All issues > Volume 60(9); 2017
Comparison of cytokine expression profiles in infants with a rhinovirus induced lower respiratory tract infection with or without wheezing: a comparison with respiratory syncytial virus
- Corresponding author: Yeo Hyang Kim, MD, PhD. Department of Pediatrics, Kyungpook National University School of Medicine, 680 Gukchaebosang-ro, Jung-gu, Daegu 41944, Korea. Tel: +82-53-200-5720, Fax: +82-53-425-6683, kimyhmd@knu.ac.kr
- Received February 14, 2017 Revised July 10, 2017 Accepted August 07, 2017
- Abstract
-
- Purpose
- Purpose
- The aim of this study was to evaluate whether infants with rhinovirus (RV) infection-induced wheezing and those with respiratory syncytial virus (RSV) infection-induced wheezing have different cytokine profiles in the acute stage.
- Methods
- Methods
- Of the infants with lower respiratory tract infection (LRTI) between September 2011 and May 2012, 88 were confirmed using reverse transcription polymerase chain reaction and hospitalized. Systemic interferon-gamma (IFN-γ), interleukin (IL)-2, IL-12, IL-4, IL-5, IL-13, and Treg-type cytokine (IL-10) responses were examined with multiplex assay using acute phase serum samples.
- Results
- Results
- Of the 88 patients, 38 had an RV infection (RV group) and 50 had an RSV infection (RSV group). In the RV group, the IFN-γ and IL-10 concentrations were higher in the patients with than in the patients without wheezing (P=0.022 and P=0.007, respectively). In the RSV group, the differences in IFN-γ and IL-10 concentrations did not reach statistical significance between the patients with and the patients without wheezing (P=0.105 and P=0.965, respectively). The IFN-γ and IL-10 concentrations were not significantly different between the RV group with wheezing and the RSV group with wheezing (P=0.155 and P=0.801, respectively), in contrast to the significant difference between the RV group without wheezing and the RSV group without wheezing (P=0.019 and P=0.035, respectively).
- Conclusion
- Conclusion
- In comparison with RSV-induced LRTI, RV-induced LRTI combined with wheezing showed similar IFN-γ and IL-10 levels, which may have an important regulatory function.
- Introduction
- Introduction
The most common upper respiratory tract infection in infants and children is infection by rhinovirus (RV)1). Classically, the most common cause of lower respiratory tract infection (LRTI) is the respiratory syncytial virus (RSV), which sometimes causes a severe LRTI, and as such, many infants with RSV infection require hospitalization2,3). Recent studies have demonstrated that the RV is also an important cause of LRTI in infants4,5). Although some reports have described the importance of the RV in causing an LRTI2,4,5,6), the data pertaining to the clinical significance of RV infections in infants are inconsistent. Some studies presented the evidences that RV is the main organism in 21% and 29% of infants with bronchiolitis, and other studies concluded that RV respiratory infection was not significantly associated with any age group.T cells play a crucial role in antiviral immunity. During invasion of respiratory viruses into the lung, innate immunity is activated to inhibit viral replication, followed by virus-specific adaptive immune responses. The helper T cells (TH) play key roles in the humoral and cellular adaptive immune responses, and are differentiated into TH1, TH2, TH17, and regulatory T cell (Treg) according to cytokine production profiles. In infections with RSV and RV, TH2 cells develop and Treg cells inhibit virus-specific T cell responses7,8). Nonetheless, few studies have compared the differences in the cytokine expression profiles among infants with wheezing according to the viral species responsible for the infection.The aim of this study was to evaluate whether infants with RV infection induced wheezing have different cytokine profiles on the acute stage in comparison to infants with RSV infection induced wheezing.
- Materials and methods
- Materials and methods
- 1. Patient population
- 1. Patient population
The enrolled patients were infants up to 12 months of age who were hospitalized from September 2011 to May 2012 with an LRTI at a single medical center. LRTI was diagnosed clinically (by presentation of crackles, wheezing, decreased breath sounds, hypoxia, and tachypnea) and radiologically (through the finding of consolidation, atelectasis, and infiltration) with or without fever. The patients with a dual viral infection or infections by metapneumovirus, adenovirus, corona virus, parainfluenza virus, influenza virus, or bocavirus were not included in the analysis. To compare the clinical characteristics and serum cytokine expression profiles, we allocated the patients into 2 groups as follows: RV infected patients and RSV infected patients.- 2. Clinical pattern
- 2. Clinical pattern
Clinical characteristics were assessed by reviewing those previously reported in the clinicians' records. Data were collected for the following parameters: age, duration of fever and cough before hospitalization, duration of fever after hospitalization, and breathing sounds. In addition, patient birth history, past history of LRTI, past history of medication use, need for oxygen therapy (evaluated via transcutaneous oxygen saturation) and corticosteroid therapy, antibiotic use, and length of hospital stay were also investigated.- 3. Sample collection and laboratory analysis
- 3. Sample collection and laboratory analysis
To identify the respiratory viral pathogens, nasal or throat swabs were taken from each patient on the day of admission. The real-time reverse transcription polymerase chain reaction (RT-PCR) assay was performed on swabs specimens (RV 12 ACE Detection, Seegene, Seoul, Korea). This RT-PCR assay was capable of detecting metapneumovirus, adenovirus, corona virus 229E/NL63, parainfluenza virus 1/2/3, influenza A/B virus, coronavirus OC43/HKU1, RV A/B, and RSV A/B.Serum samples for cytokine analysis were obtained at admission and stored at −70℃ until assayed. Ten cytokines were assayed for in the serum samples (interferon-gamma [IFN-γ], interleukin [IL]-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, and interferon-inducible protein-10 [IP-10]) using a customized Milliplex MAP Human Cytokine/Chemokine Panel (#HCYP2MAG-62K and #HCYTOMAG-60K, Millipore, Billerica, MA, USA) and the multiplex assay with the Luminex 200 Total System (Luminex Corp., Austin, TX, USA). Minimal detectable levels were as follows: IFN-γ (0.4 pg/mL), IL-2 (0.4 pg/mL), IL-4 (4.5 pg/mL), IL-5 (0.1 pg/mL), IL-6 (0.4 pg/mL), IL-8 (0.3 pg/mL), IL-10 (0.3 pg/mL), IL-13 (0.3 pg/mL), IP-10 (1.2 pg/mL).Several laboratory values were obtained on the admission day including: white blood cell count (WBC), neutrophil subset, lymphocyte subset, erythrocyte sedimentation rate (ESR), C-reactive protein levels, and liver function test results.- 4. Data analysis
- 4. Data analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Clinical data were displayed as a median and interquartile range (Table 1) and laboratory parameters were displayed as a mean and standard deviation (Table 2). Comparisons between the groups were performed using the nonparametric Mann-Whitney U test. Correlations between cytokine concentration and clinical or laboratory data were performed by calculating the Spearman correlation coefficient (r). P values of <0.05 were considered significant.- 5. Ethics
- 5. Ethics
Study approval was obtained from the Institutional Review Board (IRB) at the Keimyung University School of Medicine (2016-02-048). Written informed consent was waived by IRB.
- Results
- Results
- 1. Patients' characteristics
- 1. Patients' characteristics
Total 123 infants were confirmed by respiratory virus RT-PCR and hospitalized. Of these 123 patients, a total of 88 patients were enrolled in this study, including 38 with RV infections (RV group) and 50 with RSV infections (RSV group). The characteristics of each group are listed in Table 1.Of the patients, 62% were male, and the proportion of each sex was not significantly different between the groups. The median age at admission was 3.5 months (interquartile range [IQR], 1–6 months) in the RV group and 2.7 months in the RSV group (IQR, 2–4 months), which were not significantly different between the groups. The proportion of preterm babies that were born was not significantly different between the groups. The RV group had more patients who had a past history of LRTI or neonatal pulmonary disease compared to the RSV group (Table 1) (P<0.001)- 2. Clinical characteristics
- 2. Clinical characteristics
The clinical characteristics for each patient are listed in Table 1. The duration of fever and cough before hospitalization were not significantly different between the groups. On the 1st day of admission, 2 patients in the RV group and 2 patients in the RSV group experienced hypoxia (room air saturation<92%)9), and the frequency of hypoxia on the 1st day of admission was not significantly different between two groups. Wheezing was present in 48% of the patients (45% of patients in the RV group and 74% of patients in the RSV group), and the frequency of wheezing was higher in the RSV group than in the RV group (P=0.005).No significant differences were found between the 2 groups with regards to the duration of fever after hospitalization, administration of antibiotics, and duration of hospital stay. When patients showed aggravated respiratory symptoms (dyspnea or hypoxia) during hospitalization, we added oxygen therapy and systemic corticosteroid administration. Despite of our policy for treatment, the requirement for additional oxygen therapy was not significantly different between the 2 groups, However, the number of patients that required systemic corticosteroid administration was greater in the RSV group than in to the RV group (P=0.001).- 3. Laboratory tests
- 3. Laboratory tests
The RV group showed a higher WBC count, neutrophil subset and ESR, and a lower lymphocyte subset in comparison to the RSV group (P=0.025, P=0.003, P=0.001, and P=0.002, respectively) (Table 2). The other serologic and radiologic results were not significantly different between the 2 groups.- 4. Cytokines profiling
- 4. Cytokines profiling
Of the T-helper 1-type cytokines, the concentration of IFN-γ was lower in the RV group compared to the RSV group, although it was not significantly different (10.29±9.25 pg/mL vs. 12.23±11.23 pg/mL, P=0.474). In the RV group, the concentration of IFN-γ was higher in the patients with wheezing than in the patients without wheezing, and this finding was significantly different (14.88±9.24 pg/mL vs. 9.68±10.49 pg/mL, P=0.022) (Fig. 1). In the RSV group, the concentration of IFN-γ did not reach statistical significance between in the patients with wheezing and in the patients without wheezing (10.57±5.71 pg/mL vs. 15.19±7.96 pg/mL, P=0.105) (Fig. 1). Especially, the concentration of IFN-γ was not significantly different between the RV group with wheezing and the RSV group with wheezing (14.88±9.24 pg/mL vs. 10.57±5.71 pg/mL, P=0.155) (Fig. 1), in contrast with significant difference between the RV group without wheezing and the RSV group without wheezing (9.68±10.49 pg/mL vs. 15.19±7.96 pg/mL, P=0.019) (Fig. 1).The concentration of IL-12 was significantly higher in the RSV group than in the RV group (3.04±2.25 pg/mL vs. 2.36±2.86 pg/mL, P=0.028); however, there was no significant difference between patients with wheezing and without wheezing (2.71±2.66 pg/mL vs. 2.38±2.77 pg/mL, P=0.184). The concentration of IL-12 was not significantly different between the RV group with wheezing and without wheezing, between the RSV group with wheezing and without wheezing, between the RV group with wheezing and the RSV group with wheezing, and between the RV group without wheezing and the RSV group without wheezing.The concentration of IL-10 was not found to be different between the RV group and the RSV group (18.93±16.17 pg/mL vs. 23.34±15.44 pg/mL, P=0.08); however, there was significant difference between patients with wheezing and without wheezing (23.74±16.14 pg/mL vs. 10.77±11.95 pg/mL, P=0.001). In the RV group, the concentration of IL-10 was higher in the patients with wheezing than in the patients without wheezing, and this finding was significantly different (25.70±16.43 pg/mL vs. 11.31±11.95 pg/mL, P=0.007) (Fig. 2). In the RSV group, the concentration of IL-10 did not reach statistical significance between in the patients without wheezing and in the patients with wheezing (23.80±16.13 pg/mL vs. 21.86±12.89 pg/mL, P=0.965) (Fig. 2). Especially, the concentration of IL-10 was not significantly different between the RV group with wheezing and the RSV group with wheezing (25.70±16.43 pg/mL vs. 23.80±16.13 pg/mL, P=0.801) (Fig. 2), in contrast with significant difference between the RV group without wheezing and the RSV group without wheezing (11.31±11.95 pg/mL vs. 21.86±12.89 pg/mL, P=0.035) (Fig. 2).The T-helper 2-type cytokines (IL-4, IL-5, and IL-13) and other inflammatory cytokines (IL-6, IL-8, and IP-10) were not significantly different between the 2 groups and between patients with wheezing and without wheezing.A correlation between cytokine levels and laboratory or clinical results was not found.
- Discussion
- Discussion
In this study, we assessed cytokine profiles between RV infection induced wheezing and RSV infection induced wheezing on the acute stage of infection. We detected that both RV and RSV infections with wheezing were primarily similar expression of cytokines from T-regulatory cells such as IL-10, and with partially increased IFN-γ concentration among the 10 cytokines.RV is the main pathogen that is responsible for causing the common cold and pediatric asthma attacks10,11). In both the Emergency Department and outpatient clinics, in addition to the RSV, the RV is a common pathogen responsible for causing wheezing among infants12,13). In our study, the virus that was the major viral pathogen responsible for causing LRTI in infants was the RSV, which was responsible for LRTI in up to 40.7% of the hospitalized children and was followed by the RV (30.9%). These results are consistent with the findings of other studies1,3).The present study demonstrated that there were no significant differences in the clinical characteristics between the RV LTRI group and the RSV LTRI group, although more patients in the RV LTRI group had a history of previous respiratory infection compared with the RSV LTRI group. In a previous study that compared the clinical pattern of RV and RSV infections in infants with bronchiolitis5), they found that infants with a RV infection and those with a RSV infection showed similar clinical symptoms like the present study. They also described that the infants with a RV infection were older and more often had atopic dermatitis and eosinophilia5). They supposed that RV-associated wheezing may be significantly associated with atopy predisposition.Recent studies demonstrated that when T-helper 1, T-helper 2, and T-regulatory cell type cytokine expressions between RV and RSV induced early wheezing in pediatric patients were compared, the RV group had higher IFN-γ, IL-13, and IL-10 concentration than the RSV group14,15). They concluded that the RV seems to trigger early wheezing in children with an allergic phenotype. Although the age of the enrolled patients was different in the present study, we also experienced that the concentrations of IFN-γ and IL-10 were significantly high in the RV induced LRTI and associated with wheezing in RV-induced LRTI.IFN-γ is a pro-inflammatory and immune-regulatory cytokine related to nonspecific defense mechanism. It is secreted from neutrophils at the site of the viral infection, and inhibits viral replication11). Infants hospitalized with severe RSV disease had a reduction in IFN-γ levels in nasopharyngeal aspirates and a decreased expression of IFN-γ by peripheral blood mononuclear cells16,17). In addition, IFN-γ can induce airway hyper-responsiveness and tends to aid in the development of wheezing18). A previous study also demonstrated that the expression levels of IFN-γ were higher at the time of RV infection and played an important role in RV-induced early wheezing18). The present study showed that IFN-γ levels had not only RSV induced LRTI with or without wheezing but also RV induced LRTI with wheezing like the previous studies. IFN-γ showed a significant relation to wheezing in RV-induced LRTI.IL-10 is an anti-inflammatory cytokine whose immunomodulatory action is well-known19). In our previous pediatric study, IL-10 levels were significantly higher in H1N1 patients infected in 2009 who presented with severe pneumonia in comparison to mild pneumonia20). These findings may suggest that because of the anti-inflammatory and immunomodulatory properties of IL-10, elevated IL-10 levels present in severe disease may have additional effects that aid in the control of the disease. Similar to H1N1 infection, in previous study regarding cytokine gene expression in the induced sputum from children with virus induced acute asthma, it was demonstrated that IL-10 mRNA was increased in virus induced acute asthma, which was reduced upon the recovery phase21). During RSV induced inflammation, especially severe RSV infection, Th2-, Th9-, and Th17-related cytokines are elevated. Murine studies presented that the combined actions of regulatory factors such as CD4 regulatory T cells and IL-10 inhibits the inflammatory cytokine response and limit RSV-induced disease22). Another study also demonstrated that the IL-10 level was higher in the acute phase than the convalescent phase in both RV and RSV infections, which was significantly different in comparison to the control group14). The increased IL-10 level found during an acute viral infection may suggest resolution of the inflammatory process. There is evidence that patients with a decreased IL-10 response could have a greater inflammatory response to viral infections and an increased risk for recurrent wheezing23,24).The recent study reported the interplay between IL-10 and IFNs. They concluded that innate IFNs take the antiviral and anti-inflammatory immune response in normal subjects with viral infection, and IL-10 is secreted simultaneously and may induce regulatory T cells and control inflammation19). In the present study, IFN-γ and IL-10 levels had a significant relation to wheezing in RV-induced LRTI.Previous study in murine models showed the relation between IL-12 and IFN-γ in virus infection25). In addition, previous studies also reported that the role of Th2-type cytokines (IL-4 and IL-5), IL-2 and IL-12 in RV infections were less pronounced. These studies presented that Th2-type cytokines may reflect a chronic inflammation of lower respiratory tract and a susceptibility to more severe RV-infections26,27). Some studies compared the differences of cytokines between RV and RSV and they concluded that the RV infection had markedly higher systemic levels of IL-5 and IL-13 than RSV infection when there was a close correlation between RV infections and atopic characteristics5,28). Unlike previous studies, we could not find any significant differences of Th2-type cytokines, IL-12 and IL-13 between RV and RSV infections. These results may be related that enrolled patients were very young and did not show definite atopic characteristics from their past history.The limitation of our study includes the facts that there were a small number of subjects and a lack of an estimate of infected viral loads. Although the patients had a respiratory tract infection, we only conducted an analysis on their serum cytokine levels. In addition, there were no long-term follow-up results regarding the progress of their infection including the occurrence of infantile asthma or other allergic phenotypes.In conclusion, RV induced LRTI is as important as RSV induced LRTI, and, if wheezing is combined with RV induced LRTI, this may have clinical significance and display similar characteristics to RSV induced LRTI. In addition, RV induced LRTI combined with wheezing showed similar levels of IFN-γ and IL-10 in comparison with RSV induced LRTI. IFN-γ and IL-10 may have an important regulatory function in acute viral induced wheezing.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Blomqvist S, Roivainen M, Puhakka T, Kleemola M, Hovi T. Virological and serological analysis of rhinovirus infections during the first two years of life in a cohort of children. J Med Virol 2002;66:263–268.
[Article] [PubMed]2. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010;23:74–98.
[Article] [PubMed] [PMC]3. Fodha I, Vabret A, Ghedira L, Seboui H, Chouchane S, Dewar J, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol 2007;79:1951–1958.
[Article] [PubMed]4. Midulla F, Scagnolari C, Bonci E, Pierangeli A, Antonelli G, De Angelis D, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child 2010;95:35–41.
[Article] [PubMed]5. Jartti T, Gern JE. Rhinovirus-associated wheeze during infancy and asthma development. Curr Respir Med Rev 2011;7:160–166.
[Article] [PubMed] [PMC]6. Drysdale SB, Mejias A, Ramilo O. Rhinovirus - not just the common cold. J Infect 2017;74(Suppl 1): S41–S46.
[Article] [PubMed]7. Arruvito L, Raiden S, Geffner J. Host response to respiratory syncytial virus infection. Curr Opin Infect Dis 2015;28:259–266.
[Article] [PubMed]8. Miyauchi K. Helper T cell responses to respiratory viruses in the lung: development, virus suppression, and pathogenesis. Viral Immunol 2017;30:421–430.
[Article] [PubMed]9. British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the management of community acquired pneumonia in childhood. Thorax 2002;57(Suppl 1): i1–i24.
[Article] [PubMed] [PMC]10. Khetsuriani N, Kazerouni NN, Erdman DD, Lu X, Redd SC, Anderson LJ, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol 2007;119:314–321.
[Article] [PubMed]11. Jartti T, Waris M, Niesters HG, Allander T, Ruuskanen O. Respiratory viruses and acute asthma in children. J Allergy Clin Immunol 2007;120:216author reply 217.
[Article] [PubMed] [PMC]12. Mansbach JM, McAdam AJ, Clark S, Hain PD, Flood RG, Acholonu U, et al. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad Emerg Med 2008;15:111–118.
[Article] [PubMed] [PMC]13. Jartti T, Lehtinen P, Vuorinen T, Osterback R, van den, Osterhaus AD, et al. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis 2004;10:1095–1101.
[Article] [PubMed] [PMC]14. Jartti T, Paul-Anttila M, Lehtinen P, Parikka V, Vuorinen T, Simell O, et al. Systemic T-helper and T-regulatory cell type cytokine responses in rhinovirus vs. respiratory syncytial virus induced early wheezing: an observational study. Respir Res 2009;10:85.
[Article] [PubMed] [PMC]15. Gavala ML, Bertics PJ, Gern JE. Rhinoviruses, allergic inflammation, and asthma. Immunol Rev 2011;242:69–90.
[Article] [PubMed] [PMC]16. Christiaansen AF, Syed MA, Ten Eyck PP, Hartwig SM, Durairaj L, Kamath SS, et al. Altered Treg and cytokine responses in RSV-infected infants. Pediatr Res 2016;80:702–709.
[Article] [PubMed] [PMC]17. Aberle JH, Aberle SW, Dworzak MN, Mandl CW, Rebhandl W, Vollnhofer G, et al. Reduced interferon-gamma expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med 1999;160:1263–1268.
[Article] [PubMed]18. Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet 2005;365:142–149.
[Article] [PubMed]19. Zdrenghea MT, Makrinioti H, Muresan A, Johnston SL, Stanciu LA. The role of macrophage IL-10/innate IFN interplay during virus-induced asthma. Rev Med Virol 2015;25:33–49.
[Article] [PubMed]20. Kim YH, Kim JE, Hyun MC. Cytokine response in pediatric patients with pandemic influenza H1N1 2009 virus infection and pneumonia: comparison with pediatric pneumonia without H1N1 2009 infection. Pediatr Pulmonol 2011;46:1233–1239.
[Article] [PubMed] [PMC]21. Wood LG, Simpson JL, Wark PA, Powell H, Gibson PG. Characterization of innate immune signaling receptors in virus-induced acute asthma. Clin Exp Allergy 2011;41:640–648.
[Article] [PubMed]22. Christiaansen AF, Knudson CJ, Weiss KA, Varga SM. The CD4 T cell response to respiratory syncytial virus infection. Immunol Res 2014;59:109–117.
[Article] [PubMed]23. Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One 2007;2:e966.
[Article] [PubMed] [PMC]24. Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 2009;324:55–59.
[Article] [PubMed] [PMC]25. Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol 1996;156:1138–1142.
[Article] [PubMed]26. Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax 2002;57:328–332.
[Article] [PubMed] [PMC]
Fig. 1
Comparison of interferon gamma (IFN-γ concentrations between the rhinovirus (RV) and respiratory syncytial virus (RSV) groups. The serum concentrations of IFN-γ were compared between the wheezing and nonwheezing subgroups, and between the RV and RSV groups. Median, quartiles, and range are shown. The Mann-Whitney U test was used to compare the cytokine levels.
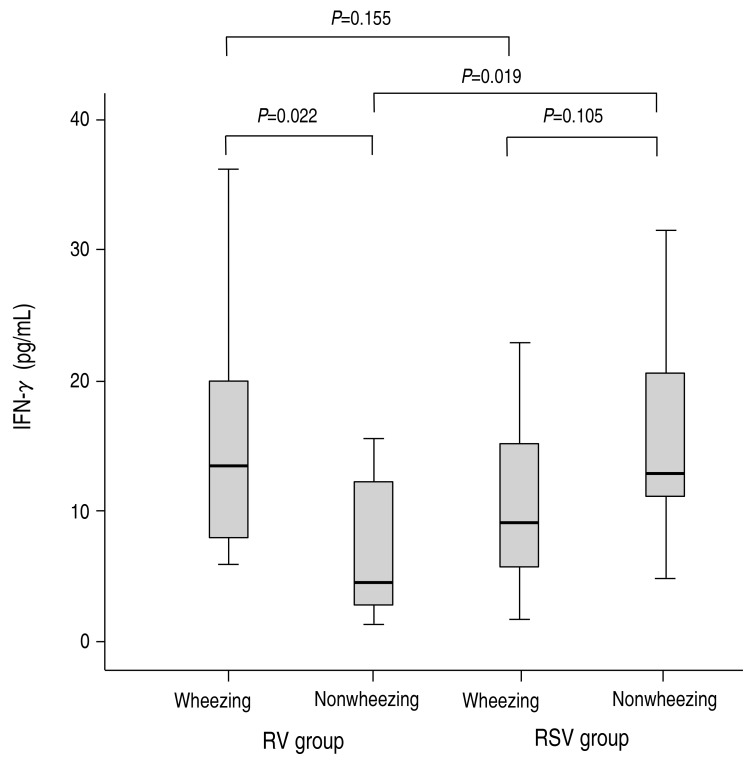
Fig. 2
The comparisons of interleukin (IL)-10 between the rhinovirus (RV) group and respiratory syncytial virus (RSV) groups. The serum concentrations of IL-10 were compared between the wheezing and nonwheezing subgroups, and between the RV and RSV groups. Median, quartiles, and ranges are shown. The Mann-Whitney U test was used to compare the cytokine levels.
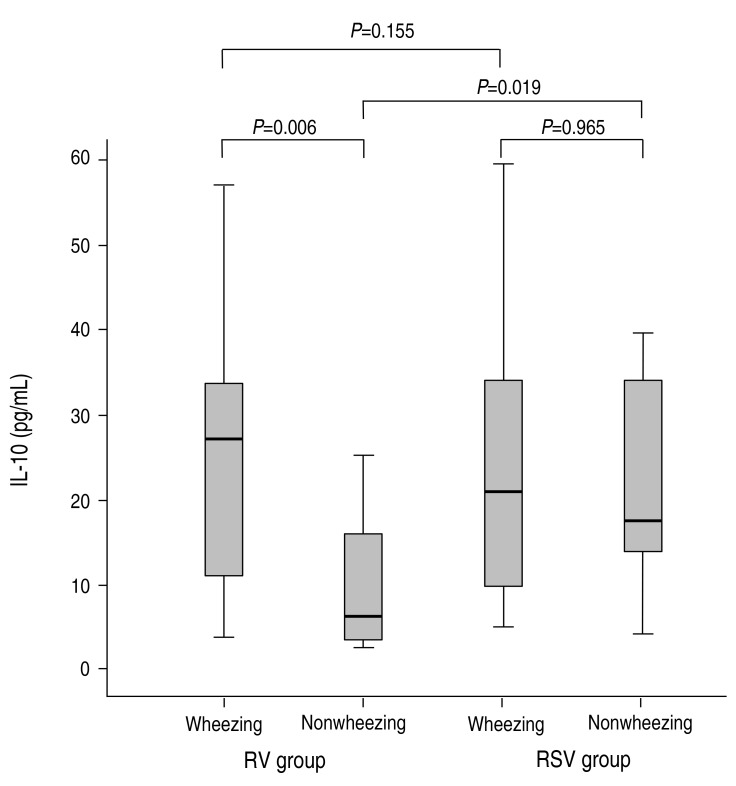
Table 1
Comparison of clinical manifestations between the RV and RSV groups
Table 2
Comparison of laboratory data between the RV and RSV groups
Values are presented as mean±standard deviation.
RV, rhinovirus; RSV, respiratory syncytial virus; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate transaminase; ALT, alanine Transaminase; WBC, total white blood cell count.
*P<0.05, statistically significant difference between the groups.

 About
About Browse articles
Browse articles For contributors
For contributors
