immediately after the anastomosis between the renal artery of the graft and his right iliac artery, and it was maintained fully. The next day, a transplant Doppler ultrasonogram and glomerular filtration rate scan with radioisotope revealed relatively good renal perfusion. His serum creatinine decreased to 1.0 mg/dL, and vital signs were stable. His urine output was about 15,000 mL per day beginning on postoperative day 1. His daily urine output was maintained at up to 10,000 mL 1 week later, despite normal graft function. Moreover, he lost 4 kg more compared to before KT, and fluid restriction had no effect on the polyuria. Thus, we checked his urine, serum osmolality, and serum ADH levels (Table 1). A partial ADH deficiency such as incomplete central DI was suspected based on his clinical and laboratory findings, and he was administered desmopressin acetate (30–40 mcg/day) nasal spray without a water deprivation test. After administering desmopressin, urine specific gravity and osmolality abruptly increased to 1.020 and 361 mosm/kg, respectively, and daily urine output declined to <5,000 mL. To exclude the possibility of a pituitary gland lesion, magnetic resonance imaging (MRI) was performed, which presented no abnormal findings (Fig. 1). The desmopressin acetate was tapered gradually and discontinued 3 months later, and ADH level normalized to 2.02 pg/mL. Graft function was good 20 months after KT, and urine output was maintained within the normal range without desmopressin (Fig. 2).
- Discussion
- Discussion
DI, characterized by excretion of a copious volume of dilute urine, can be life-threatening if not properly diagnosed and managed. It develops due to inadequate or impaired secretion of ADH from the pituitary gland (central DI) or unresponsiveness to ADH at the renal tubules (nephrogenic DI)4). This condition presents several clinical manifestations such as polyuria, polydipsia, growth retardation, and excessive thirst5). Central DI is occasionally idiopathic or originates from secondary etiologies, including a pituitary tumor, encephalitis, meningitis, cerebral infarction, trauma, or hypoxic-ischemic injury6).Although our patient did not have typical clinical manifestations of central DI, such as hypernatremia, due to closed monitoring and continuous hydration, he presented with massive polyuria, hypotonic urine, relatively high serum osmolality, low ADH level, and a dramatic response to desmopressin therapy, which were compatible with ADH deficiency similar to central DI.Then, the question was raised as to the main cause for the ADH deficiency immediately after KT. We speculated on two possible causes of the central DI-like symptoms in this patient. First, it was possible that he already had the central DI before KT. An oliguric or anuric state developing from ESRD can mask the clinical presentations of central DI with symptoms such as polyuria and polydipsia2,3). In particular, 10 months before KT, the patient was admitted to our hospital due to seizure and hypertensive encephalopathy and was discharged without any specific neurological complications. He was nearly anuric at that time. To confirm hypertensive damage in the brain architectures including the hypothalamus and the pituitary gland, we performed brain MRI, but no abnormal findings were detected. However, we could not ignore the possibility of subclinical injury to the hypothalamic-pituitary axis including the pituitary gland, which was not revealed by brain imaging. Yu et al.5 reported a case of transient central DI after an aorto-coronary bypass operation, and suggested that one of the possible causes was transient cerebral ischemia in the hypothalamus or in the hypothalamic-pituitary axis that occurred during the bypass, despite normal brain imaging. However, because the patient's clinical presentations of central DI and the necessity of desmopressin acetate therapy were transient, it was less likely that there was pre-existing central DI due to permanent brain damage before KT.Second, transient failure of ADH secretion due to his hydration state immediately before KT was another possibility. Secretion of ADH in patients with ESRD can be controlled by intravascular volume and plasma osmolality according to dialysis ultrafiltration rather than urine output. If plasma osmolality is high or intravascular volume decreases, secretion of ADH increases; however, if plasma osmolality is low or intravascular volume increases, secretion of ADH decreases. If a pediatric recipient receives a kidney from an adult donor, pediatric nephrologists have a tendency to maintain a fully hydrated state by decreasing dialysis ultrafiltration immediately before KT to facilitate the recipient's blood shifting to the graft. Because we also maintained his volume state fully hydrated for 24 hours before KT, it was speculated that this preconditioning could have suppressed secretion of ADH and produced transient central DI. In addition, corticosteroid therapy can aggravate central DI by inhibiting ADH secretion7). However, it was less likely that the transient change in ADH secretion according to his hemodynamic condition would last more than 3 months after KT. Therefore, we concluded that the response of the hypothalamic-pituitary axis was slowed down during the 3 months and suppression of ADH secretion was maintained similar to central DI, with the following combination of conditions: subclinical injury of the hypothalamic-pituitary axis due to hypertensive encephalopathy combined with transient suppression of ADH due to the patient's hemodynamic condition before KT and after KT with corticosteroid treatment.In summary, we report a case of a transient deficiency of ADH, similar to central DI, with the necessity for desmopressin acetate therapy immediately after KT. We suggest that polyuria can develop following KT not only from renal tubular dysfunction but also due to other causes such as central DI, as seen in our case. It is important that clinicians have greater awareness of this uncommon cause.
- Conflicts of interest
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Kim KM, Kim SM, Lee J, Lee SY, Kwon SK, Kim HY. Newly developed central diabetes insipidus following kidney transplantation: a case report. Transplant Proc 2013;45:2804–2806.
[Article] [PubMed]2. Henne T, Bokenkamp A, Offner G, Ehrich JH. Perioperative management of central diabetes insipidus in kidney transplantation. Pediatr Nephrol 2001;16:315–317.
[Article] [PubMed]3. Kim DD, Holdaway IM. Unmasking of undiagnosed pre-existing central diabetes insipidus after renal transplantation. Pituitary 2012;15:106–109.
[Article] [PubMed]4. Makaryus AN, McFarlane SI. Diabetes insipidus: diagnosis and treatment of a complex disease. Cleve Clin J Med 2006;73:65–71.
[Article] [PubMed]5. Yu CH, Cho JH, Jung HY, Lim JH, Jin MK, Kwon O, et al. A case of transient central diabetes insipidus after aorto-coronary bypass operation. J Korean Med Sci 2012;27:1109–1113.
[Article] [PubMed] [PMC]6. Blotner H. Primary or idiopathic diabetes insipidus: a system disease. Metabolism 1958;7:191–200.
[PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors