All issues > Volume 60(2); 2017
The serum level of 25-hydroxyvitamin D for maximal suppression of parathyroid hormone in children: the relationship between 25-hydroxyvitamin D and parathyroid hormone
- Corresponding author: Hae Soon Kim, MD. Department of Pediatrics, Ewha Womans University School of Medicine, 1071 Anyangcheon-ro, Yangcheon-gu, Seoul 07985, Korea. Tel: +82-2-2650-5569, Fax: +82-2-2650-3718, hyesk@ewha.ac.kr
- Received July 16, 2016 Revised October 18, 2016 Accepted October 25, 2016
- Abstract
-
- Purpose
- Purpose
- Serum level of 25-hydroxyvitamin D (25-OHD) is considered as the most appropriate marker of vitamin D status. However, only a few studies have investigated the relationship between 25-OHD and parathyroid hormone (PTH) in children. To this end, this study was aimed at evaluating the lowest 25-OHD level that suppresses the production of parathyroid hormone in children.
- Methods
- Methods
- A retrospective record review was performed for children aged 0.2 to 18 years (n=193; 106 boys and 87 girls) who underwent simultaneous measurements of serum 25-OHD and PTH levels between January 2010 and June 2014.
- Results
- Results
- The inflection point of serum 25-OHD level for maximal suppression of PTH was at 18.0 ng/mL (95% confidence interval, 14.3–21.7 ng/mL). The median PTH level of the children with 25-OHD levels of <18.0 ng/mL was higher than that of children with 25-OHD levels ≥ 18.0 ng/mL (P<0.0001). The median calcium level of children with 25-OHD levels<18.0 ng/mL was lower than that of children with 25-OHD levels≥18.0 ng/mL (P=0.0001). The frequency of hyperparathyroidism was higher in the children with 25-OHD levels<18.0 ng/mL than in the children with 25-OHD levels≥18.0 ng/mL (P<0.0001). Hypocalcemia was more prevalent in the children with 25-OHD levels<18.0 ng/mL than in the children with 25-OHD levels≥18.0 ng/mL (P<0.0001).
- Conclusion
- Conclusion
- These data suggest that a vitamin D level of 18.0 ng/mL could be the criterion for 25-OHD deficiency in children at the inflection point of the maximal suppression of PTH.
- Introduction
- Introduction
Vitamin D is an essential nutrient for calcium and phosphorus absorption and bone metabolism. Parathyroid hormone (PTH) concentration reflects the status of vitamin D, and these two elements are important in the calcium-phosphate homeostatic system1).Vitamin D is synthesized through exposure to the sun2,3), and it is hydroxylated to 25-hydroxyvitamin D (25-OHD) in the liver and is then finally activated to 1,25-dihydroxyvitamin D in the kidney. The serum 25-OHD level is used as an indicator of vitamin D status1,3,4,5).Serum PTH level has regarded as an index of sufficient vitamin D nutriture, especially on the basis of the level of 25-OHD at which serum PTH level increases or no longer decreases6). Vitamin D deficiency reduces serum calcium levels, leading to PTH synthesis7). Subsequently, secondary hyperparathyroidism leads to osteomalacia, irreversible bone loss, an increased risk of bone fractures, other chronic diseases, and metabolic syndrome1,3,4). In other words, the 25-OHD level is inversely correlated with PTH, and the lowest vitamin D level at which PTH begins to rise (inflection point) has been used to define vitamin D deficiency7).Vitamin D deficiency prevalence is increasing in children and adolescents in South Korea due to the limited outdoor activity and popular use of sunscreen 2,8). According to the Korean National Health and Nutrition Examination Survey 2008, the frequency of vitamin D deficiency was 47.3% in boys and 64.5% in girls7). Vitamin D deficiency affects higher risk in children9).However, there is a lack of agreement as to the definition of vitamin D deficiency possibly as a result of differences in the study populations or in the assays used to measure 25-OHD10). The Pediatric Endocrine Society has defined Vitamin D deficiency when the serum 25-OHD level is below 15 ng/mL whereas the Endocrine Society has defined it as 20 ng/mL in adults and children5,7,11).The aim of this study is thus to evaluate the relationship between 25-OHD and PTH and to define the vitamin D deficiency in a pediatric population.
- Materials and methods
- Materials and methods
A retrospective record review was carried out at Ewha Woman's Mokdong Hospital from January 2010 to June 2014 with children aged 0.2 to 18 years (n=193; 106 boys and 87 girls) undergoing simultaneous measurement of serum 25-OHD and PTH levels due to suspected vitamin D deficiency. The serum PTH, 25-OHD, calcium and phosphorus levels were determined, and children who were diagnosed with any renal disease or hypoparathyroidism were excluded from this study.The sera of the study group were analyzed for 25-OHD and PTH level via electrochemiluminescence immunoassay (Modular E170, Roche, Basel, Switzerland). The X-ray findings for rickets were checked.Scatterplots and correlation analysis were employed to assess the relationship of calcium and phosphorus with PTH. The associations between 25-OHD and PTH, calcium and phosphorus were assessed using LOESS (locally weighted regression and smoothing scatterplots). A piecewise linear regression model with the smallest mean standard error was used to identify the best inflection point between PTH and 25-OHD. The subjects were divided in 2 groups according to the PTH value at the inflection point (and its upper and lower 95% confidence limits). Then, the calcium, phosphorus and PTH levels for both groups were compared using a t test or a Mann-Whitney test, and the proportion of subjects with hyperparathyroidism (PTH>65 pg/mL), hypocalcemia (calcium<8.8 mg/dL), hypophsphatemia (phosphorus <3.8 mg/dL) and rickets (radiological finding) were compared using a chi-square test. All of the statistical analyses were conducted using the SAS ver. 9.2, (SAS Institute Inc., Cary, NC, USA).This study was approved by the Institutional Review Board of Ewha Woman's Mokdong Hospital (approval number: EUMC 2016-01-024-001). The need for informed consent was waived by the board.
- Results
- Results
The final results for 193 children between 0.2 and 18 years of age (mean±standard deviation, 2.7±4.6 years) indicated a mean serum 25-OHD level of 25.9 ng/mL (range, 3.07–79.8 ng/mL). Table 1 shows the laboratory results for the subjects.The relationship between calcium, phosphorus and the 25-OHD level is shown in Fig. 1. There was a significant, positive correlation between the 25-OHD and serum calcium levels, but there was no significant correlation between the 25-OHD and phosphorus levels. A locally weighted regression analysis revealed an inverse relationship between PTH and 25-OHD. The piecewise regression analysis indicated that the best inflection point was a 25-OHD level of 18.0 ng/mL (95% confidence interval [CI], 14.3– 21.7 ng/mL).Fig. 2 presents the distribution of serum PTH, calcium and phosphorus level with the 25-OHD level of 18.0 ng/mL, which is the inflection point of the PTH-25-OHD relation curve. The median PTH level of children with 25-OHD<18.0 ng/mL was higher than that of children with 25-OHD≥18.0 ng/mL (62.1 [5.6–445.1] ng/L vs. 24.2 [4.4–201.8] ng/L). The median calcium level of children with 25-OHD<18.0 ng/mL was lower than that of children with 25-OHD≥18.0 ng/mL (9.1 [5.6–10.5] mg/dL vs. 9.4 [7.9–10.8] mg/dL). The median phosphorus level of children with 25-OHD<18.0 ng/mL was lower than that of children with 25-OHD≥18.0 ng/mL (4.9 [2.3–7.8] mg/dL mg/dL vs. 5.2 [2.1–8.2] mg/dL).The proportion of subjects with hyperparathyroidism, hypocalcemia, hypophosphatemia, and rickets was compared between the 2 groups according to the serum 25-OHD level at the inflection point (18.0 ng/mL) (Table 2). The proportion of hyperparathyroidism (PTH>65 ng/L) was higher in children with 25-OHD <18.0 ng/mL compared to that for children with 25-OHD≥18.0 ng/mL (49.2% vs. 6.1%, P<0.0001). The frequency of hypocalcemia (calcium<8.8 mg/dL) was higher in children with 25-OHD <18.0 ng/mL compared to that for children with 25-OHD≥18.0 ng/mL (39.3% vs. 11.4%, P<0.0001). The frequency of hypophosphatemia (phosphorus<3.8 mg/dL) was higher in children with 25-OHD<18.0 ng/mL compared to that in children with 25-OHD≥ 18.0 ng/mL (24.6% vs. 9.9%, P=0.0069).The frequency of rickets was higher in children with 25-OHD <18.0 ng/mL than in children with 25-OHD≥18.0 ng/mL (9.8% vs. 0.8%, P=0.0044).
- Discussion
- Discussion
In the human body, the vitamin D level is important to maintain calcium and phosphorus homeostasis and finally bone metabolism. Vitamin D deficiency has been reported to be prevalent around the world, and it leads to several bone diseases, like rickets, as well as other chronic diseases. Serum 25-OHD is the primary circulating form of vitamin D, and it is the best reflection of the vitamin D condition12). Therefore, the serum 25-OHD level is used to diagnose vitamin D deficiency. However, various criteria have been used as a method of measurement to determine vitamin D deficiency in study groups7,10).This study evaluated the relationship between the 25-OHD level and calcium, phosphorus and the PTH in children. During the perinatal period, the relation between calcium, 25-OHD and PTH is different from that in older children13,14), so patients younger than 2 months were excluded in our study. When the serum calcium level decreases, the PTH secretion conversely increases. A positive correlation was observed for 25-OHD level and calcium at a low 25-OHD level, but calcium showed a fixed level above the specified level of 25-OHD. In teenagers with vitamin D deficiency, serum PTH secretion rises to adjust to higher rate of bone formation for growth15). The negative relation between 25-OHD and the PTH reached a plateau at the “inflection point” of 25-OHD. This inflection point means stimulation of PTH secretion when vitamin D levels reach the limit to keep calcium homeostasis16).The frequency of hypocalcemia, hyperparathyroidism and hypophosphatemia was significantly higher in the group with the 25-OHD level<18.0 ng/mL compared with the group with ≥18.0 ng/mL (=45 nmol/L). Therefore, we presumed 18 ng/mL of 25-OHD to be the criterion for vitamin D deficiency. In a study by Crews et al.16), PTH secretion increased when the 25-OHD level did not exceed 10.0 ng/mL (=25 nmol/L) while Atapattu et al.7) observed a PTH elevation with a 25-OHD level below 13.6 ng/mL (=34 nmol/L). In comparison, our criterion for vitamin D deficiency is higher than theirs.The Pediatric Endocrine Society has defined the standard of vitamin D deficiency as a serum 25-OHD level≤15 ng/mL (=37.5 nmol/L), and the Endocrine Society states ≤20 ng/mL (=50 nmol/L) in adults and children7). In our analysis, the inflection point was 18.0 ng/mL of 25-OHD, which is within the range of those 2 criteria. Therefore, we can set the diagnosis criteria for vitamin D deficiency as a serum 25-OHD level below 18.0 ng/mL (95% CI, 14.3–21.7 ng/mL), which is based on the observation of PTH elevation in pediatric subjects. Since the definition of vitamin D deficiency by the Endocrine Society (25-OHD≤20 ng/mL) is between the inflection point and the upper CI of our study, the application of this definition for Korean children can be considered.Hill et al.10) analyzed the relationship between serum 25-OHD and PTH in children and adolescents in the United States to determine the inflection point of 25-OHD for maximal suppression of PTH. Differently from adults, the relation between 25-OHD and PTH was shown to be linear with no definite inflection point in the pediatric cohort. Malabanan17) showed that adults may need serum 25-OHD above 20 ng/mL (=50 nmol/L) to reach the appropriate PTH level.Harkness and Cromer12) reported a serum 25-OHD level of 36 ng/mL (=90 nmol/L) at the PTH plateaus of adolescent girls, and Guillemant et al.18) determined the inflection point for adolescent boys to occur at 33.2 ng/mL (=83 nmol/L). Those data thus show the difference from our study. An inflection point of serum PTH according to 25-OHD was seen in adolescent girls but not in boys, and this may suggest that the relation between 25-OHD and the PTH values can be affected by gender19). The serum PTH level normally increases during growth to support bone turnover10). Therefore, the age distribution of the study group and gender should be considered in further study.The limitation to our study was that the study group had a small size, and there was only a hospital base group. We selected the patients carrying out measurement of serum 25-OHD and PTH levels to rule out vitamin D deficiency because of their high alkaline phosphatase levels, hypocalcemia, hypophosphatemia etc. as our study group. In further study, other factors to influence to bone metabolism such as age, timing for measurement, dietary calcium intake and other problems of calcium absorption should be considered. Also, we must include a healthy pediatric population in the data analysis20,21,22,23).In conclusion, we suggested that the serum 25-OHD level of 18.0 ng/mL was the inflection point of the maximal suppression of PTH, which is compatible with other criteria regarding vitamin D deficiency.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr 2008;88:500S–506S.
[Article] [PubMed]2. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80(6 Suppl): 1678S–1688S.
[Article] [PubMed]4. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc 2011;86:50–60.
[Article] [PubMed] [PMC]5. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930.
[Article] [PubMed]6. Srivastava T, Garg U, Ruiz M, Dai H, Alon US. Serum 25(OH)-vitamin D level in children: is there a need to change the reference range based on 2011 Institute of Medicine report? Clin Pediatr (Phila) 2013;52:178–182.
[Article] [PubMed]7. Atapattu N, Shaw N, Hogler W. Relationship between serum 25-hydroxyvitamin D and parathyroid hormone in the search for a biochemical definition of vitamin D deficiency in children. Pediatr Res 2013;74:552–556.
[Article] [PubMed]8. Lee A, Kim SH, Nam CM, Kim YJ, Joo SH, Lee KR. Prevalence of vitamin D deficiency and insufficiency in Korean children and adolescents and associated factors. Lab Med Online 2016;6:70–78.
[Article]9. Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, et al. Vitamin D insufficiency in Korea: a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 2011;96:643–651.
[Article] [PubMed]10. Hill KM, McCabe GP, McCabe LD, Gordon CM, Abrams SA, Weaver CM. An inflection point of serum 25-hydroxyvitamin D for maximal suppression of parathyroid hormone is not evident from multi-site pooled data in children and adolescents. J Nutr 2010;140:1983–1988.
[Article] [PubMed] [PMC]11. Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr 2004;80(6 Suppl): 1706S–1709S.
[Article] [PubMed]12. Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int 2005;16:109–113.
[Article] [PubMed]13. Schedewie HK, Odell WD, Fisher DA, Krutzik SR, Dodge M, Cousins L, et al. Parathormone and perinatal calcium homeostasis. Pediatr Res 1979;13:1–6.
[Article] [PubMed]14. Soliman A, Salama H, Alomar S, Shatla E, Ellithy K, Bedair E. Clinical, biochemical, and radiological manifestations of vitamin D deficiency in newborns presented with hypocalcemia. Indian J Endocrinol Metab 2013;17:697–703.
[Article] [PubMed] [PMC]15. Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab 2005;90:5576–5581.
[Article] [PubMed] [PMC]16. Crews BO, Moore J, Dietzen DJ. Circulating intact parathyroid hormone is suppressed at 25-hydroxyvitamin D concentrations >25 nmol/L in children. J Pediatr Endocrinol Metab 2014;27:657–660.
[PubMed]17. Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998;351:805–806.
[Article]18. Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Peres G, et al. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int 1999;10:222–225.
[Article] [PubMed]19. Hill TR, Cotter AA, Mitchell S, Boreham CA, Dubitzky W, Murray L, et al. Vitamin D status and parathyroid hormone relationship in adolescents and its association with bone health parameters: analysis of the Northern Ireland Young Heart's Project. Osteoporos Int 2010;21:695–700.
[Article] [PubMed]20. Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005;294:2336–2341.
[Article] [PubMed]21. Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 2004;158:531–537.
[Article] [PubMed]
Fig. 1
The relationships among serum calcium (A), phosphorus (B), PTH (C), and 25-OHD levels. A serum 25-OHD level of 18.0 ng/mL was the best inflection point for maximal suppression of PTH (95% confidence interval, 14.3–21.7 ng/mL). 25-OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone.
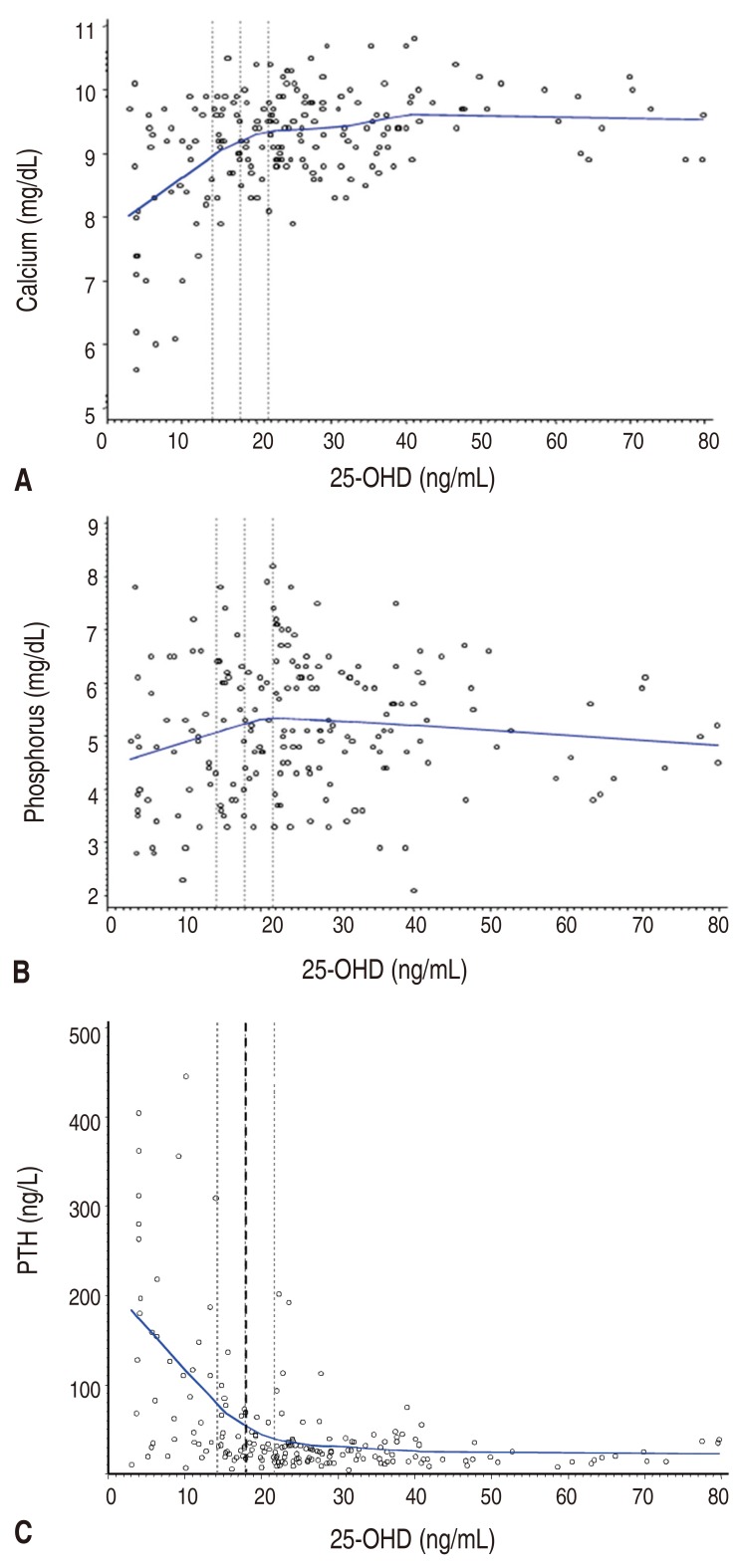
Fig. 2
The distribution of serum PTH (A), calcium (B), and phosphorus (C) levels according to serum 25-hydroxyvitamin D concentration in 18.0 ng/mL. 25-OHD, 25-hydroxyvitamin D; PTH, parathyroid hormone. (A) 25-OHD≥18.0 ng/mL. (B) 25-OHD<18.0 ng/mL.
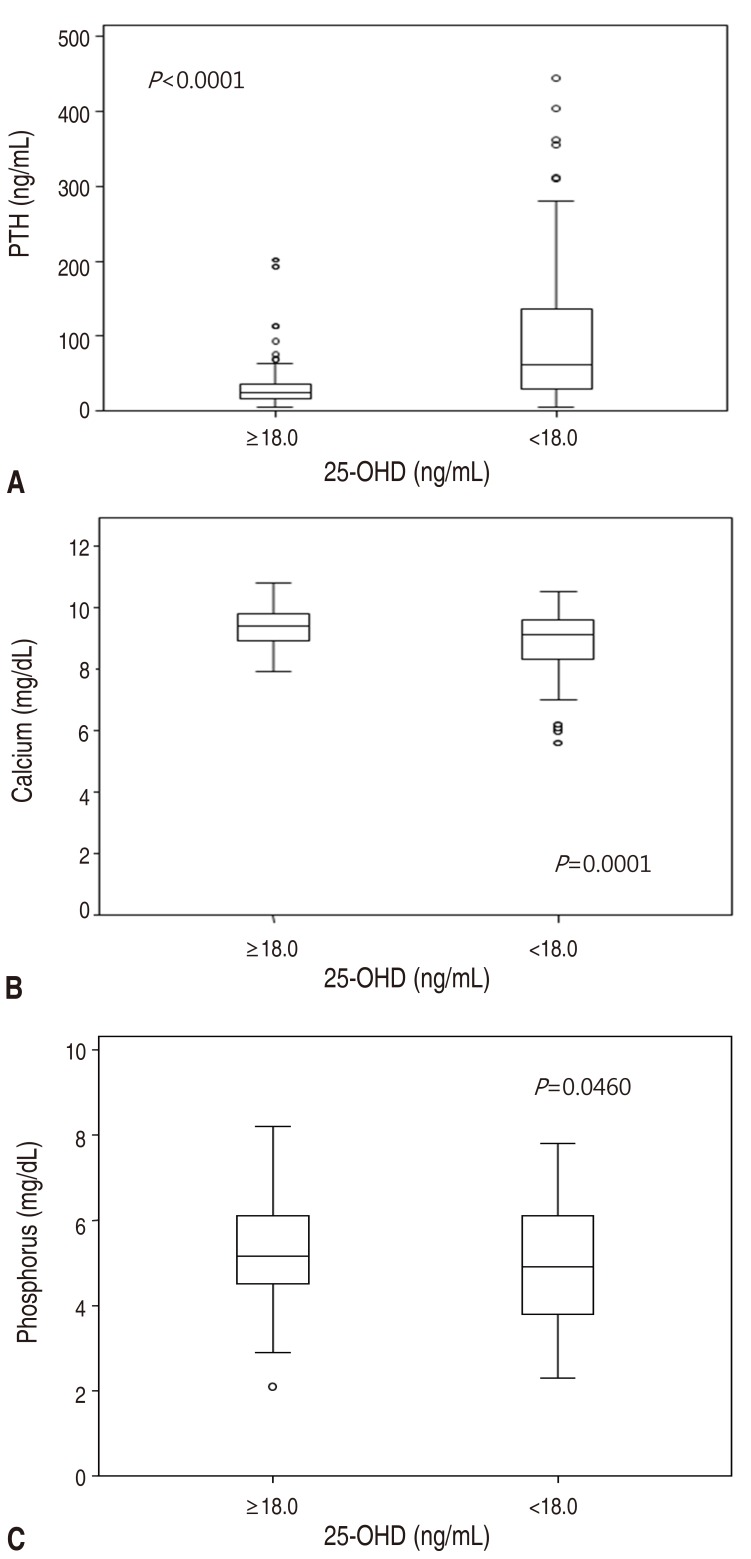
Table 1
General characteristics of subjects

 About
About Browse articles
Browse articles For contributors
For contributors
