All issues > Volume 59(11); 2016
Impact of postoperative duration of Aspirin use on longevity of bioprosthetic pulmonary valve in patients who underwent congenital heart disease repair
- Corresponding author: Sang-Yun Lee, MD. Department of Pediatrics, Sejong General Hospital, 28, Hohyeon-ro 489beon-gil, Bucheon 14754, Korea. Tel: +82-32-340-1121, Fax: +82-32-340-1236, saeng123@hanmail.net
- Received August 07, 2016 Revised September 06, 2016 Accepted September 12, 2016
- Abstract
-
- Purpose
- Purpose
- Generally, aspirin is used as a protective agent against thrombogenic phenomenon after pulmonary valve replacement (PVR) using a bioprosthetic valve. However, the appropriate duration of aspirin use is unclear. We analyzed the impact of postoperative duration of aspirin use on the longevity of bioprosthetic pulmonary valves in patients who underwent repair for congenital heart diseases.
- Methods
- Methods
- We retrospectively reviewed the clinical data of 137 patients who underwent PVR using a bioprosthetic valve between January 2000 and December 2003. Among these patients, 89 were included in our study and divided into groups I (≤12 months) and II (>12 months) according to duration of aspirin use. We analyzed echocardiographic data from 9 to 11 years after PVR. Pulmonary vale stenosis and regurgitation were classified as mild, moderate, or severe.
- Results
- Results
- The 89 patients consisted of 53 males and 36 females. Their mean age was 14.3±8.9 years (range, 2.6–48 years) and body weight was 37.6±14.7 kg (range, 14–72 kg). The postoperative duration of aspirin use was 7.3±2.9 months in group I and 32.8±28.4 months in group II. However, no significant difference in sex ratio, age, body weight, type of bioprosthetic valve, and number of early redo-PVRs. In the comparison of echocardiographic data about 10 years later, no significant difference in pulmonary valve function was found. The overall freedom rate from redo-PVR at 10 years showed no significant difference (P=0.498).
- Conclusion
- Conclusion
- Our results indicated no benefit from long-term aspirin medication (>6 months) in patients who underwent PVR with a bioprosthetic valve.
- Introduction
- Introduction
Current bioprosthetic valves have excellent hemodynamic performance and remain free from structural deterioration for up to 15 years1). But, bioprosthetic valves are heterografts made primarily of porcine or bovine tissue, each with comparable risks of thromboembolism2). Prophylaxis for thromboembolism should be based on pathogenesis and risk. The peak incidence occurs during the first 3 months after valve surgery, probably reflecting the lack of endothelialization of the newly implanted prosthetic and biologic material3,4,5).We usually use an aspirin for protection of thrombogenic phenomenon after pulmonary valve replacement (PVR) using bioprosthetic valve. However, evidence that long duration aspirin use is appropriate is currently lacking. Therefore, we aimed to analyze the impact of postoperative duration of aspirin use on longevity of bioprosthetic pulmonary valve in patients who underwent congenital heart disease repair.
- Materials and methods
- Materials and methods
We retrospectively reviewed the clinical data of 137 patients who underwent PVR using bioprosthetic pulmonary valve in Sejong Cardiovascular Institute from January 2000 to December 2003. The patients' data before 2000 years are inaccurate in Sejong General Hospital. And the purpose of this retrospective study is to analyze the longevity of bioprosthetic pulmonary valve at about 10 years after PVR. Therefore, we established the period from January 2000 to December 2003. Among them, 42 were follow-up loss. And, 6 who underwent early re-PVR due to infective endocarditis and peripheral pulmonary artery stenosis were excluded. As a result, 89 patients were included in our study and classified as group I (≤12 months) and group II (>12 months) according to duration of aspirin use. We analyzed the echocardiographic data for the amount of pulmonary valve stenosis and regurgitation from 9 to 11 years post-PVR. The amount of pulmonary valve stenosis was classified as mild (<30 mmHg), moderate (30–50 mmHg), and severe (>50 mmHg) by peak pressure gradient. The amount of pulmonary valve regurgitation was classified as mild, moderate, and severe by examiner's description. In addition, we analyzed the rate of early re-PVR due to bioprosthetic pulmonary valve failure below 10 years. Additionally, we analyzed for patients who used Hancock II and were under 13 years old. The most common type of bioprosthetic valve to be used during PVR is Hancock II in our patients. And it would be more accurate to compare the same valve, so we performed subgroup analysis for patients who used Hancock II. Furthermore, since the young patients (under 13 years old) have different calcium metabolism and immune mechanism from adults, we analyzed patients under 13 years old. The Institutional Review Board of Sejong General Hospital approved this retrospective study.
- Results
- Results
Totally 89 patients were analyzed of which, 53 were male and 36 were female. Their mean age at the time of PVR was 14.3±8.9 years old (range, 2.6–48 years) and mean body weight was 37.6±14.7 kg (range, 14–72 kg). Their congenital heart diseases consisted of tetralogy of Fallot 48, pulmonary atresia with ventricular septal defect 22, fallot type double outlet right ventricle 6, pulmonary atresia with intact ventricular septum 4 and others 3 cases (especially, aortic valve diseases [aortic stenosis, aortic regurgitation] patients underwent Ross operation). Types of bioprosthetic valve consisted of Hancock II 60, Freestyle 14, Carpentier-Edwards porcine 14, and Homograft valve 1 case (Table 1). The group I comprised 41 patients and group II was 48 patients. The postoperative duration of aspirin use was 7.3±2.9 months in group I and 32.8±28.4 months in group II (P=0.000). Sex ratio showed no difference (P=0.800). The mean age at PVR was 13.6±7.2 years old in group I and 14.9±10.1 years old in group II (P=0.493). The mean weight at PVR was 38.2±15.9 kg in group I and 37.2±13.8 kg in group II (P=0.748). In comparison of valve types, there was no statistical difference in both groups (P=0.909). The number of early re-PVR patients within 10 years was 13 of 41 patients in group I and 14 of 48 patients in group II (P=0.648) (Table 2).We analyzed echocardiographic data from 10-year post-PVR. The mean follow-up duration of echocardiography was 9.85±0.52 years in group I and 9.81±1.90 years in group II (P=0.920). In analysis of the amount of pulmonary stenosis, group I had 16 mild, 8 moderate, and 4 severe patients; and group II had 20 mild, 9 moderate, and 5 severe patients (P=0.945). In pulmonary regurgitation, group I had 23 trivial and mild, and 5 moderate patients; and group II had 30 trivial and mild, and 4 moderate patients (P=0.901). Analysis of follow-up echocardiographic data indicated no statistical difference between groups (Table 3). The 10-year re-PVR free rate was 70 to 80 percent. Overall freedom from re-PVR at 10 years showed no significant difference (P=0.597) (Fig. 1); and thromboembolic event was absent in all patients.Additionally, we analyzed 60 patients who were corrected by Hancock II valve and they were classified as group I (n=27) and group II (n=33). In comparison of aspirin use duration, Group I was 7.4±2.6 months and group II is 32.8±28.2 months (P=0.000). However, there was no difference in gender ratio (P=0.875), mean age at PVR (P=0.267), mean body weight at PVR (P=0.537), and numbers of early re-PVR (P=0.481) between both groups (Table 4). The mean follow-up duration of echocardiography was 9.95±0.52 years in group I and 9.70±2.20 years in group II (P=0.558). In comparison of pulmonary stenosis, most patients had mild degree pulmonary stenosis and there were no significant difference in both groups (P=0.789). Also, in comparison of pulmonary regurgitation, there was no difference between groups (P=0.782) (Table 5). Overall freedom from re-PVR at 10 years showed no significant difference (P=0.484) (Fig. 2A).Finally, we analyzed 49 patients under 13 years old because of the reason that we mentioned above. They were classified as group I (n=22) and group II (n=27). The mean duration of aspirin use in group I was 7.1±2.9 and group II was 31.5±26.3 months (P=0.000). However, there was no difference in gender ratio (P=0.494), mean age at PVR (P=0.522), mean body weight at PVR (P=0.995), and numbers of early redo PVR (P=0.778) between both groups (Table 6). Overall freedom from re-PVR at 10 years showed no significant difference (P=0.604) (Fig. 2B).
- Discussion
- Discussion
Patients with prosthetic valves are at increased risk for both valve thrombosis and arterial thromboembolic events6). Consequently, the anticoagulation therapy is used to lessen the thromboembolic risk. Despite anticoagulation, patients remain at risk for thromboembolic complications, and anticoagulation therapy has the additional risk of bleeding. The risk of thromboembolic and bleeding complications is present even in patients with optimal anticoagulation and is influenced by patient factors and valve position7). The optimal initial antithrombotic treatment remains an important unresolved issue in patients undergoing bioprosthetic PVR.The 2014 American Heart Association/American College of Cardiology guidelines clearly recommend the use of an anticoagulation regimen for the first 3 months after bioprosthetic aortic valve replacement and mitral valve replacement in patients to achieve an international normalized ratio of 2.5 and aspirin 75mg to 100 mg per day is reasonable in all patients with a bioprosthetic aortic or mitral valve for long term8). Also, the guideline is described as follows in European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines (version 2012). Oral anticoagulation may be considered for the first 3 months after implantation of an aortic and mitral bioprosthesis. Low-dose aspirin should be considered for the first 3 months after implantation of an aortic and mitral bioprosthesis9). The American College of Chest Physicians' guidelines recommend aspirin over warfarin (except for patients with atrial fibrillation)10). These guidelines were developed in an attempt to minimize the risk of thromboembolism during the high-risk period.On the other hand, the guidelines for the use of warfarin and aspirin after bioprosthetic PVR remain under discussion. The risk of thromboembolic complications after bioprosthetic replacement of the pulmonary valve appears to be low, and the risk of systemic embolization is essentially nonexistent7). However, patients usually use aspirin because of the potential to adversely affect the availability and durability of valve. Therefore, in our hospital, some patients were taking aspirin within 1-year post-PVR and some patients for more than 1-year post-PVR according to the cardiologist's preference. Because the duration of complete endothelialization of bioprosthetic valve in pulmonary valve might be from 3 to 6 months, we decided to 1 year as criteria for duration of aspirin use. When patients are classified according to 12 months, we expected that the mean duration of aspirin use in short duration group would be about 6 months. Actually, the mean duration of aspirin use in short duration group was 7.3 months and it was almost same with our expectation.Generally, our indication for PVR was as follows. In patient with pulmonary stenosis, we applied about 50-mmHg pressure gradient by cardiac catheterization. And, in patient with pulmonary regurgitation, the indication of PVR was right ventricular end-diastolic volume index >150 mL/m2 or right ventricular end-systolic volume index >75 mL/m2 and it was consistent with reference11).The above results suggest that taking aspirin for more than 1-year post-PVR does not affect the valve function. The appropriate duration of aspirin use for optimal valve function remains unclear and may be shorter than 6 months. Importantly, the results of our study indicated that taking aspirin for more than about 7-month post-PVR is not needed, providing the rationale to alleviate the burden of long-term aspirin use in patients who undergo bioprosthetic PVR.In most studies that analysed the outcomes of PVR in young patients, younger age and smaller valve size were believed to be the important risk factors for early reoperation for PVR12,13). A calcification of tissue valves is strongly associated with active calcium turnover by growth14) and the immunological response of patients to xenoantigens15,16), especially in young patients. In addition, it is clear that a small prosthetic valve in pediatric patients shows relative stenosis over time. Therefore, we analyzed young patients group under 13 years old and there was no significant difference in longevity of bioprosthetic pulmonary valve.Our study had several limitations of retrospective review, small number, not small follow-up loss, and heterogeneity of disease entity and bioprosthetic valve. And, although many factors can influence the progression of pulmonary stenosis, but we analyzed the longevity of bioprosthetic pulmonary valve which was classified only according to the duration of aspirin use. Therefore, it could be the limitation to assume that all other factors are on the same condition. In conclusion, long-term aspirin use above 7 months in patients who underwent PVR with bioprosthetic valve showed no benefit. Therefore, we recommend aspirin only short duration for patients with postbioprosthetic PVR. Actually, the patients who underwent PVR are taking aspirin for 6 months in our hospital as the result. Further prospective investigation is required to evaluate outcomes for aspirin use of less than 6 months.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Aramendi JI, Mestres CA, Campos V, Martínez-León J, Pontes C, Muñoz G, et al. Triflusal versus oral anticoagulation for primary prevention of thromboembolism after bioprosthetic valve replacement (TRAC): rationale and design for a prospective, randomized, co-operative trial. Interact Cardiovasc Thorac Surg 2003;2:170–174.
[Article] [PubMed]2. Leiria TL, Lopes RD, Williams JB, Katz JN, Kalil RA, Alexander JH. Antithrombotic therapies in patients with prosthetic heart valves: guidelines translated for the clinician. J Thromb Thrombolysis 2011;31:514–522.
[Article] [PubMed] [PMC]3. Stein B, Fuster V, Halperin JL, Chesebro JH. Antithrombotic therapy in cardiac disease. An emerging approach based on pathogenesis and risk. Circulation 1989;80:1501–1513.
[Article] [PubMed]4. McClung JA, Stein JH, Ambrose JA, Herman MV, Reed GE. Prosthetic heart valves: a review. Prog Cardiovasc Dis 1983;26:237–270.
[Article] [PubMed]5. Chesebro JH, Adams PC, Fuster V. Antithrombotic therapy in patients with valvular heart disease and prosthetic heart valves. J Am Coll Cardiol 1986;8(6 Suppl B): 41B–56B.
[Article] [PubMed]6. Massel D, Little SH. Risks and benefits of adding anti-platelet therapy to warfarin among patients with prosthetic heart valves: a meta-analysis. J Am Coll Cardiol 2001;37:569–578.
[Article] [PubMed]7. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American Heart Association. Circulation 2013;128:2622–2703.
[Article] [PubMed]8. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–2488.
[Article] [PubMed]9. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167–214.
[PubMed]10. Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl): e576S–e600S.
[Article] [PubMed] [PMC]11. Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation 2013;128:1855–1857.
[Article] [PubMed] [PMC]12. Lee C, Park CS, Lee CH, Kwak JG, Kim SJ, Shim WS, et al. Durability of bioprosthetic valves in the pulm onary position: long-term follow-up of 181 implants in patients with congenital heart disease. J Thorac Cardiovasc Surg 2011;142:351–358.
[Article] [PubMed]13. Caldarone CA, McCrindle BW, Van Arsdell GS, Coles JG, Webb G, Freedom RM, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg 2000;120:1022–1030.
[Article] [PubMed]14. Simionescu DT. Prevention of calcification in bioprosthetic heart valves: challenges and perspectives. Expert Opin Biol Ther 2004;4:1971–1985.
[Article] [PubMed]15. Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Studies on human naturally occurring antibodies to pig xenografts. Transplant Proc 1993;25:2917–2918.
[PubMed]
Fig. 1
Overall freedom rate from re-PVR after PVR. The overall freedom rate from re-PVR at 10 years shows no significant difference between group I (≤12 months) and group II (>12 months) according to duration of aspirin use (P=0.597). PVR, pulmonary valve replacement.
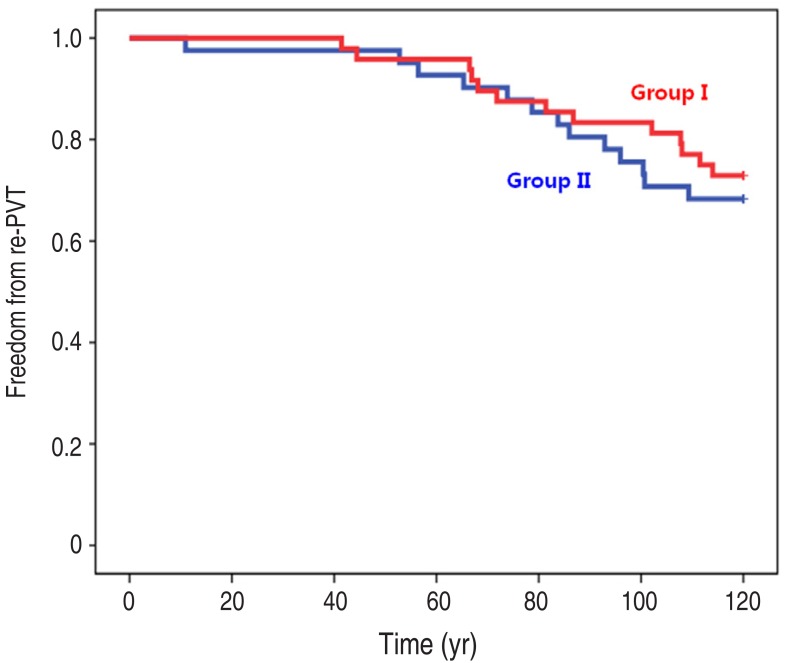
Fig. 2
Overall freedom rate from re-PVR in the patients who underwent PVR using a Hancock valve (A) and those aged <13 years (B). (A) The overall freedom rate from re-PVR at 10 years shows no significant difference in the characteristics of the patients who underwent PVR using a Hancock valve between group I (≤12 months) and group II (>12 months) according to duration of aspirin use (P=0.484). (B) Overall freedom from re-PVR at 10 years shows no significant difference in the characteristics of the patients who underwent PVR at age <13 years between group I (≤12 months) and group II (>12 months) according to duration of aspirin use (P=0.604). PVR, pulmonary valve replacement.
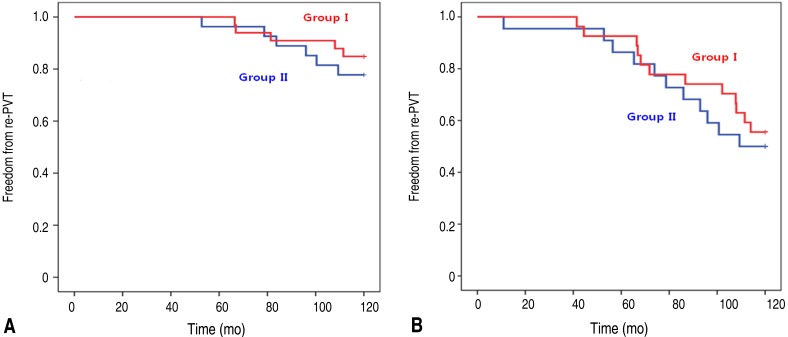
Table 1
Types of congenital heart disea
Table 2
Comparison of the basic characteristics of the subjects in groups I and II
Table 3
Echocardiographic data 10 years after pulmonary valve replacement
Table 4
Analysis of the characteristics of the patients who underwent PVR using a Hancock II valve
Table 5
Echocardiographic data of the patients who underwent pulmonary valve replacement using a Hancock II valve
Table 6
Comparison of the characteristics of the patients aged <13 years

 About
About Browse articles
Browse articles For contributors
For contributors
