All issues > Volume 59(12); 2016
Clinical predictors of chest radiographic abnormalities in young children hospitalized with bronchiolitis: a single center study
- Corresponding author: Youn Ho Sheen, MD, PhD. Department of Pediatrics, CHA Gangnam Medical Center, CHA University, 566 Nonhyeon-ro, Gangnam-gu, Seoul 06135, Korea. Tel: +82-2-3468-3032, Fax: +82-2-3468-3697, epirubicin13@gmail.com
- Received January 14, 2016 Revised May 30, 2016 Accepted June 21, 2016
- Abstract
-
- Purpose
- Purpose
- Chest radiography is often performed on patients hospitalized with typical clinical manifestations of bronchiolitis. We aimed to determine the proportion of subjects with pathologic chest radiographic findings and the clinical predictors associated with pathologic chest radiographic findings in young children admitted with the typical presentation of bronchiolitis.
- Methods
- Methods
- We obtained the following data at admission: sex, age, neonatal history, past history of hospitalization for respiratory illnesses, heart rate, respiratory rate, the presence of fever, total duration of fever, oxygen saturation, laboratory parameters (i.e., complete blood cell count, high-sensitivity C-reactive protein [hs-CRP], etc.), and chest radiography.
- Results
- Results
- The study comprised 279 young children. Of these, 26 had a chest radiograph revealing opacity (n=24) or atelectasis (n=2). Multivariate logistic regression analysis showed that after adjustment for confounding factors, the clinical predictors associated with pathologic chest radiographic findings in young children admitted with bronchiolitis were elevated hs-CRP level (>0.3 mg/dL) and past history of hospitalization for respiratory illnesses (all P<0.05).
- Conclusion
- Conclusion
- The current study suggests that chest radiographs in young children with typical clinical manifestations of bronchiolitis have limited value. Nonetheless, young children with clinical factors such as high hs-CRP levels at admission or past history of hospitalization for respiratory illnesses may be more likely to have pathologic chest radiographic findings.
- Introduction
- Introduction
Acute respiratory tract infection is one of the most common diseases encountered in the outpatient and/or Emergency Department settings in childhood1). Of these infections, bronchiolitis is the most frequent cause of hospitalization among young children2,3). According to an epidemiologic study, approximately 100,000 bronchiolitis hospitalizations occur annually in the United States4). Another study looking at 1,503,239 children under 18 years of age who visited the Emergency Department in Korea in 2012 found that 18,313 children (1.2%) were diagnosed with bronchiolitis and 6,321 children of them (34.5%) were admitted5).In a clinical setting, clinicians often perform chest radiography on children with typical manifestations of bronchiolitis in order to rule out other diseases such as pneumonia or cardio-pulmonary diseases. For example, a survey of clinical practices in the diagnosis and management of 17,397 children with bronchiolitis reported that 72% of them received chest radiographs6). Accordingly, there are several studies demonstrating that the majority of these chest radiographies done on children with bronchiolitis were read as negative and thus of little clinical use6,7,8,9,10). However, there are no studies determining the proportion of children with pathologic chest radiographic findings in Korean children admitted with bronchiolitis. Furthermore, there are only 3 studies in the literature reporting risk factors for radiographic abnormalities among young children with bronchiolitis7,9,10). Determining the proportion of children with pathologic chest radiographic findings and understanding these risk factors could help clinicians reduce the number of chest X-rays and thus avoid unnecessary radiation exposure for children.In the present study, we aimed to determine the proportion of chest radiographs showing pathologic findings inconsistent with bronchiolitis and the clinical predictors of pathologic chest radiographic findings in young children admitted with a typical presentation of bronchiolitis to an inpatient Pediatric Unit at a general hospital.
- Materials and methods
- Materials and methods
- 1. Study setting and subjects
- 1. Study setting and subjects
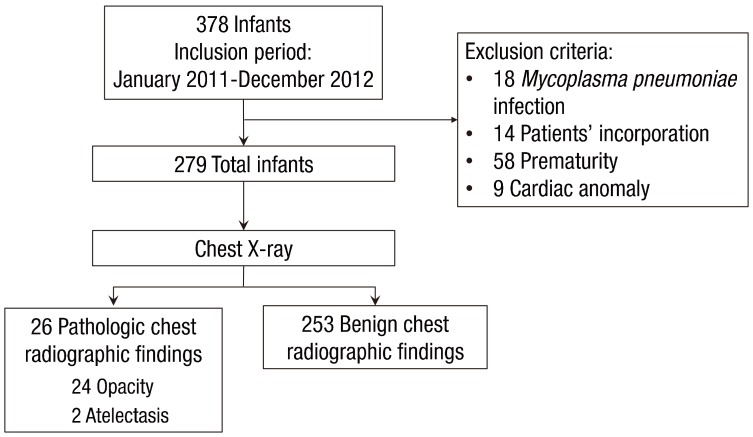
This was a retrospective chart review study of all young children hospitalized in the CHA Gangnam Medical Center, CHA University from January 2011 through December 2012 with typical clinical manifestations of bronchiolitis. Young children who met the following criteria were included: (1) children <2 years of age and (2) those presenting with typical clinical manifestations of bronchiolitis: i.e., a constellation of clinical signs and symptoms such as a viral upper respiratory prodrome followed by increased respiratory effort and wheezing11). A total of 378 young children were admitted with bronchiolitis during the study period.Exclusion criteria were as follows: (1) young children who were infected with Mycoplasma pneumoniae, determined as an initial indirect microparticle agglutinin technique (Serodia-Myco II, Fujirebio, Osaka, Japan) (titer of >1:320) or a 4-fold increasing titer on repeat specimens and positive results for anti-Mycoplasma IgM12), (2) young children whose caregivers did not answer questionnaires on the patient's past and parental history at the time of hospitalization, (3) young children who were born <37 weeks gestational age, or (4) young children who had a cardiac problem. Finally, 279 young children were included in the study (Fig. 1). Clinical factors collected at the time of admission included sex, age, the presence or absence of fever, laboratory data, and previous hospitalization for respiratory illnesses. We reviewed chest X-rays of children with previous admission due to respiratory illnesses and confirmed that their chest X-rays normalized prior to the current illness. On admission, all subjects underwent routine chest X-ray; blood samples were taken from subjects, and laboratory assessment such as complete blood count, absolute neutrophil count, total eosinophil count, and high-sensitivity C-reactive protein (hs-CRP) were performed. The study protocol was reviewed and approved by the Institutional Review Board of the CHA Gangnam Medical Center, CHA University, Seoul, Korea (approval number: GCI-16-23).All radiographs were read at admission by physicians and at a later date by a highly experienced radiologist, who is regarded as an expert in pediatric lung diseases (HKC). A second senior staff radiologist (THK) also read the initial 279 radiographs to confirm the “expert” reading. Both study radiologists acknowledged that the children were hospitalized due to respiratory tract illnesses, but were blinded to the details of presentation and the findings of the other participating radiologist.- 2. Collection of nasopharyngeal aspirate specimens and polymerase chain reaction
- 2. Collection of nasopharyngeal aspirate specimens and polymerase chain reaction
Nasopharyngeal aspirate specimens were collected at the time of hospitalization by carefully irrigating the young child's nostrils with sterile saline solution. Nasopharyngeal aspirate specimens were analyzed within 24 hours of aspiration for respiratory syncytial viruses (RSV) (A and B), human metapneumovirus, human rhinovirus, influenza A and B viruses, parainfluenza virus (A, B, and C), coronavirus (A and B), and adenovirus. Total viral RNA was obtained from nasopharyngeal aspirate specimens (300 µL) by utilizing Viral Gene-spin Viral DNA/RNA Extraction Kits (iNtRON, Seongnam, Korea) and stocked at −80℃. First-strand cDNA was manufactured by implementing Revert Aid First Strand cDNA Synthesis Kits (Fermentas Inc., Burlington, ON, Canada), followed by polymerase chain reaction (PCR) using Seeplex Respiratory Viruses Detection Kits-1 (Seegene, Seoul, Korea) and the GeneAmp PCR system 9700 (Applied Biosystems, Waltham, MA, USA). All reaction mixtures (20 µL) consisted of 3 µL of cDNA, 4 µL of 5× RV1A or 5× RV1B primer, and 10 µL of 2× Multiplex Master Mix. In short, 2.5 µL of extracted RNA was incorporated with 5× buffer, 0.2mM of each dNTP, 0.5µM of each primer13,14,15,16), 1 µL of the enzyme mix, and diethylpyrocarbonate-incorporated ultrapure water to a total volume of 25 µL. After being incubated at 50℃ for 30 minutes and at 94℃ for 15 minutes, the reactions underwent 40 cycles of denaturation for 30 seconds at 94℃, annealing for 90 seconds at 60℃, and extension for 90 seconds at 72℃, followed by a final extension at 72℃ for 10 minutes. The amplified specimens were analyzed on 2% agarose gels comprised of 0.5-g/mL ethidium bromide.- 3. Classification of chest radiographic findings
- 3. Classification of chest radiographic findings
The chest radiographic findings were divided into benign or pathologic groups17). Benign chest radiographic findings were defined as those consistent with uncomplicated bronchiolitis: normal chest radiographs, symmetric hyperinflation, parahilar peribronchial infiltrates, and diffuse interstitial markings17). Pathologic chest radiographic findings included infiltrates, atelectasis, cardiac abnormalities, foreign bodies, masses, and congenital anomalies17).The sample size was assessed for a 95% confidence interval (CI) for the primary outcome. After discussion by the researchers, we assessed the percentage of children with benign chest radiographic findings (i.e., bronchiolitis) and pathologic chest radiographic findings to be ≤3%±2%, with alpha=0.05 and beta=0.2, which produced a total required number of children with bronchiolitis of 26018).- 4. Statistical analysis
- 4. Statistical analysis
Data are expressed as median (interquartile range) or number (%) unless otherwise mentioned. Differences between groups were compared by implementing the Mann-Whitney U tests or the chi-square tests, when appropriate. Multivariate logistic regression analysis was incorporated to determine clinical risk factors for pathologic chest radiographic findings; independent variables were sex (male), age under 3 months, the presence of fever, hs-CRP>0.3 mg/dL, and a history of admission for respiratory illness. Birth weight, height, and head circumference; gestational age; respiratory rate, heart rate, and body temperature at admission; oxygen saturation at admission; duration of fever; hs-CRP; white blood cell count; and total eosinophil count were utilized as continuous variables. Sex, age under 3 months, the presence of fever at admission, hs-CRP (>0.3 mg/dL), bronchopulmonary dysplasia, a history of admission for respiratory illnesses, chest retraction, and wheezes were categorical variables. All statistical analyses were conducted using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). All statistical tests were two-sided, with a P value of less than 0.05 showing statistical significance.
- Results
- Results
- 1. General characteristics of the study subjects
- 1. General characteristics of the study subjects
The study population consisted of 279 children. The median age of the children was 8 months. Approximately 41% of the study subjects were male. The numbers of patients that had chest retraction and wheezing were 41 (14.7%) and 149 (53.4%), respectively. The median hospital stay was 4 days (1–14 days), and the number of children who received antibiotic therapy was 191 (68.5%). Only 26 of 279 children (9.3%) had pathologic chest radiographic findings on chest radiograph; of them, 24 children had opacities and two children had atelectasis.There were no differences between groups with regard to sex; age; birth weight, height, or head circumference; gestational age; respiratory rate, heart rate, body temperature, or oxygen saturation at admission; the presence of fever prior to admission; duration of fever prior to admission; the presence of chest retraction or wheezes; white blood cell count or total eosinophil count at admission; or virus detected. The total duration of fever (3 [0–5] days vs. 1 [0–3] day, P=0.007), hs-CRP (1.4 [0.4–4.1] mg/dL vs. 0.4 [0.1–1.4] mg/dL, P=0.02), hs-CRP (>0.3 mg/dL) (21/26 [80.8%] vs. 140/253 [55.3%], P=0.01) and a history of admission for respiratory illnesses (8/26 [30.8%] vs. 28/253 [11.1%], P=0.004) were significantly higher in children with pathologic chest radiographic findings than in those with benign chest radiographic findings (Table 1).- 2. Comparison of respiratory viruses in young children with pathologic and those with benign chest radiographic findings
- 2. Comparison of respiratory viruses in young children with pathologic and those with benign chest radiographic findings
Table 2 compares the respiratory viruses identified in the study subjects with pathologic and those with benign chest radiographic findings. RSV was identified in 6 subjects (23.1%) in the children with pathologic chest radiographic findings and 57 (22.5 %) in those with benign chest radiographic findings; human rhinovirus in 3 (11.5%) and 12 (4.7%), respectively; and human metapneumovirus in 1 (3.8%) and 7 (2.8%), respectively. There were no differences in the incidence of each virus between the groups (P>0.05 each).- 3. Prediction of the result of pathologic chest radiographic findings
- 3. Prediction of the result of pathologic chest radiographic findings
The diagnostic performance of hs-CRP levels (>0.3 mg/dL) was a sensitivity of 80.8% (95% CI, 60.7–93.5), specificity of 48.5% (95% CI, 42.5–54.6), positive predictive value of 13.0% (95% CI, 8.3–19.2), and negative predictive value of 96.4% (95% CI, 91.7–98.8) for predicting pathologic chest radiographic findings.- 4. Clinical predictors of pathologic chest radiographic findings in the study subjects
- 4. Clinical predictors of pathologic chest radiographic findings in the study subjects
We utilized multivariate logistic regression analysis to determine clinical predictors of pathologic chest radiographic findings in children admitted with the typical presentation of bronchiolitis. Multivariate logistic regression analyses results determined that after adjustment for potential confounding factors, including sex and age, pathologic chest radiographic findings were significantly positively associated with hs-CRP (>0.3 mg/dL) and a history of admission due to respiratory illnesses (Table 3).
- Discussion
- Discussion
In the present study, we aimed to determine the proportion of children with pathologic chest radiographic findings and the clinical predictors in young children hospitalized for bronchiolitis at a general hospital. We were able to demonstrate that the majority of children (90.7%) admitted with the typical presentation of bronchiolitis showed benign chest radiographic findings at admission; however, those with high hs-CRP levels at admission or a history of admission due to respiratory illnesses prior to the current admission were approximately 1.2 times more likely to have pathologic chest radiographic findings.Subjects with bronchiolitis may also have pneumonia detected by chest X-ray7,8,9,10). A previous study analyzing patients less than 24 months of age admitted via the emergency department with the diagnosis of bronchiolitis found that 15 subjects out of 122 patients (12.3%) showed abnormal chest X-ray findings such as atelectasis or consolidation8). Another study assessing 410 chest radiographs performed on children admitted with bronchiolitis through the Emergency Department suggested that 40 (9.7%) were considered abnormal7), a number similar to our finding of 9.3%. A previous study determined that young children with oxygen saturation ≤92% or a Respiratory Disease Assessment Instrument (RDAI) clinical score ≥10 of 17 points were more likely to have pathologic chest radiographic findings than their counterparts10). Another study found that body temperature ≥38℃ and oxygen saturation <94% were associated with infiltrates/atelectasis9). Finally, a recent study reported that the presence of fever was the only significant clinical predictor of radiographic abnormalities among their cohort7). In contrast to previous studies, we did not find fever or hypoxia to be a risk factor for chest abnormalities. We found that high hs-CRP levels at admission or a history of admission due to respiratory illnesses prior to the current admission were clinical factors predicting pathologic radiographic findings in this cohort. This discrepancy may be ascribed to differences in characteristics of the populations such as age, seasons during which the tests were performed, or causative agents.hs-CRP is a well-established biomarker that is used on a regular basis in clinical settings. It is an acute-phase serum protein in humans, and its level rises rapidly due to the cytokines released in response to infection and inflammation19). However, it has traditionally been viewed as inadequately accurate for use in the diagnosis of pneumonia, because almost all infective, autoimmune, metabolic, ischemic, and neoplastic disorders are associated with increased serum hs-CRP levels20). Nonetheless, several previous research works have corroborated the idea that it may have a diagnostic value in detecting pneumonia21) and disease severity22) in adults and in children23). Similar to previous research, we determined that a high hs-CRP level at admission is an independent clinical predictor of pathologic chest radiographic findings in this cohort of young children with bronchiolitis and has a high negative predictive value of 96.4%, indicating that if the subject's hs-CRP is less than 0.3 mg/dL at admission, the probability that the subject has a benign chest radiographic finding is high. However, it should be noted that the current practice guideline states that laboratory studies should not be obtained routinely on children with bronchiolitis24). In this regard, we speculate that hs-CRP could be used as a biomarker to help clinicians determine whether a chest X-ray should be performed only in cases where blood samples are obtained.A history of hospitalization due to respiratory illness is an important aspect of the history in children with respiratory problems in the outpatient department, as the respiratory signs and symptoms in children with previous respiratory problems tend to worsen, ultimately requiring hospitalization25). This may be due to the fact that these subjects have chronic respiratory illnesses such as asthma, thus predisposing them to developing severe respiratory illness. However, unfortunately, we did not evaluate every patient's asthma history and their family history of asthma.There are some limitations to the present study. First, the current study has inherent limitations due to its retrospective study design. Second, the present study is a single center study conducted at a secondary hospital. Third, lung function measures or objective assessments such as the RDAI clinical score were not available to determine the degree of respiratory difficulty. Fourth, this study lacks a diagnostic “gold standard” to prove the absence of bacterial infection in children with chest abnormalities. It would be ideal to perform chest radiography after a complete review of the expected benefits of chest X-ray in subjects (e.g., subjects with toxic appearance, unconventional presentations, or protracted clinical course). Furthermore, the current research work had only 2 children with atelectasis on their chest x-ray, and recognition of any factors predicting this high-risk finding was beyond calculation.In conclusion, we found that chest radiographs in young children with a typical presentation of bronchiolitis have limited value; however, those with high hs-CRP levels or a history of admission due to respiratory illnesses prior to admission were approximately 1.2 times more likely to have pathologic chest radiographic findings. Further studies with larger samples sizes are needed to corroborate this finding before a clear conclusion can be drawn.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381:1380–1390.
[Article] [PubMed] [PMC]2. Pelletier AJ, Mansbach JM, Camargo CA Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics 2006;118:2418–2423.
[Article] [PubMed]3. Stang P, Brandenburg N, Carter B. The economic burden of respiratory syncytial virus-associated bronchiolitis hospitalizations. Arch Pediatr Adolesc Med 2001;155:95–96.
[Article] [PubMed]4. Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics 2013;132:28–36.
[Article] [PubMed] [PMC]5. Roh EJ, Won YK, Lee MH, Chung EH. Clinical characteristics of patients with acute bronchiolitis who visited 146 Emergency Department in Korea in 2012. Allergy Asthma Respir Dis 2015;3:334–340.
[Article]6. Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics 2005;115:878–884.
[Article] [PubMed]7. Ecochard-Dugelay E, Beliah M, Perreaux F, de Laveaucoupet J, Bouyer J, Epaud R, et al. Clinical predictors of radiographic abnormalities among infants with bronchiolitis in a paediatric emergency department. BMC Pediatr 2014;14:143
[Article] [PubMed] [PMC]8. Galván RC, Cuello García CA. Chest x-ray: an unnecessary resource in the diagnosis of acute bronchiolitis. Bol Med Hosp Infant Mex 2011;68:195–201.9. García García ML, Calvo Rey C, Quevedo Teruel S, Martínez Pérez M, Sánchez Ortega F, Martín del Valle F, et al. Chest radiograph in bronchiolitis: is it always necessary? An Pediatr (Barc) 2004;61:219–225.
[Article] [PubMed]10. Schuh S, Lalani A, Allen U, Manson D, Babyn P, Stephens D, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr 2007;150:429–433.
[Article] [PubMed] [PMC]11. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774–1793.
[Article] [PubMed]12. Chung HL, Kim SG, Shin IH. The relationship between serum endothelin (ET)-1 and wheezing status in the children with Mycoplasma pneumoniae pneumonia. Pediatr Allergy Immunol 2006;17:285–290.
[Article] [PubMed]13. Vabret A, Dina J, Gouarin S, Petitjean J, Corbet S, Freymuth F. Detection of the new human coronavirus HKU1: a report of 6 cases. Clin Infect Dis 2006;42:634–639.
[Article] [PubMed]14. Vabret A, Mourez T, Dina J, van der Hoek L, Gouarin S, Petitjean J, et al. Human coronavirus NL63, France. Emerg Infect Dis 2005;11:1225–1229.
[Article] [PubMed] [PMC]15. Vabret A, Mouthon F, Mourez T, Gouarin S, Petitjean J, Freymuth F. Direct diagnosis of human respiratory coronaviruses 229E and OC43 by the polymerase chain reaction. J Virol Methods 2001;97:59–66.
[Article] [PubMed] [PMC]16. Bellau-Pujol S, Vabret A, Legrand L, Dina J, Gouarin S, Petitjean-Lecherbonnier J, et al. Development of three multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods 2005;126:53–63.
[Article] [PubMed] [PMC]17. Farah MM, Padgett LB, McLario DJ, Sullivan KM, Simon HK. First-time wheezing in infants during respiratory syncytial virus season: chest radiograph findings. Pediatr Emerg Care 2002;18:333–336.
[Article] [PubMed]18. McClave JT, Dietrich FH. Statistics. 4th ed. San Francisco (CA): Dellen Pub. Co., 1988.19. Du Clos TW, Mold C. The role of C-reactive protein in the resolution of bacterial infection. Curr Opin Infect Dis 2001;14:289–293.
[Article] [PubMed]20. Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med 2006;119:166.e17–166.e28.
[Article] [PubMed]21. García Vázquez E, Martínez JA, Mensa J, Sánchez F, Marcos MA, de Roux A, et al. C-reactive protein levels in community-acquired pneumonia. Eur Respir J 2003;21:702–705.
[Article] [PubMed]22. Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 2008;121:219–225.
[Article] [PubMed]23. Lakhani D, Muley P. The association of positive chest radiograph and laboratory parameters with community acquired pneumonia in children. J Clin Diagn Res 2013;7:1629–1631.
[PubMed] [PMC]
Table 1
Demographic and clinical characteristics of young children with pathologic and those with benign chest radiographic findings
Table 2
Viruses identified in young children with pathologic and those with benign chest radiographic findings
Table 3
Assessment of the independent relationships between chest X-ray abnormalities and clinical variables in young children hospitalized with bronchiolitis

 About
About Browse articles
Browse articles For contributors
For contributors