All issues > Volume 61(6); 2018
The outcome of percutaneous stent implantation in congenital heart disease: experience of a single institute
- Corresponding author: Seong Ho Kim, MD. Department of Pediatrics, Sejong General Hospital, 28, Hohyeon-ro, 489beon-gil, Bucheon 14754, Korea. Tel: +82-32-340-1121, Fax: +82-32-340-1236, shkim24926@hanmail.net
- Received September 14, 2017 Revised October 17, 2017 Accepted October 27, 2017
- Abstract
-
- Purpose
- Purpose
- The efficacy of percutaneous stent implantation for congenital heart disease (CHD) in Korea, where stent availability is limited, has not been determined. This study evaluated the acute and midterm results of stent implantation in different CHD subgroups.
- Methods
- Methods
- Stents were implanted in 75 patients with 81 lesions: (1) pulmonary artery stenosis (PAS) group, 56 lesions in 51 patients; (2) coarctation of the aorta (CoA) group, 5 lesions in 5 patients; (3) Fontan group, 13 lesions in 12 patients; (4) ductal stent group, 3 lesions in 3 patients; and (5) other CHD group, 4 lesions in 4 patients. Mean follow-up duration was 2.1 years (0.1–4 years). Medical records were reviewed retrospectively.
- Results
- Results
- The minimum lumen diameter (MLD) in PAS and CoA increased from 5.0±1.9 mm and 8.4±1.6 mm to 10.1±3.6 mm and 12.3±2.5 mm, respectively (P<0.01). In the PAS group, pressure gradient decreased from 25.7±15.6 mmHg to 10.4±10.1 mmHg, and right ventricular to aortic pressure ratio from 0.56±0.21 to 0.46±0.19. In the CoA group, the pressure gradient decreased from 50±33 mmHg to 17±8 mmHg. In the ductal stent group, the MLD of the ductus increased from 2.3 mm to 4.3 mm and arterial oxygen saturation from 40%–70% to 90%. No deaths were associated with stent implantation. Stent migration occurred in 3 patients, but repositioning was successful in all. Stent redilation was performed successfully in 26 cases after 29±12 months.
- Conclusion
- Conclusion
- Percutaneous stent implantation was safe and effective, with acceptable short and mid-term outcomes in Korean CHD patients.
- Introduction
- Introduction
Vascular stenosis in patients with congenital heart disease (CHD) is associated with significant morbidity and mortality.1) Since the first report of percutaneous dilation of pulmonary stenosis in 1953, percutaneous balloon angioplasty has been widely used for many vascular lesions of CHD because it is less invasive than surgery under cardiopulmonary bypass. In the late 1980's, percutaneous stent implantation was used to treat vascular stenosis in CHD. Numerous reports have shown that stent implantation is a safe and effective treatment that provides excellent immediate and long-term outcomes.1,2,3) Stent implantation can dilate parts of a stenotic lesion and can also provide a support structure around it to prevent recoil. Nowadays, stent implantation is used widely and effectively in patients with CHD, especially in cases of recoiling with balloon angioplasty, even though it is still considered “off label” i.e., not approved by the U.S. Food and Drug Administration for conditions such as branch pulmonary artery stenosis (PAS), coarctation of the aorta (CoA), and venous system stenotic lesions. However, in Korea, only a few reports about stent implantation in patients with CHD have been published, and they included only immediate or short-term follow-up in patients with PAS.4,5) The purpose of this study was to evaluate the safety and efficacy of intravascular stent implantation in various types of CHD, and to assess the improvement in immediate and midterm outcomes based on our experience in a single institute.
- Materials and methods
- Materials and methods
- 1. Subjects and methods
- 1. Subjects and methods
Patients with CHD who underwent percutaneous stent implantation between March 2012 and August 2016 in the Department of Pediatric Cardiology at Sejong General Hospital were enrolled in this study. A total of 75 patients with 81 vascular lesions underwent implantation of vascular stents; the sites of the lesions were as follows: (1) PAS group, 56 lesions in 51 patients; (2) CoA group, 5 lesions in 5 patients; (3) Fontan group, 13 lesions in 12 patients; (4) ductal stent group, 3 lesions in 3 patients; and (5) other CHD group, 4 lesions in 4 patients.Patients' medical records were retrospectively reviewed for demographic data.To evaluate the effectiveness and safety of catheterization, all catheterization data were obtained before and after stent implantation. The following parameters were recorded: minimum lumen diameter (MLD), systolic pressure gradient (PG) in the stenotic pulmonary artery and right ventricular to aortic pressure ratio in the PAS group; MLD and systolic PG at the narrowest portion of the aorta in the CoA group; mean PG in the Fontan group; and MLD and arterial oxygen saturation in the ductal stent group. All of patients in our study had a full preprocedural evaluation, including laboratory tests, chest radiography, electrocardiogram, echocardiography, and computed tomography angiography. Stent implantation was performed with the following indication in our study: in PAS group, PG on stenosis >20 mmHg, perfusion difference in lung perfusion scan (LPS) >2:1, or right ventricular to aortic pressure ratio >0.6; in CoA group, PG >15 mmHg; in Fontan group, mean PG >2 mmHg or LPS >2:1; in ductal stent group, although balloon valvuloplasty for pulmonary atresia with intact ventricular septum was performed, SaO2 <80% after weaning of prostaglandin E1. The indication for reintervention is the same as above, but also includes serial redilation cases. We collected the time from stent implantation to the next reintervention for patients requiring reintervention. For evaluating the effectiveness after stent implantation in each group, LPS, computed tomography angiography, and echocardiography were done before and after the procedure. We also collected data concerning complications of the stent implantation.We used various types of stent, including Omnilink (Abbott, Abbott Park, IL, USA), Express (Boston Scientific, Marlborough, MA, USA), and medium sized Genesis stents (Johnson and Johnson, New Brunswick, NJ, USA), which can be expanded to a maximum of 12 mm in diameter. In some cases, balloon angioplasty was performed first to make it easier to introduce the stent. We tried using cut Palmaz 4014 or 5014 stents (Johnson and Johnson) in the pulmonary position in several cases, but this approach was abandoned because balloon perforation occurred frequently.This study was reviewed and approved by the Institutional Review Board of Sejong General Hospital (approval number: 1741).- 2. Statistical analysis
- 2. Statistical analysis
Data are expressed as mean±standard deviation for continuous data or as numbers for categorical data. The acute outcomes of stent implantation were analyzed using the paired t test. The SPSS software package (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. A P value <0.05 and a confidence interval of 95% were considered statistically significant.
- Results
- Results
- 1. Demographic data
- 1. Demographic data
The total number of patients was 75, of whom 39 were male. Mean age at initial stent implantation was 12.9 years (range, 0–71.8 years). Mean follow-up duration after stent implantation was 2.1 years (range, 0.1–4 years). The demographic and clinical characteristics of our study participants are shown in Table 1. The underlying diseases included tetralogy of Fallot (n=16), pulmonary atresia with ventricular septal defect (PA VSD; n=19), pulmonary atresia with intact ventricular septum (PA IVS; n=6), peripheral pulmonary stenosis (n=6), CoA (n=5), univentricular hearts (n=10), hypoplastic left heart syndrome (n=2), and others (n=11). Stents were deployed in a total 81 vascular stenotic lesions. We used premounted balloon-expandable stents and self-expandable stents. The Omnilink stent (n=34, 42%) was the most commonly implanted stent for all lesions, followed by the Express (n=19, 23.5%), Palmaz (n=16, 19.7%), Hercules (Microport, Shanghai, China) (n=6, 7.4%), and Genesis (n=6, 7.4%).- 2. Acute outcomes of each group
- 2. Acute outcomes of each group
1) PAS group
1) PAS group
Fifty-one patients with PAS were identified. Median age at PAS group was 12.4 years (range, 0.7–71.8 years). In this group, tetralogy of Fallot and PA VSD were the most common underlying heart diseases. The location of stent implantation was the left pulmonary artery (LPA; 36 lesions), the right pulmonary artery (RPA; 17 lesions), or the right ventricular outflow tract (3 lesions). In this group, stent implantation resulted in a significant increase in MLD from 5.0±1.9 mm to 10.1±3.6 mm (P<0.01) (Fig. 1A). The systolic pulmonary artery PG decreased from 25.7±15.6 mmHg to 10.4±10.1 mmHg after stent implantation (P<0.01) (Fig. 1B), while the right ventricular to aortic pressure ratio decreased significantly from 0.56±0.21 to 0.46±0.19 (P<0.01) (Fig. 1C).2) CoA group
2) CoA group
This group included 5 patients, all of whom had complex coarctation accompanied by other malformations, such as aortic stenosis, bicuspid aortic valve, and Takayasu's arteritis. Mean age at this group was 18.2 years (range, 11.9–25.5 years). All patients underwent stent implantation for relieving residual aortic coarctation after surgery. The MLD of the stenotic site increased significantly from 8.4±1.6 mm to 12.3±2.5 mm (P<0.01) (Fig. 2A), while the PG decreased from 50±33 mmHg to 17±8 mmHg (P<0.01) (Fig. 2B).3) Fontan group
3) Fontan group
Stent implantation was performed in 12 patients. Mean age at Fontan group was 17.0 years (range, 0.8–24.3 years). The largest number of stents were placed in the LPA (8 lesions), followed by RPA (3 lesions), and the Fontan conduit (2 lesions). The mean pressure of the Fontan pathways before and after stenting decreased from 15.0 mmHg to 9.7 mmHg (P<0.01).4) Ductal stent group
4) Ductal stent group
Three patients were identified in this group and all were diagnosed as having PA IVS. Mean age at ductal stent group was 14.3 days (range, 7–21 days, and 2 to 16 days after balloon valvuloplasty). Stent implantation into the ductus arteriosus was performed using peripheral stent (4-mm diameter, 12–15 mm long). The MLD increased from 2.3 mm to 4.3 mm (P<0.01), and arterial oxygen saturation improved significantly from 40%–70% to 90% (P<0.01).5) Other CHD group
5) Other CHD group
Four patients were included in this group: 1 patient had double outlet right ventricle (DORV), 1 patient had PA, DORV and remote VSD, 1 had total anomalous pulmonary venous return (TAPVR) of mixed type, and 1 had congenital pulmonary vein stenosis (PVS). Mean age at this group was 6.8 years (range, 0.9–12.1 years). The deployment of the stents in this group was as follows: 2 cases of pulmonary vein stenting in DORV and congenital PVS, 1 case of superior vena cava (SVC) stenting in TAPVR mixed type, and 1 case of left ventricular outflow tract (LVOT) stenting in patient with PA, DORV, and remote VSD patient.
- 3. Complications
- 3. Complications
The overall complication rate associated with the procedure was 6.6% (5 of 75). There was only one death: a patient with congenital PVS who underwent pulmonary vein stent implantation for recurrent PVS 8 days after the procedure. However, the cause of death was pulmonary edema and sepsis, not directly related to the procedure. No acute complication, such as severe infection or massive bleeding, was observed.In 3 cases, stent migration occurred, but the stents were successfully repositioned by transcatheter manipulation. In 1 patient in the Fontan group, there was a perforation between the LPA and CoA stents. However, the perforation was tiny and there was no specific problem after the procedure. One patient in the PAS group developed hemoptysis after stent implantation, so intensive care unit care was needed to keep intubation for several hours until it resolved.- 4. Midterm outcomes
- 4. Midterm outcomes
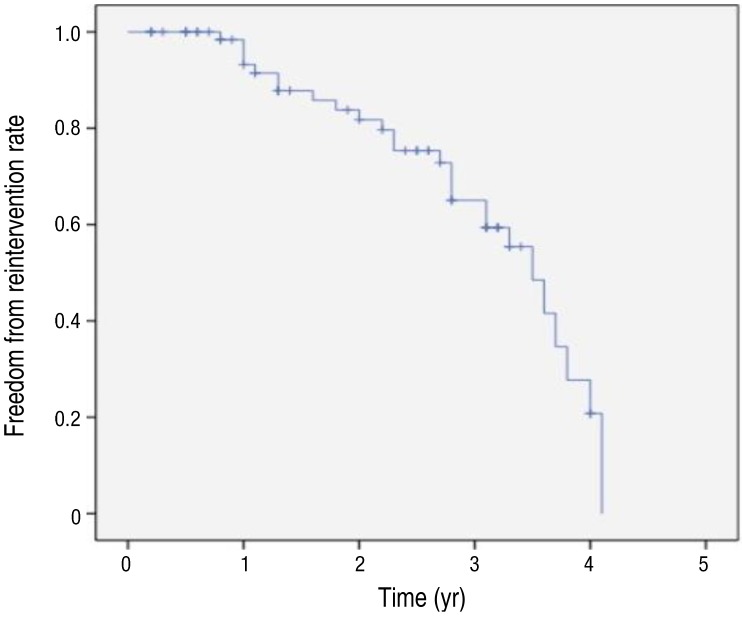
The overall survival rate was 98.6% (74 of 75). There was only 1 death, as described above. During a mean follow-up of 2.1 years, further reintervention was required in 27 patients (36%): PAS group (n=19), CoA group (n=1), Fontan group (n=5), ductal stent group (n=0), and other CHD group (n=2). The mean time to reintervention was 29±12 months. The freedom from reintervention rate of total patients in our study was shown in Fig. 3.There were 2 types of reintervention in our study. One is percutaneous catheterization, and the other is surgery. Percutaneous catheterization was performed in 26 patients, including serial redilation of stent with bigger balloon (23 of 26), or stent-in-stent implantation due to stent intimal growth (3 of 26). Only 1 patient with DORV who had implanted stent for PVS had further surgical reintervention due to recurrent PVS.
- Discussion
- Discussion
The aim of this study was to analyze the short-term outcomes of stent implantation in different groups of patients with CHD in order to assess the efficacy and safety of the method, and also to describe the midterm outcomes of stent implantation in a single institution over a 4-year period. As in previously published reports, our results from patients with CHD occur immediately after stent implantation and at follow-up in our hospital were generally excellent.Stents have contributed greatly to the development of interventional catheterization for CHD, especially PAS. Stent implantation can be considered universally superior to balloon angioplasty in the aspect of recoil. In PAS patients, elevated right ventricular pressure is associated with right ventricular failure, arrhythmias and sudden cardiac death. Aggressive management is required to improve the patient's hemodynamics.1,3) Therefore, the treatment of PAS is critical for these patients. In our study, PAS was the most common lesion over all stent implantations for CHD. We evaluated the effect of stent implantation on PAS in terms of the increase in the MLD of the stenotic lesions, the decrease in systolic PG, and the decrease in right ventricular to aortic pressure ratio; our stent implantation procedures yielded statistically significant successful outcomes for all 3 parameters. These results are consistent with studies by other investigators.2,3,6,7,8,9,10) However, the right ventricular to aortic pressure ratio of some patients did not decrease significantly after the procedure, probably because of residual distal PAS.Percutaneous stent implantation can be used to reduce the blood PG and to relieve symptoms associated with native CoA or residual coarctation after surgical intervention. According to previous data, stent implantation is similarly as effective as surgical repair with respect to acute and intermediate-term follow-up outcomes.11) In our study, stent implantation showed good efficacy with an acceptable midterm outcome in all patients, except for 1 patient with abdominal aorta stenosis who was suspicion of Takayasu's arteritis, and underwent balloon angioplasty for restenosis 14 months after stent implantation. In a recent report, self-expandable stents demonstrated good results for native and recurrent CoA.12) Two patients of our cases underwent stent implantation using self-expandable stent (Hercules). One patient had a excellent outcome, while the other patient received redilation after 14 months of stenting. Although the exact mechanism is unknown, restenosis of this patient is thought to be caused by chronic inflammation of vessels due to Takayasu's arteritis.In Fontan patients, stent is used to increase mean diameter and reduce PG in patients with cavopulmonary pathway obstruction.13) In our study, the most patients received stent implantation in branch pulmonary artery and 2 patients received stent in Fontan conduit and right SVC each other, and all patient experienced the significant decreased Fontan pathway pressure after stent insertion and improved hemodynamics. Some of them showed significant PG on LPA (PG>2 mmHg), but relatively good sized diameter superoinferiorly in anteroposterior projection. However, computed tomography showed significant stenosis on LPA anteroposteriorly, therefore, we implanted stent on LPA. Three-dimensional rotational angiography is beneficial for the diagnosis and interventional procedure in this situation. One of our patients with hypoplastic left heart syndrome in Fontan group had stent implantation on LPA, followed by stenting on descending aorta because LPA stent compressed descending aorta. Tiny hole developed between 2 stents, but was not significant hemodynamically.Stent implantation in a patent ductus arteriosus is an alternative to surgical palliation in neonatal patients with ductal-dependent pulmonary circulation.14,15) Optimal diameter of stented ductus was not established, and one of our patients showed significant pulmonary overflow after 5-mm stent, and needed a transcatheter closure of stented ductus with an Amplatzer vascular plug II (St. Jude Medical, St. Paul, NN, USA) in a month after confirming adequate right ventricle as a pulmonary pump. We supposed a 4-mm stent would be an optimal size for ductal stenting in PA IVS.PVS is rare, presenting either as primary PVS or secondary PVS after TAPVR repair surgery. In many cases, PVS is progressive in nature and can result in secondary pulmonary hypertension and RV failure. Since PVS is a challenging lesion to treat whether it occurred congenitally or postoperatively. Restenosis rate and overall mortality is still high. In our study, 2 patients underwent stent implantation for recurrent PVS despite serial balloon angioplasty or reoperation. Their immediate outcomes were good, however, they needed further intervention for PVS. And one of them died 8 days after stenting. This patient with congenital PVS was repaired surgically in neonate, and PVS developed, which recurred despite one more surgery and several times of balloon dilatation. Therefore, stent implantation was performed finally, but the patient died because of pulmonary edema and sepsis.Stenting of the systemic ventricular outflow tract can be a potential therapeutic option in rare patients with CHD who developed significant ventricular hypertension or severe ventricular dysfunction due to outflow obstruction.16) In our study, 1 patient with PA, DORV and remote VSD received LVOT stent implantation for LVOT stenosis after corrective surgery. Before stenting, this patient showed severe LV dysfunction and low cardiac output, requiring left ventricular assisted device support (LVAD). However, after relieving LVOT obstruction with LVOT stenting (Palmaz 4014 stent), PG of LVOT was decreased 45 mmHg to 7 mmHg significantly and improved LV function. It could be possible to wean LVAD the next day (Fig. 4). Stent fracture was observed on the distal portion twelve months after stenting, but the PG on cardiac catheterization was insignificant (<20 mmHg). Follow-up echocardiogram in 4 years also showed no significant PG on LVOT stent.Several complications related to stent implantation have been reported in other studies: stent migration during deployment, slippage of stent from balloon, late stent migration, stent fracture, embolism, lung congestion, gastrointestinal bleeding, paralysis of the brachial plexus, perforation of the pulmonary artery, and hemoptysis. In some previously reported multicenter studies, up to 23.1% of patients experienced an adverse event, 2.8% of patients required surgical treatment, and 10% experienced a high severity event.2,3,6,7,8,9) In our study, complication rate was 6.6%, which is lower or similar to other institutes. And unlike other studies, there were no severe complications such as massive bleeding, bacteremia, or death related to procedure. Although stent migration occurred in 3 patients, repositioning was successful in all. In our cohort, overall survival rate was 98.6% (74 of 75). There was no death apparently associated with the stents or procedures in our study.In many studies including our study, efficacy and safety of the stent is amply demonstrated. However, mid and long-term outcomes are not sufficiently completed. Although stents make up for shortcoming of balloon angioplasty in aspect of recoil, in-stent stenosis is also outstanding issue to resolve. In the studies of Tomita et al.2) and Ing et al.17), redilation rates were 42.3% (108 of 255) and 58.9% (43 of 73), respectively. In our study, 30.6% of patients (23 of 75) needed redilation after stent implantation. Especially, in case of early age intervention, as the younger patients grow, further dilation of the stent will be required to compensate for their somatic growth or because of in-stent restenosis.1,2,17) According to recent study, stents with a larger diameter appear to remain patent longer than small stents, allowing a long interval free from reintervention.2) However, deployment of an oversized stent may be potentially harmful to the vessel in small child. So in some cases, repetitive in-stent redilation can be an effective method after small stent insertion. In our study, serial redilation after stent implantation was the most common reason for reintervention, and only in 3 cases, there were stent-in-stent procedures due to intimal growth. However, our study differs from other studies in the following point. In our study, there were many cases of planned serial redilation for reduce the risk of vessel rupture. These results would influence the value of freedom from reintervention of total patients in our study (Fig. 3). And to overcome the problem of restenosis, it will be necessary to carry out close long-term follow-up and to determine the mechanism of restenosis.The Palmaz stent is still the most commonly used for lesions of great vessels, such as pulmonary arteries, aorta, and systemic veins.2,8,17) In our study, the Omnilink (premounted stent) was the most commonly used, followed by the Express (premounted stent). This differs slightly from previous studies, because, unlike other countries, the types of stent that can be used for treatment in Korea are limited. Stents with maximum expanded diameter >15 mm were not available in Korea, apart from the Palmaz 4014 or 5014 stent, which was used for CoA but was too long for pulmonary artery deployment. We tried using cut Palmaz 4014 or 5014 stents in the pulmonary position in several cases, but this approach was abandoned because balloon perforation occurred frequently.New stents have been developed and have emerged with promising results. In practice, many of the types of stent used currently in children are used “off-label,” because they have not been approved for vascular lesions in children.This study had several limitations. First, it included only a relatively small cohort and it was retrospective in design. Second, since the procedures were performed by more than 2 operators, it is possible that the postprocedural follow-up was nonuniform. Third, there is a lack of analysis for related factors or mechanism about in-stent restenosis. Furthermore, at present, several stents, such as the P308E (Cordis, Miami, FL, USA), Genesis XD (Cordis Corp., Bridgewater, NJ, USA), or Valeo (Bard Peripheral Vascular, Tempe, AZ, USA), are not available in Korea. The limited supply of stents remains a challenge to be overcome in order to achieve more successful outcomes of stent implantation.The results of our study showed that stent implantation is not only an effective and safe therapeutic modality for the treatment of vascular stenosis, but also an essential strategy in patients with CHD. However, we have no long-term results from this procedure; accordingly, the follow-up will continue so that we can detect and manage any unexpected long-term complications that may occur.
- Notes
Conflicts of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Shaffer KM, Mullins CE, Grifka RG, O'Laughlin MP, McMahon W, Ing FF, et al. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol 1998;31:661–667.
[Article] [PubMed]2. Tomita H, Nakanishi T, Hamaoka K, Kobayashi T, Ono Y. Stenting in congenital heart disease: medium- and long-term outcomes from the JPIC stent survey. Circ J 2010;74:1676–1683.
[Article] [PubMed]3. Law MA, Breinholt JP 3rd, Shamszad P, Justino H, Mullins CE, Ing FF. The outcome of pulmonary artery stents following surgical manipulation. Catheter Cardiovasc Interv 2011;77:390–394.
[Article] [PubMed]4. Ko HK, Kim YH, Yu JJ, Ko JK, Park IS, Seo DM, et al. Effectiveness and safety of percutaneous transcatheter implantation of pulmonary arterial stent in congenital heart disease. Korean Circ J 2012;42:40–45.
[Article] [PubMed] [PMC]5. Kim SH. Pulmonary artery stents-still “off label”. Korean Circ J 2012;42:8–9.
[Article] [PubMed] [PMC]6. Lewis MJ, Kennedy KF, Ginns J, Crystal MA, Torres A, Vincent J, et al. Procedural success and adverse events in pulmonary artery stenting: insights from the NCDR. J Am Coll Cardiol 2016;67:1327–1335.
[PubMed]7. O'Laughlin MP, Slack MC, Grifka RG, Perry SB, Lock JE, Mullins CE. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation 1993;88:605–614.
[Article] [PubMed]8. O'Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation 1991;83:1923–1939.
[Article] [PubMed]9. Boe BA, Zampi JD, Schumacher KR, Yu S, Armstrong AK. The use and outcomes of small, medium and large premounted stents in pediatric and congenital heart disease. Pediatr Cardiol 2016;37:1525–1533.
[Article] [PubMed]10. Danon S, Gray RG, Crystal MA, Morgan G, Gruenstein DH, Goldstein BH, et al. Expansion characteristics of stents used in congenital heart disease: serial dilation offers improved expansion potential compared to direct dilation: results from a Pediatric Interventional Cardiology Early Career Society (PICES) investigation. Congenit Heart Dis 2016;11:741–750.
[Article] [PubMed]11. Yeaw X, Murdoch DJ, Wijesekera V, Sedgwick JF, Whight CM, Pohlner PG, et al. Comparison of surgical repair and percutaneous stent implantation for native coarctation of the aorta in patients ≥ 15 years of age. Int J Cardiol 2016;203:629–631.
[Article] [PubMed]12. Khajali Z, Sanati HR, Pouraliakbar H, Mohebbi B, Aeinfar K, Zolfaghari R. Self-expandable stent for repairing coarctation of the left-circumferential aortic arch with right-sided descending aorta and aberrant right subclavian artery with Kommerell's aneurysm. Ann Vasc Surg 2017;38:318.e7–318.e10.
[Article] [PubMed]13. Mets JM, Bergersen L, Mayer JE Jr, Marshall AC, McElhinney DB. Outcomes of stent implantation for obstruction of intracardiac lateral tunnel Fontan pathways. Circ Cardiovasc Interv 2013;6:92–100.
[Article] [PubMed]14. Tola HT, Ergul Y, Saygi M, Ozyilmaz I, Guzeltas A, Odemis E. Ductal stent implantation in tetralogy of fallot with aortic arch abnormality. Tex Heart Inst J 2015;42:281–284.
[Article] [PubMed] [PMC]15. Michel-Behnke I, Akintuerk H, Thul J, Bauer J, Hagel KJ, Schranz D. Stent implantation in the ductus arteriosus for pulmonary blood supply in congenital heart disease. Catheter Cardiovasc Interv 2004;61:242–252.
[Article] [PubMed]16. Porras D, McElhinney DB, Del Nido P, Lock JE, Meadows J, Marshall AC. Clinical and stent-related outcomes after transcatheter or operative placement of bare-metal stents in the ventricular septum or subvalvar systemic outflow tract. Circ Cardiovasc Interv 2012;5:570–581.
[Article] [PubMed] [PMC]
Fig. 1
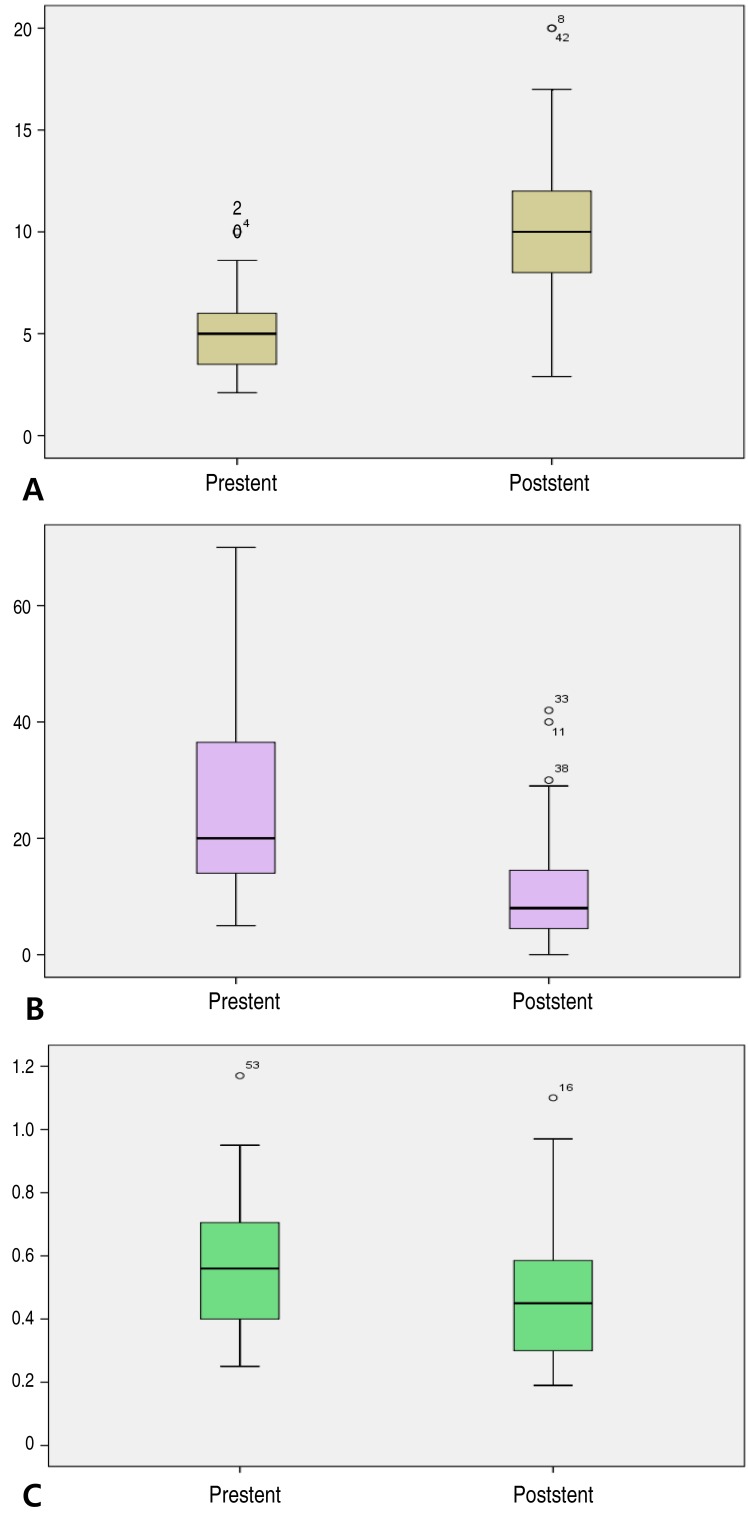
Fig. 2
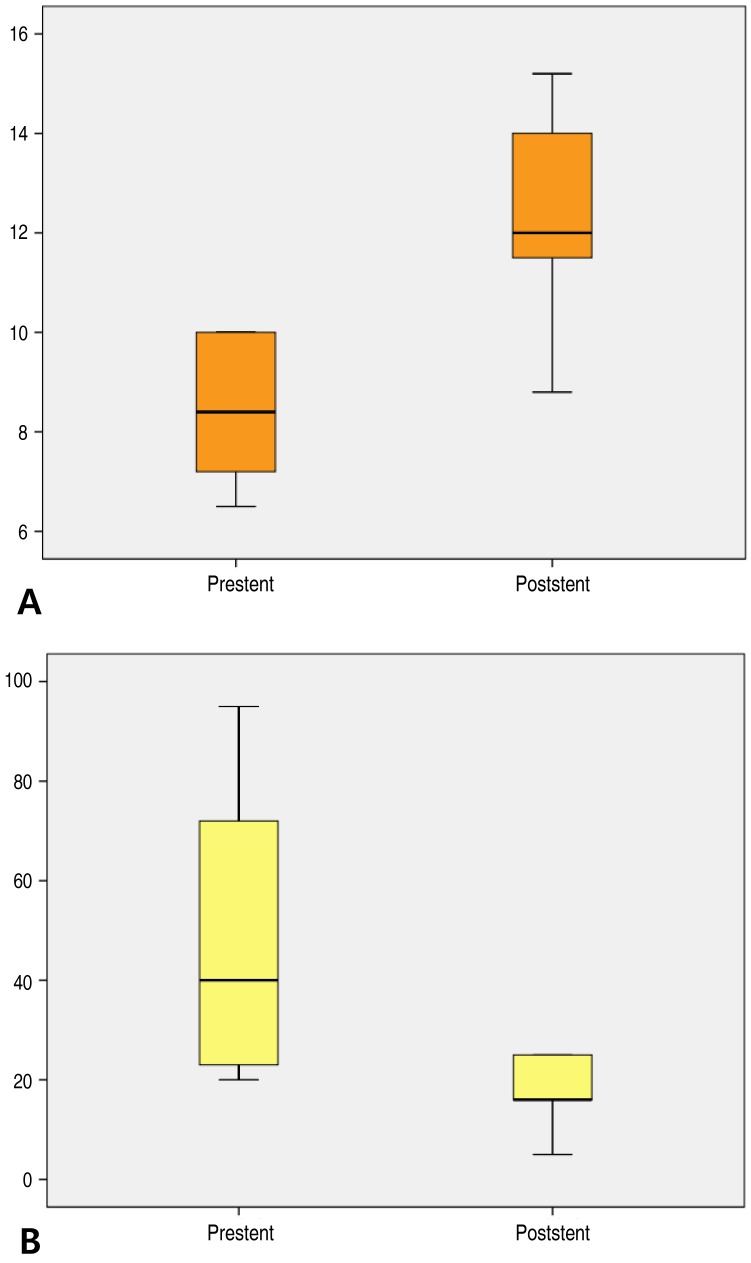
Fig. 4
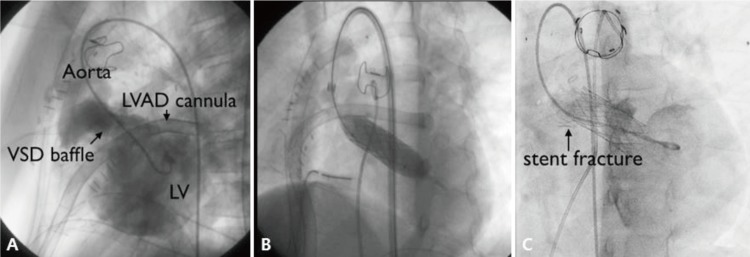
Table 1
Values are presented as median (range) or number (%).
TOF, Tetralogy of Fallot; PA VSD, pulmonary atresia with ventricular septal defect; PA IVS, pulmonary atresia with intact ventricular septum; PPS, peripheral pulmonary stenosis; CoA, Coarctation of aorta; UVH, univentricular hearts; HLHS, hypoplastic left heart syndrome; PAS, pulmonary artery stenosis; SVC, superior vena cava; LVOT, left ventricular outflow tract.
*Others: anomalous origin of the right pulmonary artery from the ascending aorta (n=2), atrial septal defect (n=1), congenital pulmonary vein stenosis (n=1), double outlet right ventricle (n=2), Takayasu's arteritis (n=1), Taussig-Bing anomaly (n=1), total anomalous pulmonary venous return mixed type (n=1), and truncus arteriosus (n=2).

 About
About Browse articles
Browse articles For contributors
For contributors