All issues > Volume 60(1); 2017
The outcome of short-term low-dose aspirin treatment in Kawasaki disease based on inflammatory markers
- Corresponding author: Hong Ryang Kil, MD, PhD. Department of Pediatrics, Chungnam National University Hospital, Chungnam National University School of Medicine, 282 Munhwa-ro, Jung-gu, Daejeon 35015, Korea. Tel: +82-42-280-7251, Fax: +82-42-255-3158, gilhongr@gmail.com
- Received September 08, 2016 Revised October 17, 2016 Accepted October 21, 2016
- Abstract
-
- Purpose
- Purpose
- Previously, Kawasaki disease (KD) treatment with low-dose aspirin was administered for 6–8 weeks after the acute phase. However, inflammatory marker levels normalize before 6–8 weeks. In this study, we aimed to investigate the clinical outcome of short-term low-dose aspirin treatment based on inflammatory and thrombotic marker levels.
- Methods
- Methods
- We performed a retrospective review of the medical records of patients with KD who were hospitalized at Chungnam National University Hospital between September 2012 and May 2014. When fever subsided, low-dose aspirin treatment was started. Inflammatory (white blood cell count, erythrocyte sedimentation rate, and C-reactive protein) and thrombotic markers (D-dimer) were monitored at follow-ups conducted in 1- to 2-week intervals. The low-dose aspirin administration was terminated when both markers were normalized and no cardiovascular complications were observed.
- Results
- Results
- Eighty-four patients with KD (complete KD, n=49; incomplete KD, n=35) were enrolled. The inflammatory and thrombotic marker levels were normalized within 3–4 weeks on average. At the beginning the low-dose aspirin treatment, 9 patients had coronary artery lesions but 75 did not. When the low-dose aspirin administration was terminated at the time the inflammatory marker levels were normalized, no new CALs developed during the follow-up at 6–8 weeks.
- Conclusion
- Conclusion
- Most of the inflammatory marker levels were normalized within 3–4 weeks after the acute phase of KD. New cardiovascular complications did not develop during the course of the short-term aspirin treatment based on the inflammatory marker levels, clinical findings, and echocardiography.
- Introduction
- Introduction
Kawasaki disease (KD) is a systemic vasculitis of unknown cause that occurs primarily in children under 6-years-old. Coronary artery lesions (CALs) occur in 15%–20% of untreated cases and in 3%–5% of properly treated cases. In developed countries, KD is the commonest cause of acquired heart disease in childhood1,2,3,4).In KD, the risk of vascular injury and thrombosis increases due to endothelial cell injury and platelet activation caused by systemic vasculitis. After the acute phase of KD, low-dose aspirin (3–5 mg/kg/day) is recommended for an antiplatelet effect5). After the inflammatory markers have normalized and no echocardiographic evidence of CALs exists, new CAL does not occur during long-term follow-up. The duration of low-dose aspirin treatment is determined to 6–8 weeks conventionally, not evidence based6). However, platelet activation has been reported to increase during the first 3 months after disease onset, but it has also persisted for over a year in some studies. The endothelial cell dysfunction can last from several months to several years7). It is difficult to assess the endothelial cell dysfunction and platelet activation as well as clinical marker to apply in clinical setting. Few studies have performed the timing of inflammatory and thrombotic marker normalization after the acute phase of KD and the cardiovascular outcome of low-dose aspirin treatment based on inflammatory markers.The present study investigated the clinical outcome of short-term low-dose aspirin treatment based on levels of inflammatory and thrombotic markers
- Materials and methods
- Materials and methods
- 1. Study population
- 1. Study population
The study group was composed of KD patients admitted to the Chungnam National University Hospital, South Korea between September 2012 and May 2014. KD diagnosis was based on clinical features established by the Japanese Circulation Society (JCS), 20132). The 6 major findings includes : (1) fever persisting for ≥5 days; (2) bilateral bulbar conjunctival injection, without exudate; (3) changes to the lips and oral cavity, including erythema, lip cracking, strawberry tongue, and diffuse injection of the oral and pharyngeal mucosa; (4) polymorphous exanthema; (5) changes in the peripheral extremities, including redness of the palms and soles, indurative edema during the acute phase, and membranous desquamation of the finger tips during the subacute phase; and (6) nonpurulent cervical lymphadenopathy (>1.5 cm). Patients with at least 5 of the above 6 major findings or with 4 of the 6 major findings in whom revealed CALs during illness diagnosed as complete KD. A diagnosis of incomplete KD is made for those who meet 4 of the 6 findings but no CALs and those who have 3 of the 6 findings and have CALs. CALs on echocardiography is defined if any of 2 conditions are met described in the American Heart Association (AHA) guidelines: (1) a left anterior descending artery or right coronary artery Z-score≥2.5; (2) coronary arteries meeting the Japanese Ministry of Health criteria for aneurysms1,2).The study groups were divided in CAL and non-CAL groups based on the echocardiographic finding at the discharge. Patients not receiving high-dose intravenous immunoglobulin (IVIG) treatment at acute phase, with recurrence, or having the onset of infectious disease during follow-up were excluded from the study.- 2. Study methods
- 2. Study methods
Clinical characteristics including the cardiovascular symptoms, laboratory data, electrocardiogram, echocardiographic findings, and Harada scores were retrospectively reviewed from medical records. White blood cell (WBC) counts, hemoglobin (Hb) levels, platelet counts, erythrocyte sedimentation rates (ESRs), and levels of aspartate transaminase (AST), alanine transaminase (ALT), C-reactive protein (CRP), and D-dimers were determined.During the acute phase of KD, IVIG (2 g/kg) and moderate-dose aspirin (30–50 mg/kg/day) were administered. Refractory to IVIG was defined as persistent or recrudescent fever ≥36 hours after completion of the initial IVIG infusion. When the fever subsided, the dose of aspirin was decreased to 3–5 mg/kg/day. Thereafter patients were discharged after 48 hours of monitoring. Follow-up observations were made every 7–14 days after discharge. In the present study, WBCs, ESRs, and CRP levels as inflammation markers and platelet numbers and D-dimer levels as thrombotic markers were investigated during follow-up. When both inflammatory and thrombotic markers normalized and no CALs detected on echocardiographic examination, low-dose acetylsalicylic acid (ASA) treatment was terminated. Normalization of inflammatory and thrombotic markers was defined as WBCs, 6000–15,000/µL; platelets, 130,000–400,000/µL; CRP, 0–0.5 mg/dL; ESR, 0–20 mm/hr, and D-dimers, 0–1.0 µg/mL.Echocardiographic examination was performed twice during hospitalization (immediately prior to IVIG administration and before discharge) and twice during the follow-up observations (upon termination of low-dose ASA administration, when inflammatory and thrombotic markers had normalized, and 6–8 weeks after the onset of KD). Echocardiographic evaluation included the regional wall motion, systolic and diastolic function, status of coronary arteries, and the presence of thrombus within coronary arteries.- 3. Statistical analysis
- 3. Statistical analysis
SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used to perform unpaired t-tests on the entire KD patient population and on each subgroup. All values were expressed as means±standard deviation, and statistical significance was set as P<0.05.- 4. Ethics statement
- 4. Ethics statement
The study protocol was approved by the Institutional Review Board of Chungnam National University Hospital (approval number: 2016-07-027-001).
- Results
- Results
- 1. Clinical and laboratory characteristics on admission
- 1. Clinical and laboratory characteristics on admission
A total of 84 patients (49 patients with complete KD and 35 patients with incomplete KD) were enrolled in this study. Among the KD patients, the sex ratio of males to females was 1.35:1, the mean age at the time of onset was 2.38±1.61 years. There were no significant differences in the patients' clinical characteristics (age, sex, Harada score)8) and laboratory findings (WBC, Hb, platelet, ESR, CRP, AST ALT, albumin, D-dimer) on admission according to the presence of CALs at discharge (Table 1).- 2. Times to inflammatory and thrombotic markers normalization
- 2. Times to inflammatory and thrombotic markers normalization
The times to normalization of the inflammatory and thrombotic markers after the fever had subsided were 11.27±11.24 days (platelets), 14.09±6.59 days (CRP), 14.05±8.79 days (D-dimer), and 22.25±7.42 days (ESR). Normalization of the platelet counts and CRP levels occurred within 2–3 weeks on average with most values normalized within 4–5 weeks; ESR levels normalization occurred within 3 weeks on average with most values normalized within 5 weeks. The platelet counts normalized earlier and ESR levels normalized later. There were no statistically significant differences according to clinical criteria of KD or the presence of CALs (Table 2).- 3. The relationship between clinical profiles and inflammatory marker normalization
- 3. The relationship between clinical profiles and inflammatory marker normalization
The times required to normalize the levels of inflammatory markers including ESR and CRP levels were not significantly different based on clinical profiles (sex, duration of fever, response to IVIG treatment, or Harada score). The ESR level tended to show the slowest improvement in the patients who were not respondent to IVIG treatment (30.88±7.43 days, P=0.38), both ESR and CRP level tended to show the slowest improvement in boys (15.42 ±4.77 and 15.84±4.63 days, P=0.56 and P=0.51) (Table 3).- 4. Cardiovascular outcomes of short-term low-dose aspirin treatment
- 4. Cardiovascular outcomes of short-term low-dose aspirin treatment
At discharge, there were 9 patients with CALs (complete KD, 6; incomplete KD, 3) and 75 patients without CALs (complete KD, 43; incomplete KD, 32). In echocardiographic examination at normalization of inflammatory and thrombotic markers, 4 of 9 patients with CALs showed regression of the CALs and the low-dose aspirin treatment were terminated. Among 75 patients without CALs at discharge, there were no evidence of new CALs, myocardial ischemia or intravascular thrombus at 6–8 weeks after acute phase of KD (Fig. 1).
- Discussion
- Discussion
KD is an acute, self-limiting vasculitis of unknown etiology that occurs predominantly in infants and young children. Even if the morphology of the coronary arteries appears normal on echocardiography, vascular endothelial injury and dysfunction may persist after the convalescent phase9). During the acute phase of KD, platelet function and fibrinolysis abnormalities appear and coagulation abnormalities may persist for several months to several years. The low-dose aspirin treatment for 6–8 weeks after the acute phase is based on studies indicating that new morphologic coronary artery abnormalities do not occur more than 6–8 weeks after the onset of KD10). A study on adult patients with ischemic heart disease reported that aspirin resistance appeared frequently due to inadequate dosages, drug interactions, and genetic polymorphism11). The clinical measurement of endothelial cell function, platelet function, and coagulation abnormalities is difficult in pediatric patients; therefore, a scientific basis does not exist which indicators should be used to determine the duration of low-dose aspirin treatment.Treatment with low-dose aspirin (3–5 mg/kg) for 6–8 weeks after the acute stage of KD is reasonably consistent, globally, since both CALs and inflammatory marker levels show improvement within this time frame. However, the duration of follow-up and the guideline of measurement of inflammatory markers and cardiovascular function parameters varies by institutes. Oh et al.12) conducted follow-up examinations on 282 KD patients, 6 weeks after low-dose aspirin therapy. ESRs and CRP levels had not normalized in 12.4% and 4.3% of the patients, respectively. They also found thrombocytosis and WBC abnormalities in 12.8% and 5.3% of these patients, respectively. However, in the present study, CRP, platelet, and D-dimer levels generally normalized within 2 weeks and the ESRs within 4 weeks, inconsistent with previous study. The differences in these findings may be attributed to differences between the subjects in the 2 studies, as well as the recent increased prevalence of incomplete KD. Moreover, because the KD group consisted of children ≤5-years-old, the prevalence of infectious disease was higher than in other age groups, which may have impacted inflammatory marker levels. In the present study, patients developing any infectious disease during the follow-up period were excluded. The results suggested that the most of inflammatory and thrombotic markers had mostly normalized within 3–4 weeks after the acute phase.In KD, the known risk factors of developing CALs include clinical characteristics, such as age, sex, duration of fever, and refractory to IVIG, as well as inflammatory marker levels, such as Hb, platelet, neutrophil, band fraction, serum sodium levels, albumin and immunoglobulin. Several scoring systems have been developed to identify children at high risk of IVIG resistance and developing CAL2,13,14,15,16,17). In the present study, there were no significant differences in the times to CRP and ESR normalization associated with sex, fever duration, retreatment, Harada score, or the presence of CALs at discharge. These results suggest that although these risk factors may influence IVIG responsiveness or the occurrence of acute-phase CALs, they have little effect on normalization of inflammatory markers following the acute phase.Scott et al.18) reported that most patients with echocardiographic abnormalities at 2 weeks after CAL onset do not demonstrate new CAL development in any later examinations. Echocardiography is mandatory at the time of diagnosis and 4–6 weeks after onset, but additional echocardiography is recommended for patients with cardiovascular abnormalities, beyond the standard 4–6 weeks. A multicenter study by McMorrow Tuohy et al.19) showed that patients with normal coronary arteries at 2 months after KD did not show any new CAL occurrences. Additionally, although CALs were present during the early stage of KD, they resolved within 1–2 months, without further progression. Based on these results, follow-up observation of patients without CALs are recommended, by the AHA, at 4–6 weeks and 6–12 months from disease onset1); by the JCS, at 1, 2, 6, and 12 months from disease onset2). In the present study, when the serum inflammatory markers had normalized and CALs were not evident on echocardiograms, low-dose aspirin treatment was terminated about 3–4 weeks of KD onset. New CALs were not subsequently discovered at 6- to 8-week follow-up in patients without CAL at discharge. Similarly, four of the 9 patients with CALs at discharge showed regression of CAL during the follow-up period, consistent with previous studies. The echocardiography results were similar between short-term aspirin therapy based on inflammatory markers and conventional therapy. Low-dose aspirin treatment with antiplatelet effect targeted to abnormal platelet activation, which may persist for months after acute KD as demonstrated by increased serum markers of platelet activation and enhanced platelet aggregation6). So there are some limitations of inflammatory markers as surrogate determining the duration of low-dose aspirin treatment. Present study suggested that if aspirin treatment duration is based on inflammatory markers, clinical findings, and echocardiography, the duration of low-dose aspirin treatment may be reduced without negatively impacting the occurrence of cardiovascular lesions.The limitations of the present study include its being a single-center study with a small sample size; its retrospective design; the determination of incomplete KD is made for those who meet 4 of the 6 findings but no CALs and those who have 3 of the 6 findings and have CALs, possibly resulting in over-diagnosis; and the absence of data regarding platelet activation and aggregation, endothelial function, blood coagulation status, and aspirin resistance as well as long-term outcome after 6–8 weeks of low-dose aspirin.In conclusion, this study showed that the majority of the inflammatory marker levels were normalized within 3–4 weeks after the acute phase of KD. Additionally, new cardiovascular complications did not develop in short-term aspirin treatment based on inflammatory markers, clinical findings, and echocardiography.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004;114:1708–1733.
[Article] [PubMed]2. JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest version. Circ J 2014;78:2521–2562.
[Article] [PubMed]3. Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J 2014;33:24–27.
[Article] [PubMed]4. Kang HJ, Kim GN, Kil HR. Changes of clinical characteristics and outcomes in patients with Kawasaki disease over the past 7 years in a single center study. Korean J Pediatr 2013;56:389–395.
[Article] [PubMed] [PMC]5. Akagi T, Kato H, Inoue O, Sato N. Salicylate treatment in Kawasaki disease: high dose or low dose? Eur J Pediatr 1991;150:642–646.
[Article] [PubMed]6. Williams RV, Tcheng WY, Minich LL. Anticoagulation in the acute and long-term management of Kawasaki disease. Prog Pediatr Cardiol 2004;19:179–188.
[Article]7. Israels SJ, Michelson AD. Antiplatelet therapy in children. Thromb Res 2006;118:75–83.
[Article] [PubMed]8. Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn 1991;33:805–810.
[Article] [PubMed]9. Yamakawa R, Ishii M, Sugimura T, Akagi T, Eto G, Iemura M, et al. Coronary endothelial dysfunction after Kawasaki disease: evaluation by intracoronary injection of acetylcholine. J Am Coll Cardiol 1998;31:1074–1080.
[Article] [PubMed]10. Dhillon R, Clarkson P, Donald AE, Powe AJ, Nash M, Novelli V, et al. Endothelial dysfunction late after Kawasaki disease. Circulation 1996;94:2103–2106.
[Article] [PubMed]12. Oh IJ, Moon KH, Hong ME, Kim YS, Lee CW, Yoon HS. Clinical significance of follow-up laboratory tests, performed at 6 weeks after the onset of Kawasaki disease. Korean J Pediatr 2006;49:672–676.
[Article]13. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics 2000;105:E78
[Article] [PubMed]14. Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J Pediatr 2000;137:177–180.
[Article] [PubMed]15. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 2006;149:237–240.
[Article] [PubMed]16. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 2007;166:131–137.
[Article] [PubMed]17. Kobayashi T, Inoue Y, Tamura K, Morikawa A, Kobayashi T. External validation of a scoring system to predict resistance to intravenous immunoglobulin. J Pediatr 2007;150:e37
[Article] [PubMed]
Fig. 1
The cardiovascular outcome of short-term low-dose aspirin treatment. KD, Kawasaki disease; CAL, coronary artery lesion; D/C, discharge.
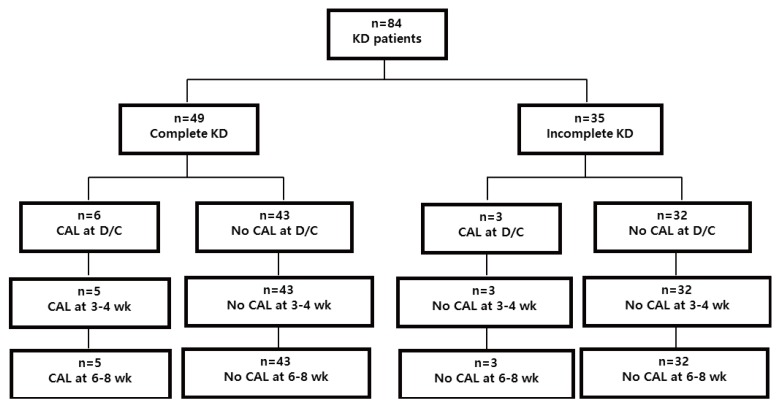
Table 1
Clinical and laboratory characteristics of the patients with Kawasaki disease on admission
Values are presented as mean±standard deviation unless otherwise indicated.
KD, Kawasaki disease; CAL, coronary artery lesions; WBC, white blood cells; Hb, hemoglobin; Hct, hematocrit; Plt, platelets; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate transaminase; ALT, alanine transaminase.
P>0.05, Non-CAL vs. CAL and complete KD vs. incomplete KD.
Table 2
Times to normalization of the inflammatory marker levels of the patients with Kawasaki disease according to the clinical criteria or CAL formation
Table 3
The relationship between the clinical profiles and the times to normalization of the inflammatory marker levels of the patients with Kawasaki disease

 About
About Browse articles
Browse articles For contributors
For contributors
