All issues > Volume 61(5); 2018
Clinical implications in laboratory parameter values in acute Kawasaki disease for early diagnosis and proper treatment
- Corresponding author: Kyung-Yil Lee, MD. Department of Pediatrics, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, 64 Daeheung-ro, Jung-gu, Daejeon 34943, Korea. Tel: +82-42-220-9540, Fax: +82-42-221-2925, leekyungyil@catholic.ac.kr
- Received September 08, 2017 Revised October 13, 2017 Accepted October 26, 2017
- Abstract
-
- Purpose
- Purpose
- This study aimed to analyse laboratory values according to fever duration, and evaluate the relationship across these values during the acute phase of Kawasaki disease (KD) to aid in the early diagnosis for early-presenting KD and incomplete KD patients.
- Methods
- Methods
- Clinical and laboratory data of patients with KD (n=615) were evaluated according to duration of fever at presentation, and were compared between patients with and without coronary artery lesions (CALs). For evaluation of the relationships across laboratory indices, patients with a fever duration of 5 days or 6 days were used (n=204).
- Results
- Results
- The mean fever duration was 6.6±2.3 days, and the proportions of patients with CALs was 19.3% (n=114). C-reactive proteins (CRPs) and neutrophil differential values were highest and hemoglobin, albumin, and lymphocyte differential values were lowest in the 6-day group. Patients with CALs had longer total fever duration, higher CRP and neutrophil differential values and lower hemoglobin and albumin values compared to patients without CALs. CRP, albumin, neutrophil differential, and hemoglobin values at the peak inflammation stage of KD showed positive or negative correlations each other.
- Conclusion
- Conclusion
- The severity of systemic inflammation in KD was reflected in the laboratory values including CRP, neutrophil differential, albumin, and hemoglobin. Observing changes in these laboratory parameters by repeated examinations prior to the peak of inflammation in acute KD may aid in diagnosis of early-presenting KD patients.
- Introduction
- Introduction
Kawasaki disease (KD) is an acute self-limiting infection-related immune-mediated systemic inflammation of childhood, which may be triggered by substances produced after exposure to unknown agents.1) Clinical phenotype and natural course of KD varies across individuals, and the majority of patients recover from the disease without complication within an average 10 to11 days of fever duration.2) However, some severely affected patients have prolonged fever and/or complications including severe coronary artery aneurysms.3) Since KD is a systemic inflammatory process, the severity of systemic inflammation is reflected in a variety of laboratory indices. Therefore, some parameters, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell (WBC) count and differentials, albumin, hemoglobin, alanine transaminase (ALT), platelet count, and pyuria have been used for the diagnosis of incomplete KD.4) Furthermore, changes in some of these laboratory parameters after intravenous immunoglobulin (IVIG) infusion also reflect the degree of systemic inflammation in individuals. Thus, evaluation of these changes is helpful to decide further treatment modalities for IVIG nonresponders.5)Recently in Korea, there have been a few bacterial diseases clinically similar KD, and a few viral diseases showing similar laboratory characteristics to KD. However, early diagnosis of KD is still problematic due to early presentation of patients before the appearance of full clinical criteria and the change to a mild KD phenotype with increasing incomplete KD.6) Therefore, proper guidelines for early diagnosis in these patients may be needed in Korea.In the present study, we evaluated relationships across laboratory parameters that are associated with inflammation in the acute stage of KD. Also, we discuss reasonable methods for the early diagnosis and proper treatment of KD based on changes in laboratory indices in the acute stage of KD.
- Materials and methods
- Materials and methods
A total of 615 subjects were selected for this study from 2 university hospitals in Daejeon, Korea, Chungnam National University Hospital (n=330) and The Catholic University of Korea Daejeon St. Mary's Hospital (n=285), during 2-time periods between 2000 and 2004 (n=284) and between 2010–2014 (n=331). The study was approved by the Institutional Review Board of The Catholic University of Korea, Daejeon St Mary's Hospital (DC17RESI0061). Data from these subjects was previously used for our published paper in which recent patients with KD were shown to have milder clinical phenotypes and less severe CRP, platelet, albumin and hemoglobin values compared to patients admitted a decade ago.6)Subjects with classic KD satisfied with the diagnostic criteria as defined by the Japanese Kawasaki Disease Research Committee and the American Heart Association (AHA): the patient had a fever duration of ≥5 days with at least 4 of the 5 principal clinical signs: (1) bilateral conjunctival injection, (2) changes in the lips and oral cavity, (3) polymorphous skin rash, (4) changes in the peripheral extremities, and (5) acute nonpurulent cervical lymphadenopathy.4,7) The subjects in this study were included patients with incomplete KD as well as classic KD. Incomplete KD was defined as having fever for ≥5 days with 3 or fewer principal signs, with or without cardiac lesions once other KD-like diseases with similar findings had been excluded.6) All patients underwent 2-dimensional echocardiography of the coronary arteries during hospitalization and again at approximately 1 or 2 months after discharge. Echocardiographic findings that were performed during hospitalization were analysed. With respect to coronary artery lesions (CALs), coronary artery ectasia (dilatation) was defined if the internal diameter was up to 1.5 times that of the adjacent coronary artery, and as presenting a luminal dilatation up to 3 mm in children under the age of 5 years or 4.0 mm in children 5 years of age or older. Coronary artery aneurysm was defined when the internal diameter was greater than 1.5 times or ≥3 mm in children under the age of 5 years or ≥4.0 mm in children 5 years of age or older based on the criteria established by the Japanese Ministry of Health and Welfare guidelines.7)The following laboratory parameter levels were examined at the time of admission: WBC with differential, hemoglobin, platelet, ESR, CRP, total protein, albumin, ALT, and aspartate aminotransferase (AST). The values of laboratory parameters were obtained using automatic analyser at both institutions, and no significant differences were seen in the values between the institutions. We evaluated the laboratory indices according to the duration of fever at presentation, and patients were classified into 6 groups, the ≤3rd, 4th, 5th, 6th, 7th, and the ≥8th day groups. Also, we evaluated the clinical and laboratory data between the patients with CALs (n=119) and those without CALs (n=496). It is well-known that some laboratory values vary according to fever duration and age. Additionally, since we wanted to evaluate relationship across laboratory indices in acute KD more clearly, we used the patient group with fever duration of 5 days or 6 days (n=204) for analysis.All calculations were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±standard deviation. One-way analysis of variance (ANOVA) test and Student t test for continuous variables was used for comparisons between groups. The Pearson chi-square test was used for categorical variables. To determine the significance of the relationships between laboratory indices, correlation analysis was performed and Pearson correlation coefficients were calculated. A P value of <0.05 was considered statistically significant.
- Results
- Results
- 1. Clinical and laboratory parameters according to fever duration at presentation
- 1. Clinical and laboratory parameters according to fever duration at presentation
The clinical and laboratory data for each patient group with different fever duration are presented in Table 1. Mean age was 29.7±21.4 months, and the male-to-female ratio was 1.6:1 (375/240). The mean total fever duration was 6.6±2.3 days. The proportion of CALs was 19.3%, and 8 patients had coronary artery aneurysms among patients with CALs. The proportion of IVIG retreatment was 9.6%. Incomplete KD was 37.1% of all KD. Among each group with different fever duration, there were relatively similar proportions in CALs, IVIG retreatment and incomplete KD, although there was a trend for a higher proportion of these parameters in the ≥8th day group (Table 1).In the laboratory findings, WBCs, neutrophil differential and CRP values were the highest, and albumin, hemoglobin, and lymphocyte differential values were the lowest in the 6th day group. The platelet count tended to increase after peak day along with increasing days of fever, and showed highest value in the ≥8th day group. Total protein level was the highest in the ≥8th day group, and the ESR values did not fluctuate significantly. The AST and ALT values were highest in the ≤3rd day group, but 46.7% of patients did not show increased ALT values during the acute stage of KD. In statistical analysis using 1-way ANOVA test, hemoglobin, platelet, CRP, total protein, albumin, AST, and ALT showed statistical significances across the 6 groups (Table 1).- 2. Clinical and laboratory parameters between patients with and those without CALs
- 2. Clinical and laboratory parameters between patients with and those without CALs
The clinical and laboratory data between patient with CALs (n=119) and patients without CALs (n=496) are presented in Table 2. The mean age was not different in both groups, but the male-to-female ratio was higher in the CALs group (P=0,004). The proportion of incomplete KD was not different in the both groups. The total fever duration and the rate of repeated IVIG treatment cases were longer and higher in the CALs group. In laboratory findings, WBC, neutrophil differential, ESR and CRP values were higher, and the values of hemoglobin and albumin were lower in the CALs group compared to patients without CALs. There was no difference in platelet value at presentation, but significant higher value at follow-up examination in the CAL group. There were no differences in total protein, AST, and ALT between the groups (Table 2).- 3. Relationship among laboratory indices around peak inflammation in acute KD
- 3. Relationship among laboratory indices around peak inflammation in acute KD
We analysed relationships between laboratory indices using the patients with fever duration of 5 or 6 days (n=204). There were negative correlations between the CRP and albumin and between the CRP and hemoglobin, and positive correlation between the CRP and neutrophil differential (P<0.01 or P<0.05). There were positive correlation between the albumin and hemoglobin (P<0.01) (Fig. 1). There was a negative correlation between albumin and neutrophil differential (P<0.05), but between hemoglobin and neutrophil differential, there was no statistical correlation (r2=0.142). Except these parameters, there were no laboratory indices that had a statistical correlation each other, across WBC, platelet, ESR, total protein, AST, and ALT (data not shown).
- Discussion
- Discussion
In Korea, recent KD patients manifest a higher incidence of incomplete KD together with a lower incidence of severe CALs compared with their historic counterparts, suggesting that KD has changed to a milder phenotype over time.6,8,9,10,11) In the present study, there were 228 incomplete KD patients (37.1%) among the total 615 subjects. It has been reported that the laboratory values and the rate of CALs in patients with incomplete KD were compatible to those with complete KD.12,13) We also found that patients with incomplete KD had similar incidence of CALs, but they had less severe values in some laboratory findings such as CRP and albumin compared with patients with complete KD.6)Individual patients with classic KD have showed various clinical phenotypes. The order of appearance and severity of the clinical diagnostic criteria varies in each patient. Other organ involvements also differ in each KD patient, and activated laboratory indices for specific organ involvement are noted in a part of patients with KD.14) Cardiac involvement including CALs affects 5%–25% of patients, and aseptic meningitis was noted in 46.6% of patients in our previous observation.15) Other organ involvement signs such as arthritis, gall bladder hydrops, and anterior uveitis are less frequently seen.16,17) The proportion of CALs and the cases with elevation of ALT in this series were 19.1% and 53.3%, respectively.Laboratory indices such as CRP, ESR, and WBC are universally activated in all patients with KD. We previously found that the intensity of systemic inflammation gradually increases and reaches a peak on the 6th day after fever onset.18) Accordingly, severely affected patients may have more prolonged fever duration and a later peak fever day with a high risk of severe CALs. In this study, we reconfirmed these findings with larger number of subjects. The neutrophil differential and CRP were highest, and albumin, hemoglobin and lymphocyte differential were lowest in the 6th day group. In addition, we found that laboratory values such as CRP, albumin, hemoglobin, and neutrophil differential correlated with each other during the peak stage of systemic inflammation (5th or 6th days). These findings support evidence that these parameters correctly reflect systemic inflammation in vivo. Indeed, these laboratory indices have been used for diagnosing incomplete KD. Suspected KD patients with fever duration of ≥5 days are recommended to be examined daily after confirmation of elevated CRP (>3.0 mg/dL) and ESR (40 mm/hr) values according to the AHA guideline.4,14) Patients with incomplete KD can be treated by IVIG, when patients have at least 3 of the following 6 activated laboratory indices: albumin ≤3 g/dL, low hemoglobin for age, elevation of ALT, platelets after 7 days ≥450,000/mm3, WBC count ≥15,000/mm3, and pyuria (urine ≥10 WBCs/high-power field).As shown in this study, a large number of patients are admitted with 2 to 4 days of fever duration (n=339, 55.1%). It is believed that most paediatricians in Korea may easily distinguish KD from other febrile infectious diseases in the early stages of KD including incomplete KD within 5 days after fever onset, using the clinical and laboratory findings, including elevated CRP and ESR levels in the early stage. For example, because nearly all children (>95%) in Korea received bacillus Calmette-Guerin (BCG) vaccination within 1 month of age, BCG site inflammation is noted in over 80%–90% of infants in the early stages of KD,19,20) and older children received ophthalmologic consultation for anterior uveitis.17,21) Moreover, suspected KD patients may have received repeated laboratory examinations in the early stage of the disease as suggested by the AHA guideline. Since early admitted incompletely presented KD patients can show additional clinical signs during admission, it is possible that early IVIG treatment may affect the appearance of clinical diagnostic criteria for patients with potentially complete KD. These findings could explain increased number of incomplete KD diagnoses in Korea.6,9) The AHA diagnostic criteria for incomplete KD patients are helpful for the diagnosis of KD in Korea as well as in Western countries22); however, because of early presentation of KD patients before the appearance of full clinical criteria and the increase in incomplete KD cases in Korea, the criteria have obstacles such as low sensitivity in the early stage of the disease20,23) and waiting for 1 to 3 days to start IVIG treatment after suspected diagnosis. Since early treatment as soon as possible for patients with KD as well as for patients with acute infectious or immune-mediated diseases is a natural concept, early IVIG treatment for preventing formation and/or progression of CALs may be essential. Therefore, it may be reasonable that pediatricians confirm the further elevation of CRP and neutrophil differential values together with reduction in albumin and hemoglobin values, and the appearance of elevated transaminases or pyuria in repeated examinations in the early stage of the disease, especially before the peak of inflammation.1,5,6,18) In our experience, the majority of suspected KD patients showed increased CRP and neutrophil differential values together with an approximately 5%–10% reduction of albumin and hemoglobin values within 24 hours in repeated examinations (unpublished observation).The change in laboratory values could be used for the treatment of severely affected patients. Since most patients with KD experience no CALs or mild ectasia of CALs, pediatricians should focus on the early detection of severely affected patients who potentially progress to severe coronary artery aneurysms. It is well-known that severity of KD inflammation is reflected in laboratory values not only before but also after IVIG infusion. In the present study, we found that the total fever duration and the proportion of repeated IVIG treated cases were longer and higher in the patients with CALs. Also, the CRP value was higher, and the values of hemoglobin and albumin were lower in the CALs groups compared to patients without CALs. Many studies also have shown that patients with CALs or/and IVIG nonresponsiveness have more activated laboratory values in the acute stage of KD, and some study groups have used scoring systems for the prediction of severe KD patients.24,25,26,27) Since some laboratory values in the score systems before IVIG treatment may be influenced by confounding factors such as the stage of inflammation response as shown in this study, variable involvement of organ tissues in individuals, patient age, and possibly ethnicity, the sensitivity and specificity of the prediction for severe KD may be different across the score systems.1,28) The treatment policies based on score systems may have obstacles such as overtreatment of patients with no CALs.It was reported that sustained activated values after IVIG treatment, including higher WBC and CRP levels, were observed in the IVIG nonresponsive group.29,30) We also previously reported that sustained higher WBC and CRP values were observed in the IVIG nonresponsive group at 24 hours after IVIG termination (>38℃), whereas the IVIG responsive group showed an approximately 40%–60% reduction of these indices.5) At the present time, the definition of IVIG nonresponsiveness is not clearly determined; the observation periods after termination of IVIG are different (24, 36, or 36–48 hours) and the basis of body temperature for nonresponsiveness (37.5℃ or 38.0℃) is also not identical among study groups. Initial IVIG nonresponders also consist of patients having different inflammation intensity. In the present study, CALs incidence and repeated IVIG cases were relatively similar in the different fever-duration groups but they were the highest in the ≥8th day group. This suggests that CALs may begin to develop and progress in the early stage of the disease before the peak of inflammatory intensity, and that prompt further treatment for severely affected patients is essential. It is proposed that patients who have no clinical improvement, such as a lack of well-being with sustained fever ≥38℃, and show no change or increased CRP and/or WBC at 24 hours after IVIG infusion, should be treated as soon as possible with next step treatments such as intravenous methylprednisolone (30 mg/kg) with/without additional IVIG (1 g/kg). If patients do not respond to this treatment, third step treatments such as infliximab and cytotoxic drugs also can be tried according to the laboratory and clinical findings performed the next day.1,5) This treatment modality based on laboratory indices may be more reasonable and is expected to reduce the incidence of severe CALs. However, it was reported that early IVIG with additional methylprednisolone pulse therapy (30 mg/kg, one dose) could not prevent aneurysm formation in some severely affected patients.31) Moreover, patients who did not received IVIG with spontaneous defervescence or those who responded to initial IVIG or next step treatments, on occasion, can progress to severe aneurysms after defervescence.32,33) These findings suggest that there may be etiologic substances that induce continuous inflammation in coronary artery pathologic lesions, and that severity and chronicity of CALs may depend on immune status of the host against KD insults. The immune status of patients with giant aneurysms may not control the etiologic substances properly and may induce ongoing inflammation in coronary artery pathologic lesions. Therefore, immune-modulators such as IVIG and corticosteroids may be limited in ability to remove the substances inducing sustained coronary artery injury.1,6,34)Increase of platelet count or thrombocytosis (>400,000/mm3) is noted in the early convalescent stage of KD. As shown in this study, platelet count may begin to increase after the peak of inflammation. 18) All patients with KD in this study also showed increased platelet values during admission.6) Recently we reported that platelet count and IgG, IgM, and IgA levels were concurrently increased in the early convalescent stage. Platelet and each immunoglobulin level were statistically correlated. In this study, only the patients with fever duration of ≥8 days showed the highest total protein value (6.8 g/dL) as well as platelet value with similar albumin value compared to earlier fever day groups. Since total protein is consist of mainly albumin and gamma globulin fraction (immunoglobulins and other proteins), this finding suggests that immunoglobulins may also begin to increase after the peak of inflammation in KD. Moreover, it was reported that lower total protein level after initial IVIG treatment was a characteristic of initial IVIG-nonresponders or nonresponders of second line treatment.5,30) These results suggest that platelets, immunoglobulins, and other anti-inflammatory proteins are associated with the recovery reaction from KD insults. Additionally, the increased extent of these indices in the subacute stage may reflect the extent of systemic inflammation. Therefore, confirmation of this characteristic of KD inflammation could be helpful for KD patient selection after IVIG treatment.35)In conclusion, KD is a self-limited systemic inflammatory process and the intensity of systemic inflammation gradually increases and reaches a peak at mean 6th day after fever onset. The severity of systemic inflammation is reflected in laboratory indices such as CRP, WBC differentials, albumin and hemoglobin, and these parameters correlated with each other. Repeated daily examinations of laboratory indices before the peak of inflammation of KD could be helpful in early diagnosis for early-presented patients with KD and incomplete KD. The same modality after IVIG treatment is also helpful for proper treatment for IVIG nonresponsive or severely affected patients.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J 2012;53:262–275.
[Article] [PubMed] [PMC]2. Kawasaki T. Acute febrile mucocutaneous lymph node syndrome in young children with unique digital desquamation. Arerugi 1967;16:178–222.
[PubMed]3. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379–1385.
[Article] [PubMed]4. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 2004;114:1708–1733.
[Article] [PubMed]5. Hwang JY, Lee KY, Rhim JW, Youn YS, Oh JH, Han JW, et al. Assessment of intravenous immunoglobulin non-responders in Kawasaki disease. Arch Dis Child 2011;96:1088–1090.
[Article] [PubMed]6. Kil HR, Yu JW, Lee SC, Rhim JW, Lee KY. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr Rheumatol Online J 2017;15:60
[Article] [PubMed] [PMC]7. Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo (Japan): Ministry of Health and Welfare, 1984.8. Kang HJ, Kim GN, Kil HR. Changes of clinical characteristics and outcomes in patients with Kawasaki disease over the past 7 years in a single center study. Korean J Pediatr 2013;56:389–395.
[Article] [PubMed] [PMC]9. Rhim JW, Youn YS, Han JW, Lee SJ, Oh JH, Lee KY. Changes in Kawasaki disease during 2 decades at a single institution in Daejeon, Korea. Pediatr Infect Dis J 2014;33:372–375.
[Article] [PubMed]10. Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, et al. Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009–2011. Pediatr Infect Dis J 2014;33:24–27.
[Article] [PubMed]11. Kim GB, Park S, Eun LY, Han JW, Lee SY, Yoon KL, et al. Epidemiology and clinical features of Kawasaki disease in South Korea, 2012–2014. Pediatr Infect Dis J 2017;36:482–485.
[Article] [PubMed]12. Yu JJ. Diagnosis of incomplete Kawasaki disease. Korean J Pediatr 2012;55:83–87.
[Article] [PubMed] [PMC]13. Manlhiot C, Christie E, McCrindle BW, Rosenberg H, Chahal N, Yeung RS. Complete and incomplete Kawasaki disease: two sides of the same coin. Eur J Pediatr 2012;171:657–662.
[Article] [PubMed]14. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135:e927–e999.
[Article] [PubMed]15. Chung HS, Lee KY, Han JW, Cha SW, Lee DJ, Whang KT. Clinical analysis and comparison of Kawasaki disease between patients younger than one year of age and those over one year of age. J Korean Pediatr Soc 1999;42:936–942.16. Lee KY, Oh JH, Han JW, Lee JS, Lee BC. Arthritis in Kawasaki disease after responding to intravenous immunoglobulin treatment. Eur J Pediatr 2005;164:451–452.
[Article] [PubMed]17. Lee KJ, Kim HJ, Kim MJ, Yoon JH, Lee EJ, Lee JY, et al. Usefulness of anterior uveitis as an additional tool for diagnosing incomplete Kawasaki disease. Korean J Pediatr 2016;59:174–177.
[Article] [PubMed] [PMC]18. Lee KY, Han JW, Hong JH, Lee HS, Lee JS, Whang KT. Inflammatory processes in Kawasaki disease reach their peak at the sixth day of fever onset: laboratory profiles according to duration of fever. J Korean Med Sci 2004;19:501–504.
[Article] [PubMed] [PMC]19. Kang JH, Hong SJ, Seo IA, Kwak MH, Cho SM, Kim DK, et al. Early detection of Kawasaki disease in infants. Korean Circ J 2015;45:510–515.
[Article] [PubMed] [PMC]20. Seo JH, Yu JJ, Ko HK, Choi HS, Kim YH, Ko JK. Diagnosis of incomplete kawasaki disease in infants based on an inflammation at the bacille calmette-guérin inoculation site. Korean Circ J 2012;42:823–829.
[Article] [PubMed] [PMC]21. Choi HS, Lee SB, Kwon JH, Kim HS, Sohn S, Hong YM. Uveitis as an important ocular sign to help early diagnosis in Kawasaki disease. Korean J Pediatr 2015;58:374–379.
[Article] [PubMed] [PMC]22. Yellen ES, Gauvreau K, Takahashi M, Burns JC, Shulman S, Baker AL, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics 2010;125:e234–e241.
[Article] [PubMed] [PMC]23. Jun HO, Yu JJ, Kang SY, Seo CD, Baek JS, Kim YH, et al. Diagnostic characteristics of supplemental laboratory criteria for incomplete Kawasaki disease in children with complete Kawasaki disease. Korean J Pediatr 2015;58:369–373.
[Article] [PubMed] [PMC]24. Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr 1986;108:388–392.
[Article] [PubMed]25. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 2006;149:237–240.
[Article] [PubMed]26. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006;113:2606–2612.
[Article] [PubMed]27. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 2007;166:131–137.
[Article] [PubMed]28. Rigante D, Andreozzi L, Fastiggi M, Bracci B, Natale MF, Esposito S. Critical overview of the risk scoring systems to predict non-responsiveness to intravenous immunoglobulin in Kawasaki syndrome. Int J Mol Sci 2016;17:278
[Article] [PubMed] [PMC]29. Fukunishi M, Kikkawa M, Hamana K, Onodera T, Matsuzaki K, Matsumoto Y, et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr 2000;137:172–176.
[Article] [PubMed]30. Seo E, Yu JJ, Jun HO, Shin EJ, Baek JS, Kim YH, et al. Prediction of unresponsiveness to second intravenous immunoglobulin treatment in patients with Kawasaki disease refractory to initial treatment. Korean J Pediatr 2016;59:408–413.
[Article] [PubMed] [PMC]31. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med 2007;356:663–675.
[Article] [PubMed]32. Tewelde H, Yoon J, Van Ittersum W, Worley S, Preminger T, Goldfarb J. The Harada score in the US population of children with Kawasaki disease. Hosp Pediatr 2014;4:233–238.
[Article] [PubMed]33. Takahashi T, Sakakibara H, Morikawa Y, Miura M. Development of coronary artery lesions in indolent Kawasaki disease following initial spontaneous defervescence: a retrospective cohort study. Pediatr Rheumatol Online J 2015;13:44
[Article] [PubMed] [PMC]
Fig. 1
Correlations among laboratory indices at the peak inflammation in acute Kawasaki disease. CRP, C-reactive protein.
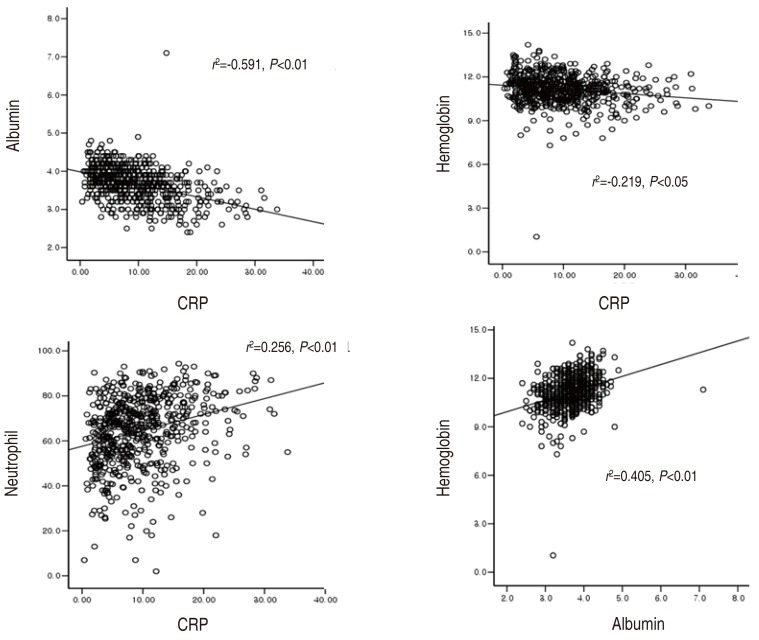
Table 1
Clinical and laboratory characteristics of patients with Kawasaki disease (KD) according to fever duration
Values are presented as mean±standard deviation or number (%). Data were analyzed by 1-way analysis of variance test.
CAL, coronary artery lesion; IVIG, intravenous immunoglobulin; WBC, white blood cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*Re-IVIG, IVIG retreatment case.
Table 2
Clinical and laboratory parameters in patients with and without coronary artery lesions

 About
About Browse articles
Browse articles For contributors
For contributors

