All issues > Volume 59(8); 2016
Clinical outcome of patients with refractory Kawasaki disease based on treatment modalities
- Corresponding author: Hong Ryang Kil, MD. Department of Pediatrics, Chungnam National University Hospital, Chungnam University School of Medicine, 282 Munhwa-ro, Jung-gu, Daejeon 35015, Korea. Tel: +82-42-280-7251, Fax: +82-42-255-3158, gilhong@cnu.ac.kr
- Received January 09, 2016 Revised March 16, 2016 Accepted April 11, 2016
- Abstract
-
- Purpose
- Purpose
- Although a significant number of reports on new therapeutic options for refractory Kawasaki disease (KD) such as steroid, infliximab, or repeated intravenous immunoglobulin (IVIG) are available, their effectiveness in reducing the prevalence of coronary artery lesions (CAL) remains controversial. This study aimed to define the clinical characteristics of patients with refractory KD and to assess the effects of adjuvant therapy on patient outcomes.
- Methods
- Methods
- We performed a retrospective study of 38 refractory KD patients from January 2012 to March 2015. We divided these patients into 2 groups: group 1 received more than 3 IVIG administration+ steroid therapy, (n=7, 18.4%), and group 2 patients were unresponsive to initial IVIG and required steroid therapy or second IVIG (n=31, 81.6%). We compared the clinical manifestations, laboratory results, and echocardiographic findings between the groups and examined the clinical utility of additional therapies in both groups.
- Results
- Results
- A significant difference was found in the total duration of fever between the groups (13.0±4.04 days in group 1 vs. 8.87±2.30 days in group 2; P=0.035). At the end of the follow-up, all cases in group 1 showed suppressed CAL. In group 2, coronary artery aneurysm occurred in 2 patients (6.4 %). All the patients treated with intravenous corticosteroids without additional IVIG developed CALs including coronary artery aneurysms.
- Conclusion
- Conclusion
- No statistical difference was found in the development of CAL between the groups. Prospective, randomized, clinical studies are needed to elucidate the effects of adjunctive therapy in refractory KD patients.
- Introduction
- Introduction
Kawasaki disease (KD) is an acute febrile, systemic vasculitic syndrome of unknown etiology, occurring primarily in children younger than 5 years of age. Administration of intravenous immunoglobulin (IVIG) as a single 2 g/kg within the first 10 days after onset of fever in combination with high-dose aspirin reduces the risk of coronary artery lesion (CAL) to 3% to 5%1). However, 10% to 20% of patients with KD do not respond to the conventional therapy and requires second line of treatments, such as repeated doses of IVIG, pulse methylprednisolone, cyclophosphamide, methotrexate, plasmapheresis or infliximab2,3,4,5,6,7). The effectiveness of these treatment options on CAL is not clear in patients with refractory KD. The risk of coronary artery aneurysm (CAA) is increased in these patients, and few studies have been conducted on optimal management of such cases3,8).A previous study reported that in exceptional patients with KD fail to respond to second (or third, if necessary) 2 g/kg dose of IVIG9). However, the relative value of repeated doses of IVIG in refractory KD remains unknown. Therefore, we retrospectively evaluated the baseline characteristics of 38 patients with refractory KD and compared the effects of more than 3rd IVIG infusions and other adjuvant therapies on the occurrence of CAL.
- Materials and methods
- Materials and methods
- 1. Patients and data collection
- 1. Patients and data collection
We retrospectively analyzed the medical records of 38 patients with refractory KD from Eulji University Hospital and Chungnam National University Hospital between January 2012 and March 2015. We defined patients of refractory KD as those who had persistent fever (≥38.0℃) that lasted for more than 24 to 36 hours after the end of initial IVIG infusion. These patients with refractory KD were divided into 2 groups. Group 1 consisted of 7 patients (18.4%) who were responsive to 3 or more IVIG infusions, and group 2 consisted of 31 patients (81.6%) who were unresponsive to initial IVIG and required additional IVIG or steroid therapy.Laboratory data prior to IVIG treatment was analyzed and the data included a complete blood count, erythrocyte sedimentation rate, albumin, aspartate aminotransferase (AST), sodium, N-terminal fragment of B-type natriuretic peptide and C-reactive protein (CRP). Laboratory findings were compared between groups 1 and 2.We also estimated a risk score8) with point scores and cutoff values for each variables as follows: 2 points each for sodium 133 mmol/L or less, initial treatment given on day 4 of illness or earlier, AST 100 IU/L or more, and neutrophils at least 80%; and 1 point for platelet count 30.0×104/mm3 or less, CRP at least 10 mg/dL, and age 12 months or less.- 2. Echocardiography
- 2. Echocardiography
GE echocardiography system Vivid 7 (GE healthcare Korea, Seoul, Korea) was used at two medical centers. Echocardiography was performed within 8 days of the onset of fever or before IVIG administration. The left ventricular percent fractional shortening (FS) was calculated as the difference between the dimensions at end diastolic and end systolic divided by end-diastolic dimension. Tissue Doppler imaging (TDI) and conventional echocardiography were performed. Systolic velocity, e' velocity and a' velocity were measured at the basal level of the interventricular septum. In addition, pulsed wave Doppler recording of mitral leaflet tips provided mitral inflow velocity pattern from which early diastolic velocity (E), late diastolic velocity with atrial contraction (A), and E/A ratio. Coronary artery dilatation was defined as positive if the coronary artery diameter ≥3 mm in children under 5 years or ≥4 mm in children aged ≥5 years, and aneurysms were diagnosed if ≥4 mm and giant aneurysm if ≥8 mm10). We obtained these data by reviewing the charts.- 3. Treatments for refractory KD
- 3. Treatments for refractory KD
Initially, all the patients with KD were treated with IVIG, 2 g/kg in a single infusion over 10–12 hours along with aspirin (50 mg/kg/day). Patients with a fever lasted for more than 24 to 36 hours after completion of IVIG treatment without another possible source of fever were retreated with IVIG (1–2 g/kg) and continued to receive aspirin therapy. When fever persisted, intravenous methylprednisolone (30 mg/kg/dose) was administered for 3 consecutive days. However, corticosteroid monotherapy without additional IVIG was identified in 3 cases.Infliximab (5 mg/kg) was administered as a single infusion when fever persisted despite additional IVIG or corticosteroid treatments. A Mantoux skin test for tuberculosis was done before infliximab treatment, and the results were recorded at 48 hours. Antibody levels to infliximab were not measured. Adverse effects were classified according to severity and possible association with KD or other treatments.- 4. Statistical analysis
- 4. Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA), and data was expressed as mean±standard deviation unless otherwise stated. We compared the categorical variables between the 2 groups using a 2-tailed Fisher exact test. We used Mann-Whitney test to analyze the continuous variables. In addition, we utilized multivariate logistic regression analysis to determine the relationships between the development of CAL on follow-up echocardiography and clinical variables in the study subjects.- 5. Ethics statement
- 5. Ethics statement
The study protocol was reviewed and approved by Institutional Review Board of Eulji University Hospital (approval number: 2015-10-008-001).
- Results
- Results
- 1. Baseline characteristics of the patients
- 1. Baseline characteristics of the patients
The series of patients in the present study included 29 boys and 9 girls, whose mean age at diagnosis was 33.47±16.66 months (range, 8–67 months). Of the 38 patients, 32 patients (84.2%) were diagnosed with typical KD and 6 patients (15.8%) with incomplete KD. The mean time until start of IVIG treatment was 4.0±0.57 days in group 1 and 4.9±1.37 days in group 2, respectively (P=0.012).Among 38 patients with refractory KD, 63% (n=24) received antibiotics intravenously due to concurrent respiratory infection or high procalcitonin level (range, 0.52–41 ng/mL). Retropharyngeal abscess was confirmed on computed tomography in 3 patients (7.8%). Incision and drainage of retropharyngeal abscess was not required in these cases.Children in the 2 groups did not differ significantly in sex distribution, age at presentation, and body mass index (Table 1). A significant difference was found in the total duration of fever between the 2 groups (13.0±4.04 days in group 1 vs. 8.87±2.30 days in group 2, P=0.035). In addition, Kobayashi et al.8) scores were higher in group 1 than group 2 (6.57±2.07 in group 1 vs. 3.84±2.22 in group 2, P=0.005).- 2. Laboratory findings
- 2. Laboratory findings
The laboratory data of the 2 groups on admission were not significantly different with regards to white blood cell counts, hemoglobin, platelet counts, and CRP levels (Table 2). The mean albumin level was 4.03±0.25 mg/dL in group 1 and 3.55±6.19 mg/dL in group 2 (P=0.003). The mean procalcitonin level was 3.64 mg/dL in group 1 and 0.91 mg/dL in group 2 (P=0.078). In addition, the levels of triglyceride and high density lipoprotein-cholesterol did not differ between the 2 groups.- 3. Echocardiographic findings
- 3. Echocardiographic findings
There was no significant difference between the groups in development of CAL. At discharge, CAA occurred in 2 patients in group 2. At discharge and 2 months after treatment, no patients had CAA in group 1. There was no difference between the 2 groups in the degree of left ventricular FS and the early transmitral flow velocity (E)/the early diastolic mitral annular velocity(e') ratio measured by TDI (Table 3).- 4. Treatments for refractory KD
- 4. Treatments for refractory KD
1) IVIG retreatment
1) IVIG retreatment
In the present study, the second dose of IVIG was 2 g/kg in 23 patients and 1 g/kg in 12 patients. The first IVIG infusion was initiated significantly earlier in group 1 than in group 2.In group 1, the second and the third IVIG infusion was administered at 6.57±0.53 fever days and 9.80±1.95 fever days, respectively. Seven patients received third or fourth IVIG infusion following methylprednisolone pulse therapy (MPT). The starting point of steroid treatment was coincident with the time of the third IVIG infusion in group 1 except 1 patient (range, 6–12 fever days). One patient received 3 doses of MPT with the second IVIG infusion, but required additional IVIG infusion due to persistent fever. Two patients failed to become persistently afebrile despite these treatments including a third IVIG infusion. Consequently, they received infliximab and the fourth IVIG treatment, respectively (Fig. 1).2) Corticosteroid treatment
2) Corticosteroid treatment
Overall, 21 patients received 2 to 6 doses of pulse methylprednisolone (18 patients with additional IVIG vs. 3 patients without additional IVIG). Seventeen patients responded to a second IVIG treatment without corticosteroid treatment. Among 18 patients with crossed-over MPT with a second IVIG, 10 patients (55.5%) became afebrile (Fig. 1). The time of the MPT infusion was significantly earlier in group 2 than group 1 (9.0±2.0 fever days in group 1 vs. 7.14±1.40 fever days in group 2, P=0.023).One patient in group 2 received second IVIG (1 g/kg) on fever day 6. However, fever and other Kawasaki features persisted. On fever day 8, MPT (30 mg/kg/dose) was infused consecutively and was tapered by oral prednisolone due to subsided fever. However, the patient showed a giant coronary aneurysm (8 mm) in the right main coronary artery on follow-up echocardiography.3) Infliximab infusion
3) Infliximab infusion
Overall, 4 patients received a single infusion of 5 mg/kg of infliximab because of persistent fever despite steroid pulse therapy or additional IVIG on mean 12.75±2.59 fever days. One patient received infliximab on fever day 10 after MPT without additional IVIG. The next day, his fever subsided but the patient had a giant CAA on follow-up echocardiography.Two patients who received infliximab on fever day 12 had mild coronary artery dilation. The patient who received infliximab on fever day 17 after the third IVIG infusion had no CAL. In the present study, a 31-month boy developed severe neutropenia (absolute neutrophil count 180/µL) and whole body rash after infliximab infusion even though the fever subsided. However, this adverse event resolved spontaneously. No patient had anaphylactic reaction during infliximab infusion.
- 5. Clinical outcome
- 5. Clinical outcome
At discharge and 2 months after treatment, there was no significant difference in the incidence of CALs between the 2 groups (Table 3). At the end of follow-up, no patients in group 1 had CALs. Coronary artery aneurysm occurred in 2 patients in group 2 (6.4%). Two patients in group 2 had giant aneurysms measuring 8 to 10 mm in the left main coronary artery and right proximal coronary artery, respectively and were on the medications of warfarin and aspirin. One patient in group 1 had a small aneurysm (4 mm) in the left main coronary artery in the acute stage, but showed normalization of the vessel on follow-up echocardiography.- 6. Association between development of CAL and clinical variables
- 6. Association between development of CAL and clinical variables
Patients were divided in 2 groups with based on final coronary outcomes. Six patients had CALs including CAAs in group 2 (Table 3). After multivariate logistic regression analysis, the serum albumin level at admission was the risk factor for developing CALs. There was no correlation between other clinical variables and CALs on follow-up echocardiography (Table 4).
- Discussion
- Discussion
This study was conducted to determine the role of additional therapies in patients with refractory KD and to investigate the occurrence of coronary artery complications according to treatment modalities. There was no statistical difference between the 2 groups in the development of CALs in the present study due to small sample size. However, 3 patients treated with corticosteroid monotherapy without additional IVIG was associated with CALs including CAAs. Moreover, IVIG retreatment ≥10 days after illness onset was beneficial for preventing progression of CALs.KD is an acute febrile vasculitis of unknown origin that occurs predominantly in infants and young children. The use of high-dose IVIG and aspirin lowered the frequency of coronary artery complications caused by KD to below 5%11). Prompt reduction of inflammation and duration of fever, definitely before illness day 8 or 9, is essential when CALs begin to appear. However, 10% to 20% of patients with KD do not respond to conventional therapy and have continued fever, and such cases are in need of an additional rescue therapy12,13). Only limited data are available to guide rescue therapy after initial IVIG resistance.Treatment strategies have varied between institutions, and guidelines for additional treatment have not been established. Most experts recommend the administration of a second 2 g/kg dose of IVIG as the first-line rescue therapy before considering corticosteroids, although only two-thirds of these patients respond to the second IVIG14). In the present study, 27 patients (79%) responded to the second IVIG with or without MPT. Three patients received MPT without a second IVIG and showed CALs including CAA.The dose-response effect of IVIG forms the theoretical basis for repeated IVIG infusion in refractory KD. Although its mechanism of action is not clear, immunoglobulin has systemic anti-inflammatory and immunomodulating action. IVIG binds to the Fc receptor of B and T lymphocytes, inhibiting the production of antibody by B cells, and altering regulatory T-cell helper or suppressor activities15). Recent data demonstrate that IVIG has direct inhibitory effects on leukocyte recruitment in vitro and in vivo via the inhibition of selectin and integrin functions16). The potential benefit of the third or fourth IVIG in refractory KD is uncertain, in part because our understanding of the mechanism underlying the effects of IVIG in the pathogenesis of KD is incomplete. In the present study, a 33-month-old boy with a small CAA who is resistant to the third IVIG and steroid pulse therapy showed improvement immediately after the infusion of the 4th IVIG on fever day 18. It is remarkable that seven patients who received more than 3rd IVIG had no CAL despite the long fever day. These cases indicate that the more severe inflammation persists in a patient with KD, the more IVIG doses are required, and IVIG treatment ≥10 days after illness onset could be beneficial for preventing progression of CALs. However, these cases leave something to be desired because other intensive second line treatments besides IVIG may shorten the hospital length.Results of studies on the use of corticosteroid as second or third line therapy for refractory KD are controversial. The guidelines state that corticosteroids should be restricted to children in whom ≥2 infusions of IVIG have been ineffective in alleviating fever and acute inflammation14). In the present study, the timing of intravenous corticosteroids was after the second dose of IVIG (1–2 g/kg) in 18 patients. Corticosteroid monotherapy without additional IVIG was identified in 3 cases. These cases were associated with coronary artery complications although fever subsided after MPT infusion.The results of a retrospective study suggested that administration of MPT for IVIG-resistant KD may reduce the risk of CALs17). A major benefit from early suppression of vasculitis would result if the corticosteroid therapy administrated procedes vascular remodeling. However, in a recent prospective study, MPT did not reduce the risk of development of CAL and does not seem to be beneficial as single agent therapy for IVIG resistant KD18). The present study showed that corticosteroid monotherapy without additional IVIG resulted in CAA, although infliximab was administered subsequently.In the present study, 4 patients with refractory KD received infliximab infusion. Blockade of the inflammatory cascade at the level of tumor necrosis factor (TNF)-α is a rational step in the treatment of refractory KD, because circulating levels of TNF-α are markedly elevated during acute KD. The degree of elevation of TNF-α correlates with the coronary artery damage and the development of aneurysms19). Infliximab is a chimeric monoclonal IgG1 antibody that almost completely suppresses the biological activity of TNF-α. Burns et al.20) reported that both infliximab and a second IVIG infusion were safe and well tolerated in the subjects with KD who were resistant to standard IVIG treatment. However, recent data shows that infliximab was still detected 48 hours after infusion in the serum of a patient with disease refractory to infliximab, suggesting unknown factors or cytokines other than TNF-α were responsible for the unresponsiveness and disease progression21). The present study showed that an early infliximab-treated patient without a second IVIG infusion had CAA in the follow-up echocardiography. One patient treated by infliximab after 3 doses of IVIG and methylprednisolone pulse infusion had transient coronary artery dilatation that resolved in follow up echocardiography. These cases indicate that the timing of infliximab infusion for patients with refractory KD should be after at least 2 doses of IVIG.Infliximab is an immunosuppressive medication associated with increased risk of infection22,23). Infusion reactions, ranging from flushing and dyspnea to anaphylaxis, are thought to be related to formation of antibodies directed against infliximab in patients receiving repeated doses for chronic infection24,25). So far, the adverse effects of single infusion of infliximab in refractory KD were not frequently reported.All the patients received a single high dose IVIG without steroids as a primary treatment of KD in this study. A recent study revealed that the adjunct use of corticosteroids with IVIG for the initial treatment reduced the risk of coronary artery abnormalities in a meta-analysis of 9 clinical studies26). They showed that IVIG plus corticosteroid therapy significantly reduced risk of coronary abnormality with no significant difference in the incidence of severe adverse events between the IVIG and IVIG plus steroid group. Recent trends regarding treatment choices for patients that do not respond to initial IVIG treatment have changed from additional therapies to initial intensive treatment for patients with high-risk KD.This study has some limitations: first, this was a retrospective study at 2 medical centers. The dosage of the second IVIG was 2 g/kg in 1 hospital and 1 g/kg in the other hospital. We could not determine whether these different IVIG doses influenced the coronary artery outcome; second, we enrolled a small number of patients, and other additional treatments such as plasma exchange or other cytotoxic agents were not used in the present study.In this study, KD patients who required more than third IVIG infusion are more likely to have high risk factors including clinical characteristics and laboratory findings suggesting high level of inflammation. However, there was no difference between the 2 groups in the development of CALs. Because there are no consensus recommendations for the treatments of refractory KD, further prospective study is necessary to determine the optimal adjunctive therapies for patients with refractory KD.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 1991;324:1633–1639.
[Article] [PubMed]2. Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr 1993;123:657–659.
[Article] [PubMed]3. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr 1996;128:146–149.
[Article] [PubMed]4. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics 2000;105:E78
[Article] [PubMed]5. Lee MS, An SY, Jang GC, Kim DS. A case of intravenous immunoglobulin-resistant Kawasaki disease treated with methotrexate. Yonsei Med J 2002;43:527–532.
[Article] [PubMed]6. Imagawa T, Mori M, Miyamae T, Ito S, Nakamura T, Yasui K, et al. Plasma exchange for refractory Kawasaki disease. Eur J Pediatr 2004;163:263–264.
[Article] [PubMed]7. Burns JC, Mason WH, Hauger SB, Janai H, Bastian JF, Wohrley JD, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr 2005;146:662–667.
[Article] [PubMed]8. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006;113:2606–2612.
[Article] [PubMed]9. Shulman ST. Is there a role for corticosteroids in Kawasaki disease? J Pediatr 2003;142:601–603.
[Article] [PubMed]10. Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Japanese Ministry of Health and Welfare, 1984.11. Royle J, Burgner D, Curtis N. The diagnosis and management of Kawasaki disease. J Paediatr Child Health 2005;41:87–93.
[Article] [PubMed]12. Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther 2010;8:197–203.
[Article] [PubMed] [PMC]13. Shulman ST, Rowley AH. Advances in Kawasaki disease. Eur J Pediatr 2004;163:285–291.
[Article] [PubMed]14. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004;110:2747–2771.
[Article] [PubMed]15. Ramesh S, Schwartz SA. Therapeutic uses of intravenous immunoglobulin (IVIG) in children. Pediatr Rev 1995;16:403–410.
[Article] [PubMed]16. Gill V, Doig C, Knight D, Love E, Kubes P. Targeting adhesion molecules as a potential mechanism of action for intravenous immunoglobulin. Circulation 2005;112:2031–2039.
[Article] [PubMed]17. Ogata S, Bando Y, Kimura S, Ando H, Nakahata Y, Ogihara Y, et al. The strategy of immune globulin resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. J Cardiol 2009;53:15–19.
[Article] [PubMed]18. Teraguchi M, Ogino H, Yoshimura K, Taniuchi S, Kino M, Okazaki H, et al. Steroid pulse therapy for children with intravenous immunoglobulin therapy-resistant Kawasaki disease: a prospective study. Pediatr Cardiol 2013;34:959–963.
[Article] [PubMed]19. Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol 1990;56:29–36.
[Article] [PubMed]20. Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr 2008;153:833–838.
[Article] [PubMed] [PMC]21. Mori M, Imagawa T, Hara R, Kikuchi M, Hara T, Nozawa T, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: report of an open-label case series. J Rheumatol 2012;39:864–867.
[Article] [PubMed]22. Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–2285.
[Article] [PubMed]23. van Ingen J, Boeree MJ, Dekhuijzen PN, van Soolingen D. Mycobacterial disease in patients with rheumatic disease. Nat Clin Pract Rheumatol 2008;4:649–656.
[Article] [PubMed]24. Riegert-Johnson DL, Godfrey JA, Myers JL, Hubmayr RD, Sandborn WJ, Loftus EV Jr. Delayed hypersensitivity reaction and acute respiratory distress syndrome following infliximab infusion. Inflamm Bowel Dis 2002;8:186–191.
[Article] [PubMed]
Fig. 1
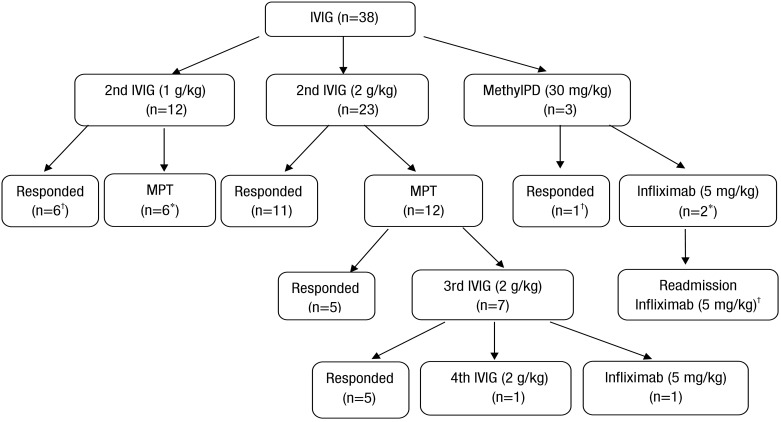
Table 1
Table 2
Values are presented as mean±standard deviation.
IVIG, intravenous immunoglobulin; AST, aspartate aminotransferase; ALT, alanine aminotransferase, ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NT-proBNP, N-terminal fragment of B-type natriuretic peptide; HDL-C, high density lipoprotein-cholesterol.
*Mann-Whitney test.
Table 3
Values are presented as number (%) or mean±standard deviation.
IVIG, intravenous immunoglobulin; CAL, coronary artery lesion; LV, left ventricle; E, base peak early diastolic E-wave velocity (cm/sec); A, base peak early diastolic A-wave velocity (cm/sec); e', peak early diastolic myocardial velocity (cm/sec); F/U, follow-up at 2 months after treatment.

 About
About Browse articles
Browse articles For contributors
For contributors
