All issues > Volume 61(1); 2018
Quality improvement in pediatric care
- Corresponding author: Moon Sung Park, MD, PhD. Department of Pediatrics, Ajou University School of Medicine, 206 World cup-ro, Yeongtong-gu, Suwon 16499, Korea. Tel: +82-31-219-5006, Fax: +82-31-216-6657, drparkms@aumc.ac.kr
- Received November 10, 2017 Revised December 12, 2017 Accepted December 21, 2017
- Abstract
-
We often overlook the importance of several safety issues such as identification of patients, timeout procedure, hand hygiene, handoff communication, and many others. This ignorance, along with many other issues, leads to medical error being ranked as a third leading cause of death in the U.S. Consequently, quality improvement (QI) has become one of the major subjects in healthcare despite a relatively short history. Improving quality is about making healthcare safe, effective, patient-centered, timely, efficient, and equitable. Understanding the need and methodology of QI as well as participation is now essential for physicians. Although basic QI methodology has not changed, one of the most fascinating changes in recent QI is conducting large-scale QI projects through multicenter networks. Prospective multicenter QI projects utilizing the Korean Neonatal Network are a substantial initiation of pediatric QI in Korea. The Korean Pediatric Society should set ambitious goals for QI activities for every primary care pediatrician and pediatric subspecialist.
- Introduction
- Introduction
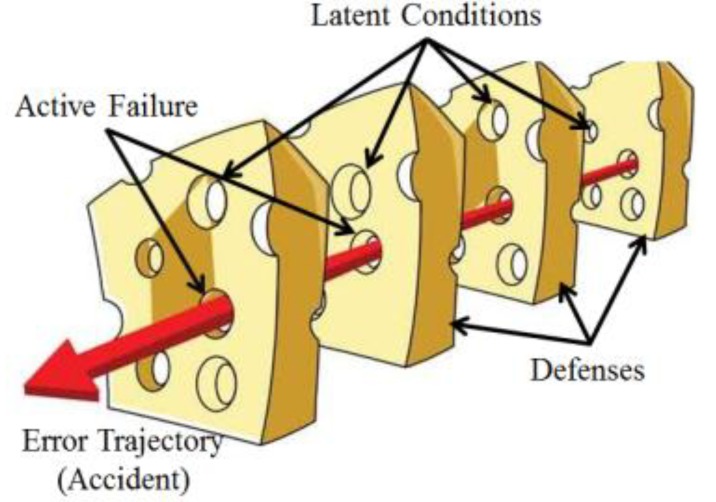
Many people have a rosy picture of the quality of their healthcare system. They fantasize that it will provide highly updated and new research-proven practice in a strict policy-oriented manner. They sometimes find some problems involving error—no one is perfect—but, overall, they think the quality of care will be excellent. Unfortunately, there is a large gap between reality and fantasy.Intrathecal injection of vincristine, which leads to catastrophic results for the patient, was first reported in 1968, and, since then, over 60 cases have been reported worldwide.1) Because of its fatal outcome caused by a simple human mistake, it occasionally receives media attention. Although many guidelines have been proposed to ensure safe administration of this drug, this error occurs constantly. In May of 2010, this accident happened to a 9-year-old pediatric patient at a university hospital, precipitating the issuance of the patient safety act in Korea.Errors happen in all lines of work when performed by humans. Hospital workers are no exception; rather, they are exposed to more accidental risks through inadequate policies that may be due to the continuous introduction of new treatment methods, multiple communication channels involving various occupations, and many other reasons. In 1991, a review of the medical records of over 30,000 patients revealed that about 3.7% had experienced adverse events, and about 30% of the adverse events were due to human error.2) Based on this study and multiple others, the U.S. Institute of Medicine (IOM) published a historical report called “To Err is Human: Building a Safer Health System”,3) which concluded that between 44,000 and 98,000 patients die each year in the U.S. as a result of preventable medical errors. This report resulted in increased awareness of medical errors and foundation of future planning in the U.S. Moreover, a recent study4) reported that medical error could be ranked as the third leading cause of death in the U.S., and yet, unfortunately, in Korea, we have no statistical data in this area.From decades of experience, we know that adverse events, even due to human error, occur not because bad people intentionally hurt patients but because the system of healthcare today is so complex that successful outcome depends mostly on a range of factors, not just the competence of an individual healthcare provider. The most popular way to explain this concept is the “Swiss Cheese Model” by James Reason.5) In this model (Fig. 1), each slice of cheese represents an organization's defense system against failure while holes in the slices represent weaknesses in these defenses system, which open and close at random. The presence of holes in any one “slice” does not normally cause a bad outcome, because other slices may compensate for the weakness. When, by chance, all holes are aligned, permitting (in Reason's words) “a trajectory of accident opportunity,” the system produces failure, and the hazard reaches the patient. Reason's Swiss Cheese Model has become the dominant paradigm for analyzing medical errors and patient safety incidents, which is one of the main concepts of quality improvement (QI). In this paper, I would like to introduce general ideas of QI in medicine, especially in pediatrics.
- What is QI in healthcare service?
- What is QI in healthcare service?
It would be worth understanding what is meant by “quality” at the starting point of improving quality and outcomes in health systems. Without this understanding, confusion may arise during the QI process. There have been many definitions of quality in the healthcare system, but perhaps the best-known definition is that offered by the IOM: “the degree to which health services for individuals and populations increases the likelihood of desired health outcomes and are consistent with current professional knowledge”.6) In the healthcare system, there are always opportunities to redesign, optimize, and develop in a proven, effective way to improve care and satisfy the patient. Therefore, QI is a science of analyzing and evaluating tools and techniques to change healthcare systems that fall short of providing evidence-based high-quality care. Furthermore, QI in healthcare services should be a systematic, continuous process and an integral part of everyone's work, regardless of role or position within the organization.A follow-up to the groundbreaking IOM patient safety report, “To Err is Human: Building a Safer Health System,” the IOM also published “Crossing the Quality Chasm,” which advocates for a fundamental redesign of the U.S. healthcare system. This report identified and recommend improvements in 6 domains in which the healthcare system functions at far lower levels than it can and should7); those are named and described below.(1) Safe: avoiding injuries to patients from the care that is intended to help them.(2) Effective: providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit (avoiding underuse and overuse, respectively).(3) Patient-centered: providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.(4) Timely: reducing waits and sometimes harmful delays for both those who receive and those who give care.(5) Efficient: avoiding waste, including waste of equipment, supplies, ideas, and energy.(6) Equitable: providing care that does not vary in quality because of personal characteristics such as gender, ethnicity, geographic location, and socioeconomic status.From the IOM's perspective, the gap between “what we do” and “what can do” would be placed within these six domains. Therefore, these 6 frameworks of quality set by the IOM make it easier for healthcare workers to classify and analyze quality measures of each institute. In most healthcare institutes, the vast majority of measures address safety and effectiveness, a smaller number examine timeliness and patient-centeredness, and very few assess the efficiency or equity of care. Still, each institute should make its own decision to set a quality framework based on their risk assessment.
- How can QI be achieved?
- How can QI be achieved?
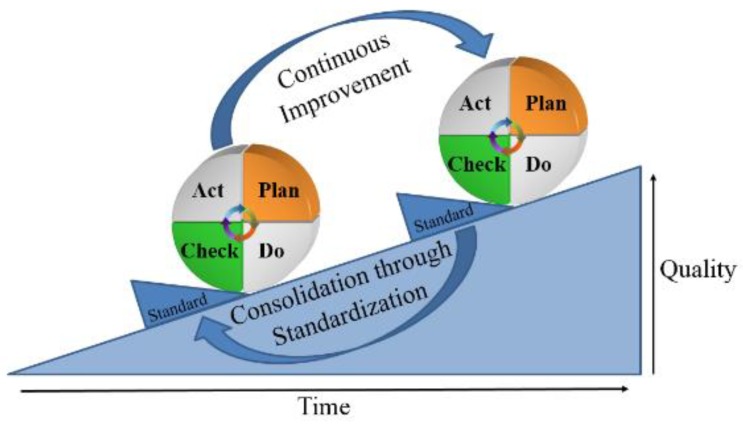
QI is data-driven. Collecting and using data effectively improves decision-making, especially when data are used to target areas for improvement. Peter Drucker, a management consultant, is often quoted as saying, “If you can't measure it, you can't improve it.” This means that you would not be able to find out whether you are successful or unsuccessful unless success is defined and tracked. For example, it is nearly impossible to decrease sepsis rates in the neonatal intensive care unit without defining and tracking the measures. Without clear objectives, you are stuck in a constant state of guessing. Performance measures are not only tools to assess healthcare against recognized standards and of importance to patients, providers, payers, and policymakers but also the foundations of current efforts to improve healthcare quality.There are various QI models currently in use including the Care model, Lean models, Six Sigma, Focus-Analyze-Develop-Execute, total quality management, and the Plan-Do-Check-Act (PDCA) cycle. Because each QI model can offer a systematic approach for assessing and improving care services, it is not essential for an organization to understand and use all of the QI models listed above. The PDCA cycle, also known as the “Shewhart cycle,” is one of the most frequently used management methods in QI to test changes on a smaller scale that allows continuous improvement process. During the “Plan” phase, the first task is to decide “What are the desired improvements?” Ideas for healthcare QI come from many sources. Leaders or teams may identify issues of QI based on an organization's mission and vision, regulatory requirements, patient issues, staff issues, or sometimes leadership priorities. Sometimes, quality indicators from public reporting or new best practice guidelines serve as an impetus for QI projects. This list of areas for improvement should be prioritized based on the impact and likelihood of each consequence. Once a QI project has been selected, the organization creates a QI team made up of all stakeholders. Then, the second task is to allow the improvement to be visualized by data measures. Because not all changes for improvement are successful, it is important to track the measure by using well-defined datasets to provide the changes that are true improvement. These measures are usually divided in 4 groups, such as structural, process, balance, and outcome measures. The structural measure quantifies physical and/or personnel capacity to provide healthcare. The process measure quantifies specific steps in a process that lead to a particular outcome measure. The balance measure quantifies any unexpected consequences in other parts of the system due to the changes of improvement. The outcome measure quantifies a patient's health status resulting from the improvement. During the QI process, it is essential to choose an appropriate set of measures that accurately reflects the system. The third task is to find “what change can we make that will result in improvement?” To accomplish this task, the team should investigate the current situation, fully understand the nature of any problem to be solved, and develop potential solutions to the problem that will be tested. Then, detailed action plans, such as “who is responsible for each task,” “target population,” “improvement targets for each task,” and “data collection and analysis methods,” are set before proceeding with the plan. The “Do” phase involves testing the action plan and collecting and analyzing the data as planned. In the “Check” phase, the effects of the changes are analyzed to decide if they will be adapted or abandoned by the organization during the “Act” phase.As mentioned, QI in healthcare services is a continuous process, and the fundamental principle of the PDCA cycle is iteration. Once the change shows optimal results, it is time to standardize your process and educate all your staff for implementation. Moreover, the next step is to restart the PDCA cycle with other changes repeatedly until the ultimate goal is achieved (Fig. 2).
- QI vs. research
- QI vs. research
Because QI activities are data-driven and involve human participants, it is not surprising that determining if an activity requires Institutional Review Board (IRB) approval can be challenging. The purpose of research is to test a hypothesis or establish theories to develop or contribute to generalized knowledge. Therefore, research uses rigid protocols with a large number of participants who may be at risk. On the contrary, the purpose of QI is to assess or improve a process, program, or system through established/accepted knowledge usually preceded by adaptive, iterative design with a small number of participants who usually are not at any risk. For this reason, all research on humans requires IRB approval while most QI activities do not. However, when QI activity involves humans to test a service or program with insufficient evidence, it should be subject to IRB review and approval.8) For example, a QI project to determine which evidence-based practice is better through comparative randomized intervention is regarded as research involving humans and subject to IRB review and approval. Moreover, compared to internal funding to improve a program, external funding may make a difference in distinguishing between QI and research.
- Collaborative improvement networks in pediatrics
- Collaborative improvement networks in pediatrics
There have been numerous reports on quality and safety improvement in the pediatric area including central line-associated blood stream infection (CLABSI), ventilator-associated pneumonia, handoffs communication, adverse drug events, and other serious safety issues.9,10,11,12) In the U.S., the initiatives for these QI activities have traditionally been supported by a national institute such as the National Quality Forum, Joint Commission, Institute for Healthcare Improvement (IHI), and Agency for Healthcare Research and Quality.Despite individual success of these QI activities, significantly improving many children in different hospital settings will require a more systematic method of verification for generalization. Moreover, these QI issues could also be found in adult patients, but, as pediatricians were taught that “children are not little adults,” it is not surprising that different strategies may be required for QI. Because children are physiologically, socially, and psychologically different from adults, the specific care processes cannot simply be adapted from adult evidence. According to the IOM, it takes an average of 17 years to translate new evidence and processes into daily practice,7) and even then, application is highly uneven. Especially in the field of pediatrics, because of the small number of patients in a subspecialty, it is difficult to learn what works best at any one center. Therefore, making a significant improvement in outcomes through substantial system change requires more than short-term efforts from multiple organizations or centers.One of the most striking phenomena in QI in the past decade has been the rapid development of multisite networks conducting large-scale QI projects. In 2002, the Quality in Pediatric Subspecialty Care workgroup, chartered by the American Board of Pediatrics, developed an improvement network model by using the IHI's Breakthrough Series model to accelerate this process and improve care and outcomes for children.13) Currently, 9 of 14 pediatric subspecialties have implemented a collaborative network14) to close the quality gap by implementing their collective experience and data into practice, including the Joint Council on Congenital Heart Disease National Pediatric Cardiology Quality Improvement Collaborative, the Children's Hospital Association Quality Transformation Network, the Cystic Fibrosis Foundation Network, the Vermont Oxford Network, and so on.In 2013, the Korean Society of Neonatology, with support from the Korea Centers for Disease Control and Prevention, launched the Korean Neonatal Network (KNN), which is a nation-wide multicenter prospective web-based registry for very low birth weight infants (VLBWIs).15) The KNN is designed to be not just a national registry for VLBWIs but also a platform for research and QI similar to the Vermont Oxford Network. The KNN just started an evidence-based prospective multicenter QI project for improving neonatal sepsis. Briefly, among 70 KNN participating hospitals, 3 hospitals from the upper 25th percentile and one from the lower 10th in late-onset neonatal sepsis rate based on KNN registry data were selected with permission as QI participating and benchmarking hospitals, respectively. QI teams from each participating hospital set their own QI strategies from which the KNN suggested evidence-based protocols, such as CLABSI control, indwelling catheter use, antibiotic use, and proper use of newly introduced products including needleless connectors and so on. They also visit the benchmarking hospital to learn effective ways to carry out the strategies. The late-onset neonatal sepsis rate, which is the QI measure, will continue to be recorded in the KNN data and will be reported at the regular KNN data monitoring visit of participating hospitals. Because this 2-year QI project uses currently active KNN data as it is, the recent evidence-based practice can simultaneously be applied to multiple institutions, and the effect can be confirmed in real time. Additionally, it is easy to carry out the QI project in the same way when the target measure is included in the KNN dataset.The American Academy of Pediatrics (AAP) has maintained a committee for QI, currently named the Steering Committee on Quality Improvement and Management, for more than 20 years. This committee works on a variety of QI issues including identifying problems, developing measures, adopting evidence-based clinical practice guidelines, determining methodologies for implementation, and educational programs.16) As the AAP has been a trusted source for policies and evidence-based guidelines for pediatric care, the Korean Pediatric Society should build up a framework to start these QI activities.
- Conclusion
- Conclusion
Despite the tremendous efforts to accomplish continuing problems in pediatric safety and healthcare quality, there are multiple gaps between what we are doing and what we can do. QI is filling these gaps to prevent latent error, as shown in the Swiss Cheese Model of accident causation. Unfortunately, in the pediatric subspecialty, there is always a relatively small number of patients with any given condition in one center to answer best practice and quality questions. Therefore, linking multiple hospitals in networks, such as the KNN, to share data and, ultimately, standardizing practice will be a powerful tool for both research and QI in the future. I cautiously recommend that the Korean Pediatric Society take the lead to set ambitious goals of QI activities for every primary care pediatrician and pediatric subspecialist.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Reddy GK, Brown B, Nanda A. Fatal consequences of a simple mistake: how can a patient be saved from inadvertent intrathecal vincristine? Clin Neurol Neurosurg 2011;113:68–71.
[Article] [PubMed]2. Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 1991;324:370–376.
[Article] [PubMed]3. Kohn LT, Corrigan J, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press, 2000.4. Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ 2016;353:i2139.
[PubMed]5. Reason JT. Human error. New York: Cambridge University Press, 1990.6. Institute of Medicine, Division of Health Care Services, Committee to Design a Strategy for Quality Review and Assurance in Medicare. In: Lohr KN, editor. Medicare: a strategy for quality assurance. Washington, DC: National Academy Press, 1990.7. Institute of Medicine, Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press, 2001.8. Hockenberry M. Quality improvement and evidence-based practice change projects and the institutional review board: is approval necessary? Worldviews Evid Based Nurs 2014;11:217–218.
[Article] [PubMed]9. Piazza AJ, Brozanski B, Provost L, Grover TR, Chuo J, Smith JR, et al. SLUG Bug: quality improvement with orchestrated testing leads to NICU CLABSI reduction. Pediatrics 2016;1;137(1): [Epub].10. Chinnadurai K, Fenlason L, Bridges B, Espahbodi M, Chinnadurai S, Blood-Siegfried J. Implementation of a sustainable ventilator-associated pneumonia prevention protocol in a Pediatric Intensive Care Unit in Managua, Nicaragua. Dimens Crit Care Nurs 2016;35:323–331.
[Article] [PubMed]11. Patton LJ, Tidwell JD, Falder-Saeed KL, Young VB, Lewis BD, Binder JF. Ensuring safe transfer of pediatric patients: a quality improvement project to standardize handoff communication. J Pediatr Nurs 2017;34:44–52.
[Article] [PubMed]12. Tham E, Calmes HM, Poppy A, Eliades AB, Schlafly SM, Namtu KC, et al. Sustaining and spreading the reduction of adverse drug events in a multicenter collaborative. Pediatrics 2011;128:e438–e445.
[Article] [PubMed]13. Lannon CM, Peterson LE. Pediatric collaborative improvement networks: background and overview. Pediatrics 2013;131(Suppl 4): S189–S195.
[Article] [PubMed]14. Lannon CM, Peterson LE. Pediatric collaborative networks for quality improvement and research. Acad Pediatr 2013;13(6 Suppl): S69–S74.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors