All issues > Volume 61(2); 2018
Discrimination of Kawasaki disease with concomitant adenoviral detection differentiating from isolated adenoviral infection
- Corresponding author: Ji-Eun Ban, MD, PhD. Department of Pediatrics, Ewha Womans University College of Medicine, 1071 Anyangcheon-ro, Yangcheon-gu, Seoul, 07985, Korea. Tel: +82-2-2650-2663, Fax: +82-2-2653-3718, jeban@naver.com
- Received February 07, 2017 Revised June 09, 2017 Accepted September 17, 2017
- Abstract
-
- Purpose
- Purpose
- Human adenovirus infection mimics Kawasaki disease (KD) but can be detected in KD patients. The aim of this study was to determine the clinical differences between KD with adenovirus infection and only adenoviral infection and to identify biomarkers for prediction of adenovirus-positive KD from isolated adenoviral infection.
- Methods
- Methods
- A total of 147 patients with isolated adenovirus were identified by quantitative polymerase chain reaction. In addition, 11 patients having KD with adenovirus, who were treated with intravenous immunoglobulin therapy during the acute phase of KD were also evaluated.
- Results
- Results
- Compared with the adenoviral infection group, the KD with adenovirus group was significantly associated with frequent lip and tongue changes, skin rash and changes in the extremities. In the laboratory parameters, higher C-reactive protein (CRP) level and presence of hypoalbuminemia and sterile pyuria were significantly associated with the KD group. In the multivariate analysis, lip and tongue changes (odds ratio [OR], 1.416; 95% confidence interval [CI], 1.151–1.741; P=0.001), high CRP level (OR, 1.039; 95% CI 1.743–1.454; P= 0.021) and sterile pyuria (OR 1.052; 95% CI 0.861–1.286; P=0.041) were the significant predictive factors of KD. In addition, the cutoff CRP level related to KD with adenoviral detection was 56 mg/L, with a sensitivity of 81.8% and a specificity of 75.9%.
- Conclusion
- Conclusion
- Lip and tongue changes, higher serum CRP level and sterile pyuria were significantly correlated with adenovirus-positive KD.
- Introduction
- Introduction
Various studies have been carried out to find out the etiology of Kawasaki disease (KD). Viral infection is thought to be one of the causes of KD, but it is still controversial1,2,3,4). Although several potential viral causes of KD are identified, the diagnosis of KD with concomitant viral infection is a little unclear to confirm KD.Adenoviral infection is frequently involved in young children less than 3 years and is characterized with prolonged high fever, upper and lower respiratory tract symptom, conjunctivitis and gastrointestinal symptom with hepatic involvement. Inflammatory biomarker such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are mostly elevated in patients with adenoviral infection. Because of strong inflammatory response, adenoviral infection closely resembles bacterial infection and KD5,6,7,8). Although the majority patients of adenoviral infection presented respiratory condition, extrapulmonary manifestation of conjunctivitis, skin rash, mucous membrane changes, cervical lymphadenopathy and encephalitis can be associated. The various clinical manifestation of adenovirus infections are particularly similar to KD regarding the clinical aspects9,10,11,12,13).A recent study demonstrated the distinction of clinical and virologic characteristics of adenoviral infection differentiating from KD with incidental adenoviral detection14). Because of clinical similarities, early and acute diagnosis and intravenous immunoglobulin (IVIG) therapy are essential to prevent coronary artery abnormalities in KD. The aim of this study is to compare the clinical and laboratory characteristics of KD patients with adenovirus detection and those with only adenoviral infection and to identify biomarkers that can differentiate KD from adenovirus infections at the initial hospitalization stage. In addition, we also assessed the clinical and laboratory distinction of KD with adenovirus from KD without adenovirus infection.
- Materials and methods
- Materials and methods
- 1. Study population
- 1. Study population
We conducted a retrospective study from August 2012 to June 2016. A total of 129 patients with KD were diagnosed during this period. The diagnosis of KD was established in patients who fulfilled the diagnostic criteria of KD. Typical KD was diagnosed in the presence of fever for at least 5 days combined with at least 4 of the 5 following clinical features; the lip and tongue change, bilateral bulbar conjunctivitis, skin rash, erythema and edema of the hands and feet and cervical lymphadenopathy with diameter of ≥1.5 cm. Incomplete KD was diagnosed if the patients had symptoms that were associated with KD met the inflammatory biomarker criteria15).Among 129 KD patients, respiratory virus polymerase chain reaction was performed in 107 patients. Respiratory virus panel included influenza A and B, adenovirus, parainfluenza virus types 1 through 4, respiratory syncytial virus types A and B, bocavirus types 1 through 4, coronavirus NL 63, OC 43, and 229E, enterovirus, human metapneumovirus, and rhinovirus types A, B, and C.In addition, we retrospectively analyzed 147 patients with isolated adenoviral infection. The most common symptoms were fever more than 4 days (81.3%), cough (73.2%), sputum (68.8%), rhinorrhea (65.2%). The most common diagnosis at admission was acute pharyngotonsillitis (57.3%), followed by pneumonia (16.1%), bronchiolitis (12.5%), and gastroenteritis (5.9%). The pharyngoconjunctival fever was initially diagnosed in 12 patients (8.2%).We compared 147 patients with adenoviral infection and 11 patients with KD with adenovirus detection. In addition, we also compared 11 patients with KD with adenovirus and 87 KD without adenovirus detection. This study protocol was approved by the Institutional Review Board of Hallym University Kangdong Sacred Heart Hospital (KANGDONG 2018-01-004). Written informed consent was obtained from all patients.- 2. Laboratory & clinical characteristics analysis
- 2. Laboratory & clinical characteristics analysis
All patients with KD were examined for inflammatory markers such as total white blood cell count, neutrophil count, neutrophil/lymphocyte ratio, ESR, CRP, lactate dehydrogenase (LD), and B-type natriuretic peptide (BNP).In addition, other laboratory findings such as hemoglobin, serum sodium, serum albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and urinalysis were also assessed. However, BNP was not detected in most patients with isolated adenoviral infection.Regarding the criteria of typical KD15), the acute clinical features such as conjunctival injection, lip and tongue changes, skin rash, cervical lymphadenopathy, and changes in extremities were also evaluated.- 3. KD treatment and echocardiographic analysis
- 3. KD treatment and echocardiographic analysis
All of the KD patients were initially treated with IVIG (2 g/kg) for 12 hours and additionally treated with IVIG in case of IVIG resistance. IVIG resistance was defined as a persistent or recrudescent fever for >48 hours after initial IVIG completion. In the case of recurrent or persistent fever with twice IVIG therapy, we managed with the fever with methylprednisolon pulse therapy or infliximab. After the acute phase, all patients have been given low dose aspirin for 8 weeks. Low dose aspirin was discontinued unless the coronary artery abnormalities persisted.In all patients with KD, transthoracic echocardiography was performed to define the involvement of the coronary arteries during the acute phase and also at an outpatient clinic at 2–3 weeks and 8 weeks. The coronary abnormalities analysis by echocardiography was performed off-line retrospectively. Echocardiographic recording at 3 different times was reviewed in each patient. Measurement were conducted at the left main coronary artery, the left anterior descending artery and the right coronary artery. To identify the coronary abnormalities, we assessed the z value of coronary arteries according to the body surface area. We defined the coronary abnormalities as a coronary z score of more than 2.5, the presence of wall irregularity, no acute tapering of the coronary artery branch and saccular or diffuse coronary aneurysm15).- 4. Statistical analysis
- 4. Statistical analysis
Continuous variables were expressed as the median (interquartile range) and categorical variables as either a number or a percentage. For continuous variables, the comparisons were performed using Mann-Whitney U test. Categorical variables were compared using Fisher's exact test, or chi-square analysis, as appropriate. Multivariate analysis was performed to detect the independent variables associated with KD with adenoviral detection using all parameters with a P value<0.05 via univariate analysis. The area under the receiver operating characteristics (ROC) curve was used to evaluate the discrimination of KD with adenoviral detection from isolated adenoviral infection and then to derive sensitivity and specificity values for CRP. The cutoff value of CRP parametric variable was determined by ROC curve. A P-value ≤0.05 was considered statistically significant.
- Results
- Results
- 1. Clinical and laboratory findings of KD with adenovirus
- 1. Clinical and laboratory findings of KD with adenovirus
Various kinds of virus were detected in 42 out of 107 (39.2%) patients in KD. Fig. 1 showed the virus distribution of KD in virus positive patients. Rhinovirus was the most commonly detected (40.5%). Additionally, 12 adenoviruses were identified in KD. Two patients had coinfection: RSV type B and rhinovirus in 1 patient and adenovirus and coronavirus in 1 patient. We excluded 1 adenovirus patient with co-infection of coronavirus.Among 129 patients with KD, adenovirus without coinfection was identified in 11 patients. Baseline clinical and laboratory characteristics of KD with adenoviral detection are shown in Table 1. The median age of these patients was 36 months (range, 5–109 months). Nine patients (81.8%) were diagnosed as typical KD. Most of the KD patients had 4 or more clinical symptoms satisfying the KD criteria, and 2 incomplete KD patients had 3 clinical features of KD accompanied by coronary artery abnormalities. In the laboratory findings, CRP was more than 50 mg/L as a whole. BNP was found to be higher than 100 pg/mL in only 3 patients. AST/ALT elevation more than 45 IU/L was detected in 3 patients and sterile pyuria defined as ≥10 WBC/high-power field was shown in 6 patients. Four patients had developed coronary artery abnormalities.- 2. Comparison between KD with adenovirus and isolated adenoviral infection
- 2. Comparison between KD with adenovirus and isolated adenoviral infection
We compared 11 patients with KD with adenovirus and 147 patients with isolated adenoviral infection. Table 2 showed the comparison of clinical and laboratory variables between the 2 groups. In patients with KD with adenovirus detection, the median fever duration was 6 days and was not different from isolated adenoviral infection group (P=0.356). Regarding the clinical features of KD, lip and tongue changes and skin rash and changes in extremities were more frequently demonstrated in the KD with adenoviral detection group than the adenoviral infection group (81.8% vs. 15.6%, P<0.001 in lip and tongue changes; 72.7% vs. 36.7%, P=0.007 in skin rash; 63.6% vs. 10.2%, P=0.001 in changes in extremities). We also compared the differences in laboratory parameters between the 2 groups. There were significant differences in CRP (67.5 [58.5–78.1] mg/L vs. 31.9 [19.2–48.1] mg/L, P=0.002), hypoalbuminemia (9.1% vs. 0.7%, P=0.009) and the presence of sterile pyuria (54.5% vs. 10.9 %, P=0.001) between the KD with adenovirus group and the isolated adenoviral infection group. In addition, the neutrophil/lymphocyte ratio had the borderline statistical significance (P=0.053). There were, however, no significant differences in WBC count, ESR, LD, hyponatremia, or AST/ALT between the 2 groups.By multivariate logistic regression analysis, lip and tongue changes (odds ratio [OR], 1.416; 95% confidence interval [CI], 1.151–1.741; P=0.001), CRP (OR, 1.039; 95% CI, 1.743–1.454; P=0.021) and sterile pyuria (OR, 1.052; 95% CI, 0.861–1.286; P=0.041) were independent predictors of KD with adenoviral detection (Table 3).The cutoff value of CRP that differentiated adenovirus-positive KD from isolated adenoviral infection was 56 mg/L, which resulted in a sensitivity of 81.8% and a specificity of 75.9% (Fig. 2).- 3. Comparison between KD with adenovirus and KD without adenovirus detection
- 3. Comparison between KD with adenovirus and KD without adenovirus detection
In a total of 129 patients with KD, the clinical and laboratory characteristics of patients with KD who were adenovirus positive (n=11) were compared with those who were adenovirus-negative (n=87). We excluded the patients with other virus isolation (n=31) in the adenovirus-negative group. The age, sex, and fever duration did not differ between patient groups. Fourteen patients (16.1%) in the adenovirus-negative KD group were diagnosed with incomplete KD compared with 2 patients (22.2%) in the adenovirus-positive KD group. There were no statistically significant differences in the clinical manifestation of KD and laboratory parameters between the two groups. In addition, coronary artery complications were not different between the 2 groups (36.4% [4 of 11] vs. 33.3% [29 of 87], P=0.868).
- Discussion
- Discussion
- 1. Main findings
- 1. Main findings
Our findings demonstrate that lip and tongue changes, higher serum CRP level and sterile pyuria were significantly related to the adenovirus-positive KD differentiating from isolated adenoviral infection. The cutoff CRP level was 56 mg/L, with 81.8% sensitivity and 75.9% specificity. The development of coronary artery abnormalities was not different between the adenovirus positive group and adenovirus negative group in KD patients.- 2. Clinical and laboratory consideration in adenovirus positive KD
- 2. Clinical and laboratory consideration in adenovirus positive KD
This study demonstrated the clinical and laboratory characteristics of KD with incidental adenoviral detection that may help differentiate isolated adenoviral infection. Although the clinical features of adenovirus-positive KD are similar to an isolated adenoviral infection, adenoviral-positive KD usually had frequent lip and tongue change, skin rash and changes in extremities compared to adenoviral infection. However, cervical lymphadenopathy was not differently observed between the 2 groups. In the present study, CRP, hypoalbuminemia and sterile pyuria could be differentiated between the adenovirus-positive KD group and adenoviral infection group. In addition, the cutoff CRP level of 56 mg/L aids in the discrimination of diagnosis of KD from adenoviral infection. This distinct clinical and laboratory pattern was characteristic of adenovirus positive KD. However, high CRP level could also be observed in adenoviral infection with secondary bacterial infection. Although the American Heart Association guidelines suggest that a CRP ≥30 mg/L or ESR ≥40 mm/hr may consider incomplete KD15), a recent study demonstrated that a CRP ≥30 mg/L has a lack specificity for KD. Additionally, a higher CRP ≥70 mg/L suggesting the discrimination of KD from adenoviral infection was consistently observed in the KD group14).- 3. Clinical implications of adenoviral detection in KD
- 3. Clinical implications of adenoviral detection in KD
Previous studies investigated viral etiology in KD and the role of viral infection associated with KD. Several potential viruses including rhinovirus, coronavirus, parainfluenza virus, human metapneumovirus and adenovirus were detected in KD patients1,2,3,4). In contrast, no association of coronavirus and bocavirus with KD was demonstrated in the other study16).Although the relationship between virus and KD was not precisely identified, clinical presentation of adenovirus still mimics KD9,10,11,12). It remains to be determined whether this virus causes KD with triggering pathophysiology, leading to similar clinical features as adenoviral infection. This study has several important clinical implications. The early differential diagnosis of KD from adenoviral infection was important to prevent inadvertent coronary artery complications. The suggested clinical features and inflammatory markers could be useful in differentiating KD from isolated adenoviral infection in the early period of disease. In addition, other inflammatory markers such as BNP and procalcitonin should be assessed, because no specific inflammatory biomarker was established during the acute phase.However, there were no significant differences in clinical and laboratory findings between the adenovirus-positive and adenovirus-negative KD groups. The development of coronary artery abnormalities was also not different. Therefore, the adenovirus detection could not be excluded in KD, but might not affect the occurrence of coronary artery abnormalities.A better understanding of the pathophysiological mechanisms of virus associated KD and detection of specific biomarkers of KD are crucial to diagnose and treat without untoward coronary complications.- 4. Study Limitations
- 4. Study Limitations
Although we found clinical manifestations and laboratory parameters differentiating adenovirus-positive KD from isolated adenoviral infection, no specific adenoviral type was identified in 11 patients with adenovirus-positive KD. In addition, there was no relationship between adenoviral detection and coronary artery abnormality in KD.In the study, we did not routinely examine for BNP in the adenoviral infection, so we could not assess the difference between the 2 groups.This was a retrospective study and we evaluated a relatively small number of adenovirus-positive KD patients; further prospective studies with larger numbers of patients are warranted to confirm our results.- 5. Conclusions
- 5. Conclusions
Despite the similarities between KD and adenoviral infection, clinical and laboratory features can be used to differentiate KD with incidental adenoviral detection from adenoviral infection. Serum CRP can be used as a biomarker to predict KD from isolated adenoviral infection at the early stage of hospitalization.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Jordan-Villegas A, Chang ML, Ramilo O, Mejias A. Concomitant respiratory viral infections in children with Kawasaki disease. Pediatr Infect Dis J 2010;29:770–772.
[Article] [PubMed] [PMC]2. Chang LY, Lu CY, Shao PL, Lee PI, Lin MT, Fan TY, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc 2014;113:148–154.
[Article] [PubMed] [PMC]3. Turnier JL, Anderson MS, Heizer HR, Jone PN, Glodé MP, Dominguez SR. Concurrent respiratory viruses and Kawasaki disease. Pediatrics 2015;136:e609–e614.
[Article] [PubMed]4. Kim JH, Yu JJ, Lee J, Kim MN, Ko HK, Choi HS, et al. Detection rate and clinical impact of respiratory viruses in children with Kawasaki disease. Korean J Pediatr 2012;55:470–473.
[Article] [PubMed] [PMC]5. Rocholl C, Gerber K, Daly J, Pavia AT, Byington CL. Adenoviral infections in children: the impact of rapid diagnosis. Pediatrics 2004;113(1 Pt 1): e51–e56.
[Article] [PubMed]6. Tabain I, Ljubin-Sternak S, Cepin-Bogović J, Markovinović L, Knezović I, Mlinarić-Galinović G. Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes. Pediatr Infect Dis J 2012;31:680–684.
[Article] [PubMed]7. Kwon HJ, Rhie YJ, Seo WH, Jang GY, Choi BM, Lee JH, et al. Clinical manifestations of respiratory adenoviral infection among hospitalized children in Korea. Pediatr Int 2013;55:450–454.
[Article] [PubMed] [PMC]8. Sun Q, Jiang W, Chen Z, Huang L, Wang Y, Huang F, et al. Epidemiology and clinical features of respiratory adenoviral infections in children. Eur J Pediatr 2014;173:441–444.
[Article] [PubMed]9. Okano M, Thiele GM, Sakiyama Y, Matsumoto S, Purtilo DT. Adenovirus infection in patients with Kawasaki disease. J Med Virol 1990;32:53–57.
[Article] [PubMed]10. Barone SR, Pontrelli LR, Krilov LR. The differentiation of classic Kawasaki disease, atypical Kawasaki disease, and acute adenoviral infection: use of clinical features and a rapid direct fluorescent antigen test. Arch Pediatr Adolesc Med 2000;154:453–456.
[Article] [PubMed]11. Barton LL. Differentiation of adenoviral infection and Kawasaki disease. Arch Pediatr Adolesc Med 2001;155:96–97.12. Shike H, Shimizu C, Kanegaye JT, Foley JL, Schnurr DP, Wold LJ, et al. Adenovirus, adeno-associated virus and Kawasaki disease. Pediatr Infect Dis J 2005;24:1011–1014.
[Article] [PubMed]13. Jaggi P, Kajon AE, Mejias A, Ramilo O, Leber A. Human adenovirus infection in Kawasaki disease: a confounding bystander? Clin Infect Dis 2013;56:58–64.
[Article] [PubMed]14. Song E, Kajon AE, Wang H, Salamon D, Texter K, Ramilo O, et al. Clinical and virologic characteristics may aid distinction of acute adenovirus disease from Kawasaki disease with incidental adenovirus detection. J Pediatr 2016;170:325–330.
[Article] [PubMed]
Fig. 1
Respiratory virus distribution in Kawasaki disease. Rhinovirus was most commonly detected. Adenovirus was detected in 11 patients. RSV B, respiratory syncytial virus type B.
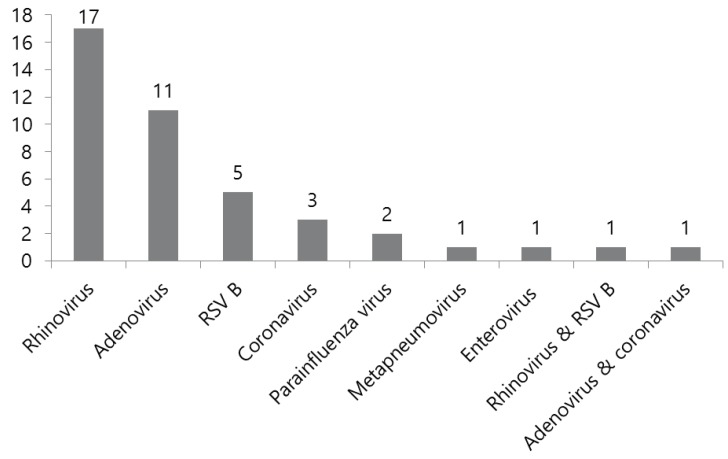
Fig. 2
Receiver-operating characteristic curves for C-reactive protein (CRP). The cutoff CRP level for differentiating adenovirus-positive Kawasaki disease from isolated adenoviral infection was determined to be 56 mg/L with 81.8% sensitivity and 75.9 % specificity. AUC, area under the receiver-operating characteristic curve; CI, confidence interval.
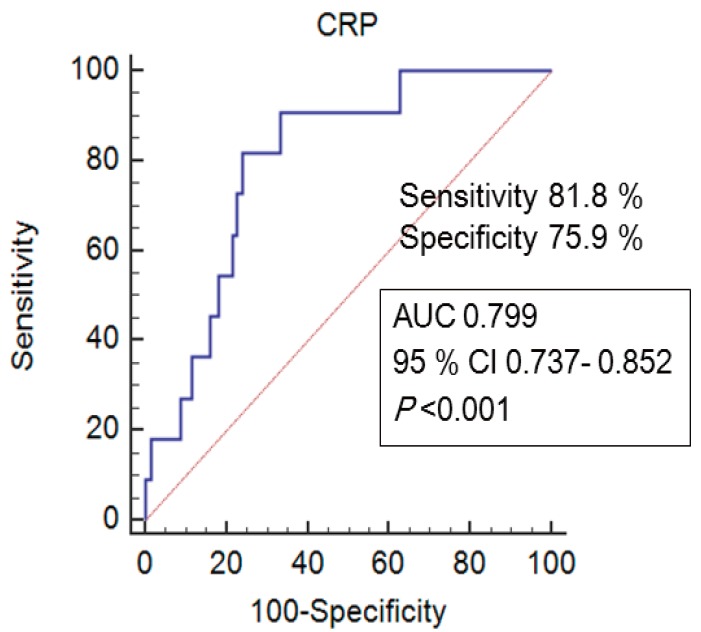
Table 1
Baseline characteristics of Kawasaki disease with adenoviral detection (n=11)
Table 2
Comparison of clinical and laboratory variables between the Kawasaki disease with adenoviral infection group and the adenoviral infection group
Table 3
Multivariate logistic regression analysis for independent predictors for Kawasaki disease with adenoviral detection

 About
About Browse articles
Browse articles For contributors
For contributors
