All issues > Volume 59(Suppl 1); 2016
Hashimoto thyroiditis with an unusual presentation of cardiac tamponade in Noonan syndrome
- Corresponding author: Hwa Jin Cho, MD. Department of Pediatrics, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea. Tel: +82-62-220-6646, Fax: +82-62-222-6103, chhj98@gmail.com
- Received July 09, 2014 Revised September 01, 2014 Accepted September 22, 2014
- Abstract
-
Noonan syndrome is an autosomal dominant, multisystem disorder. Autoimmune thyroiditis with hypothyroidism is an infrequent feature in patients with Noonan syndrome. A 16-year-old boy was admitted because of chest discomfort and dyspnea; an echocardiogram revealed pericardial effusion. Additional investigations led to a diagnosis of severe hypothyroidism due to Hashimoto thyroiditis. The patient was treated with L-thyroxine at 0.15 mg daily. However, during admission, he developed symptoms of cardiac tamponade. Closed pericardiostomy was performed, after which the patient's chest discomfort improved, and his vital signs stabilized. Herein, we report a case of an adolescent with Noonan syndrome, who was diagnosed with Hashimoto thyroiditis with an unusual presentation of cardiac tamponade.
- Introduction
- Introduction
Noonan syndrome is an autosomal dominant, multisystem disorder with heart defects, cerebrovascular abnormalities, short stature with delayed puberty, cryptorchidism, and delayed language1). However, autoimmune disorders are not commonly reported manifestation of Noonan syndrome2). Autoimmune thyroiditis has been reported in some literatures however thyroiditis with hypothyroidism is even less frequent in Noonan syndrome3).The pericardial effusion can be due to various conditions such as malignancies, infections, metabolic processes, trauma, connective tissue diseases, and endocrinologic disorder, such as hypothyroidism4). Pericardial effusion is rare in hypothyroid patients with a reported incidence of 3% to 6%; cardiac tamponade in these patients is even more infrequent5). Herein, we report a case of 16-year-old male, who had Hashimoto thyroiditis with an unusual presentation of cardiac tamponade in Noonan syndrome.
- Case report
- Case report
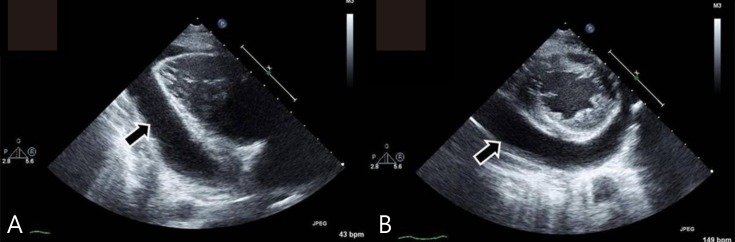
A 16-year-old male visited our clinic for evaluation of mild respiratory discomfort. On past history, he had been normally sized at birth but had pulmonary valve stenosis, for which he underwent percutaneous transluminal pulmonary valvuloplasty at 1 year of age. Also, at the age of 3 years, he had unilateral gonadal agenesis with suspected cryptorchidism, but exploratory laparotomy failed to reveal a second testicle. On examination, his height was 136.2 cm (<3rd percentile), weight was 36.7 kg (<3rd percentile) and body mass index was 19.8 kg/m2. Physical examination revealed hypertelorism, low set posteriorly rotated ears, micrognathia, thick lips, and short neck with excess nuchal skin, shield chest, and mild mental retardation. Considering his facial features, short stature and the medical history, the diagnosis of Noonan syndrome was made. In addition to the Noonan syndrome, he also had symptoms of hypothyroidism which were distended abdomen with symptoms of severe constipation, dry skin, puffy face and decreased muscle tone.At admission, his heart rate was 54 bpm, and the initial blood pressure was 100/60 mmHg. Chest radiography showed cardiomegaly with a water-bottled configuration (Fig. 1A) Electrocardiogram revealed a low voltage pattern without ST changes, and transthoracic echocardiogram demonstrated massive pericardial effusion with compression of the right ventricle during diastole (Fig. 2). There was no evidence of pericarditis or myocarditis with a normal left ventricular ejection fraction and normal findings for the following laboratory values: pro brain natriuretic peptide (20.15 pg/mL), Troponin-I (<0.015 ng/mL), erythrocyte sedimentation rate (22 mm/hr), and C-reactive protein (0.36 mg/dL). A thyroid function test was performed, which revealed high thyroid stimulating hormone (>150.0 µIU/mL) and extremely low T3 (<0.1 ng/mL) and free T4 (0.27 ng/dL). Considering these findings, additional studies were performed where the autoantibodies were elevated with an antimicrosome antibody of 320.1 IU/mL (reference, 0–34 IU/mL) and antithyroglobulin antibody of 1,700 IU/mL (reference, 0–115 IU/mL). The ultrasonogram of thyroid revealed diffuse hypoechogenicity of thyroid gland with internal striation. Also, the thyroid scan (Tc-99m pertechnetate) was consistent with hypothyroidism and ectopic thyroid was not found. All these findings were compatible with primary hypothyroidism due to Hashimoto thyroiditis. The patient was treated with L-thyroxine at 0.15 mg daily.In wards, the patient continuously reported the chest discomfort with dyspnea. He had mild tachypnea with a respiration rate of 25–29/min and his blood pressure was 70–75 mmHg (systolic) and 30–35 mmHg (diastolic). Cardiac tamponade was suspected and he underwent aseptic pericardiostomy with a pigtail catheter (8 Fr) under simultaneous sonographic and radiographic guidance, which yielded up to 360 mL of serous, yellowish clear pericardial fluid. After closed pericardiostomy, chest radiography revealed significantly reduced cardiomegaly. The patient's chest discomfort improved and his vital signs stabilized. Pericardial fluid revealed a white blood cell count of 88/mm3 (neutrophil 47%, lymphocyte 33%, and monocyte 20%), a protein level of 2.7 g/dL, and an albumin level of 2.3 g/dL. Transthoracic echocardiography showed no pericardial effusion after 3 days of closed pericardiostomy, at which point the catheter was removed. Additionally, abdominal computed tomography revealed an undescended right testis in the right inguinal area and he underwent right orchiectomy after 3 months. PTPN11 mutation testing was performed and the sequence analysis did not identify the mutation in the PTPN11 gene coding region.Over a 6-month follow-up period, levels of thyroid-stimulating hormone and free T4 normalized on L-thyroxine medication. The patient is still on medication. On chest X-ray, 6 months after the pericardial drainage, the cardiothoracic ratio was normal and there was no evidence of the cardiomegaly. (Fig. 1B)
- Discussion
- Discussion
Noonan syndrome is an autosomal dominant disorder characterized by certain facial features, short stature and congenital heart defects. It is reported at an incidence of between 1 in every 1,000 to 2,500 newborns6). Although it is an inherited disorder, the patient can be a newly diagnosed Noonan syndrome without history of disorder in other family members. The mutation in the PTPN11, SOS1, RAF1, KRAS, NRAS, and BRAF genes are reported to cause the Noonan syndrome6). Among these mutations, PTPN11 gene mutations account for about 50%, SOS1 gene mutation for 10%–15%, RAF1 gene mutation for 5%–10% of Noonan syndrome. KRAS, NRAS, and BRAF genes account for relatively small percentage of Noonan syndrome6). Our patient, who had typical clinical characteristics of Noonan syndrome, underwent PTPN11 mutation testing, however it did not identify the mutation in the PTPN11 gene coding region. The negative PTPN11 gene mutation could not rule out the diagnosis of Noonan syndrome in our case.According to scoring system of Noonan syndrome, this patient can be definitely diagnosed of Noonan syndrome. The scoring system for diagnosis of Noonan syndrome was developed by Van der Burgt et al.7) in 1997 which are made of 6 major features and 6 minor features. In this system, Noonan syndrome can be diagnosed with typical facial feature plus 1 major or 2 minor characteristics or suggestive facial feature plus 2 major or 3 minor signs. Our patient had typical facial features with 4 major criteria of pulmonary valve stenosis, short stature (below 3rd percentile), mental retardation and cryptorchidism which are definitive for Noonan syndrome. The most common congenital heart defect is pulmonary valve stenosis (50%–62%) and hypertrophic obstructive cardiomyopathy with asymmetrical septal hypertrophy is also reported in 20%8). The growth hormone levels are in normal range and somatomedin levels are sometimes elevated8). Mental retardation is present in 15%–35% which is usually mild8). Cryptochidism (undescended testicle) are also very common in Noonan syndrome (77%) as our patient also had exploratory laparotomy at age of 3 without success and had right orchiectomy at age of 16 years8).Hashimoto thyroiditis is an autoimmune disease in which the thyroid gland is attacked by cell- and antibody-mediated immune processes. The cause of autoimmune thyroiditis is unknown, but factors that may predispose to the condition include genetics, high iodine consumption, and age9). Thyroid antibodies are found more commonly in Noonan syndrome, but, hypothyroidism is not more common in Noonan syndrome compare to general population10,11). Pericardial effusion can be caused by isolated clinical problems or manifestations of various systemic diseases such as hypothyroidism12). Although mild pericardial effusion is more common in patients with hypothyroidism, moderate-to-severe pericardial effusion associated with cardiac tamponade is extremely rare. Pericardial effusion develops due to increased capillary permeability that can decrease leakage of albumin, leading to protein rich fluids in the pericardium13). Cardiac tamponade is a life threatening condition which requires immediate pericardiocentesis and/or pericardiostomy. With oral L-thyroxine replacement, mild pericardial effusion can be resolved within 2–12 months12). However, in patients with hemodynamic compromise with cardiac tamponade, pericardial fluid drainage should be urgently performed. Our patient who was diagnosed to Noonan syndrome, had severe hypothyroidism with diagnosis of Hashimoto thyroiditis. He was started oral L-thyroxine replacement immediately after the diagnosis of hypothyroidism was made, however, pericardial effusion has been aggravated and led to cardiac tamponade which required pericardial fluid drainage. Consequently, massive pericardial effusion has improved after pericardiostomy and has not recurred after the treatment of hypothyroidism which can rule out the other causes and support that the main cause of the massive pericardial effusion is hypothyroidism.In conclusion, cardiac tamponade is infrequent in hypothyroid patients with Hashimoto thyroiditis. Adolescent patients with Noonan syndrome and Hashimoto thyroiditis presenting with cardiac tamponade have not been reported previously. In patient with cardiomegaly, thorough physical examination is essential and clinicians should be aware of the possibility of concurrent autoimmune conditions in patients with Noonan syndrome. Also, appropriate clinical and laboratory evaluations should be performed, considering that pericardial effusion or cardiac tamponade can be the first symptom of autoimmune disease.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet 2013;381:333–342.
[Article] [PubMed] [PMC]3. Amoroso A, Garzia P, Vadacca M, Galluzzo S, Del Porto F, Mitterhofer AP, et al. The unusual association of three autoimmune diseases in a patient with Noonan syndrome. J Adolesc Health 2003;32:94–97.
[Article] [PubMed]4. Saito Y, Donohue A, Attai S, Vahdat A, Brar R, Handapangoda I, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography 2008;25:321–327.
[Article] [PubMed]5. Patil VC, Patil HV, Agrawal V, Patil S. Cardiac tamponade in a patient with primary hypothyroidism. Indian J Endocrinol Metab 2011;15(Suppl 2): S144–S146.
[PubMed] [PMC]6. Agarwal P, Philip R, Gutch M, Gupta KK. The other side of Turner's: Noonan's syndrome. Indian J Endocrinol Metab 2013;17:794–798.
[Article] [PubMed] [PMC]7. van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet 1994;53:187–191.
[Article] [PubMed]9. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med 1996;335:99–107.
[Article] [PubMed]10. Vesterhus P, Aarskog D. Noonan's syndrome and autoimmune thyroiditis. J Pediatr 1973;83:237–240.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors