All issues > Volume 59(Suppl 1); 2016
Idiopathic midaortic syndrome with malignant hypertension in 3-year-old boy
- Corresponding author: Gi Beom Kim, MD, PhD. Department of Pediatrics, Seoul National University Children's Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea. Tel: +82-2-2072-0266, Fax: +82-2-743-3455, ped9526@snu.ac.kr
- Received July 30, 2014 Revised August 25, 2014 Accepted August 26, 2014
- Abstract
-
Midaortic syndrome (MAS) is a rare vascular disease that commonly causes renovascular hypertension. The lumen of the abdominal aorta narrows and the ostia of the branches show stenosis. MAS is associated with diminished pulses in the lower extremities compared with the upper extremities, severe hypertension with higher blood pressure in the upper rather than lower extremities, and an abdominal bruit. The clinical symptoms are variable, and recognition in children with hypertension can aid early diagnosis and optimal treatment. Hypertension with MAS is malignant and often refractory to several antihypertensive drugs. Recently, radiologic modalities have been developed and have led to numerous interventional procedures. We describe the case of a 3-year-old boy presenting with left ventricular hypertrophy whose severely elevated blood pressure led to the diagnosis of idiopathic MAS. This case highlights the importance of measuring blood pressure and conducting a detailed physical examination to diagnose MAS. This is the first reported case of idiopathic MAS diagnosed in childhood in Korea.
- Introduction
- Introduction
Midaortic syndrome (MAS) is a rare disease characterized by luminal narrowing of the abdominal aorta, and involves stenosis of the major branches in the abdomen. In 1963, the first paper was published describing the narrowing of the descending aorta as MAS, unlike Takayasu's syndrome that involves the aortic arch and Leriche syndrome that involves aortic bifurcation1). Clinical manifestations typically include malignant hypertension, claudication, or abdominal angina in adolescents and young adults. In children, hypertension is an undervalued problem, which can be fatal or lead to a life-threatening emergency if untreated. Hypertension with MAS is severe and often unresponsive to several antihypertensive drugs, ultimately requiring surgical intervention. Here, we report a case of a 3-year-old boy presenting with left ventricular hypertrophy, who was diagnosed with idiopathic MAS following identification of severely elevated blood pressure (BP).
- Case report
- Case report
A 3-year-old boy visited the hospital outpatient department with a heart murmur. An echocardiogram showed mild left ventricle (LV) hypertrophy, and he was referred to Seoul National University Children's Hospital for further management. Transthoracic echocardiogram revealed mild LV hypertrophy with increased LV mass index (89 g/m2 or 52 g/m2.7, Fig. 1A). The patient showed no other cardiac anomaly including coarctation of the aorta. Even though he did not complain of any symptoms at the time, we administered carvedilol 3.125 mg every 12 hours for LV reverse remodeling. During a routine follow-up echocardiogram 9 months later, we found abnormally increased celiac artery velocity with a diastolic tail (peak velocity of greater than 4 m/sec, Fig. 1B), and the patient's right arm systolic BP measured 155 mmHg. He was hospitalized for evaluation of renovascular hypertension and BP management at 4 years of age.On admission, the patient did not complain of symptoms including headache and chest pain. The patient's body weight was 21.2 kg (>97th percentile) and height was 110.1 cm (>97th percentile). His BP was high (206/113 mmHg in the right arm and 194/104 mmHg in the right leg). A grade 2/6 systolic heart murmur was audible at the left sternal border. A chest X-ray showed no significant cardiomegaly and a 12-lead electrocardiogram revealed LV hypertrophy (Fig. 2).Laboratory findings on admission were as followings: serum blood urea nitrogen, 17.0 mg/dL; serum creatinine, 0.53 mg/dL; serum renin activity, 21.0 ng/mL/h (reference value, <6.7 ng/mL/hr); and serum aldosterone, 39.4 ng/dL (reference value, 3–35 ng/dL). The erythrocyte sedimentation rate was normal. Renal artery Doppler ultrasonography showed an intermittent tardus parvus spectrogram in both renal arteries with a smaller right kidney (Fig. 3A). Under the impression of renovascular hypertension, we performed computed tomographic (CT) angiography of the abdomen. The CT angiography revealed severe stenosis at the bilateral renal artery, the ostium of the celiac branch, and the ostium of the superior mesenteric artery with diffuse luminal narrowing of the abdominal aorta into the femoral artery (Fig. 3B).We prescribed labetalol continuous infusion, titrated up to 3 mg/kg/hr and administered concomitant oral antihypertensive medications (amlodipine 2.5 mg twice a day and atenolol 5 mg twice a day) to decrease the BP. Percutaneous transluminal renal angioplasty (PTRA) was planned and abdominal aortogram confirmed diffuse luminal narrowing of the abdominal aorta into the femoral artery (Fig. 4A) and the renal artery (Fig. 4B). PTRA was attempted with a 3–20 mm Savvy balloon catheter via the right femoral artery; however, the effect was unsatisfactory because of the resistance to the balloon dilatation. He was discharged with combinations of 3 oral antihypertensive drugs (amlodipine 2.5 mg twice a day and atenolol 12.5 mg once a day and enalapril 2.5 mg twice a day). At the time of discharge, BP was still high and measured as 134/60 mmHg in the right arm. Since three years after first admission, he has been followed up at the outpatient clinic without any complications and is taking 3 combinations of antihypertensive drugs (amlodipine 5 mg twice a day, atenolol 12.5 mg once a day and enalapril 1.25 mg once a day). His BP in the right arm has ranged from 110/55 to 133/58 mmHg.
- Discussion
- Discussion
We described a 3-year-old boy presenting with LV hypertrophy who was diagnosed with idiopathic MAS following identification of severely elevated BP. To the best of our knowledge, this is the first case of pediatric MAS in South Korea. Numerous studies have enumerated clinical features and radiologic findings2,3,4,5,6). MAS is prone to be referred to as hypoplasia or coarctation of the descending aorta, which presents with symptoms such as headache, claudication, and postprandial abdominal pain. Renovascular hypertension is a common comorbidity in 86% of patients with MAS3). Severe hypertension has been known to be the most common presentation leading to congestive heart failure, renal failure and severe lower extremities claudication7). The clinical presentations depend on the degree and site of the narrowing aorta. Previous studies have reported that MAS is commonly diagnosed within the first 3 decades8,9). Recently, the intensive school healthy screening system substantially aids the earlier detection in developed countries, although the patient has had no specific symptoms. Moreover, the widespread use of radiological modalities such as CT or aortography has led to the better recognition of MAS.The etiology of MAS is not completely understood, and it can be congenital or acquired. Congenital MAS is a developmental defect that is caused by both incomplete fusion and overfusion of the paired embryonic dorsal aorta10,11). The most common location of stenosis is the proximal renal artery (up to 80%), and at least 25% of cases may occur in the superior mesenteric artery9,11,12). Acquired MAS is caused by Takayasu's arteritis, neurofibromatosis, fibromuscular dysplasia, retroperitoneal fibrosis, mucopolysaccharidosis, and Williams syndrome13).Severe complications such as congestive heart failure, cerebrovascular disease, and hypertensive encephalopathy present frequently and may be fatal. Most complications arise from malignant hypertension. The response to antihypertensive drugs is known to be poor in hypertension with MAS and several previous studies have shown vascular surgery to be the best curative therapy14). As experience with PTRA grows, there are more trials in MAS. However, the PTRA success rate decreased with MAS compared with isolated renal artery stenosis because of the extensive stenotic nature of the aorta and renal branches15), even though PTRA is successful in reducing hypertension by using different technical approaches in the arteries from one study3).In conclusion, we report a 3-year-old boy presenting with left ventricular hypertrophy who was diagnosed with congenital MAS following the identification of severely elevated BP. Clinical suspicion and accurate examination, including BP measurement, would aid early diagnosis of MAS and optimal treatment, and are important to prevent the fatal complications from hypertension.
- Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
- References
- 1. Sen PK, Kinare SG, Engineer SD, Parulkar GB. The middle aortic syndrome. Br Heart J 1963;25:610–618.
[Article] [PubMed] [PMC]2. Panayiotopoulos YP, Tyrrell MR, Koffman G, Reidy JF, Haycock GB, Taylor PR. Mid-aortic syndrome presenting in childhood. Br J Surg 1996;83:235–240.
[Article] [PubMed]3. Tummolo A, Marks SD, Stadermann M, Roebuck DJ, McLaren CA, Hamilton G, et al. Mid-aortic syndrome: long-term outcome of 36 children. Pediatr Nephrol 2009;24:2225–2232.
[Article] [PubMed]4. Sumboonnanonda A, Robinson BL, Gedroyc WM, Saxton HM, Reidy JF, Haycock GB. Middle aortic syndrome: clinical and radiological findings. Arch Dis Child 1992;67:501–505.
[Article] [PubMed] [PMC]5. ten Dam K, van der Palen RL, Tanke RB, Schreuder MF, de Jong H. Clinical recognition of mid-aortic syndrome in children. Eur J Pediatr 2013;172:413–416.
[Article] [PubMed]6. Sethna CB, Kaplan BS, Cahill AM, Velazquez OC, Meyers KE. Idiopathic mid-aortic syndrome in children. Pediatr Nephrol 2008;23:1135–1142.
[Article] [PubMed]7. O'Neill JA Jr, Berkowitz H, Fellows KJ, Harmon CM. Midaortic syndrome and hypertension in childhood. J Pediatr Surg 1995;30:164–171.
[Article] [PubMed]8. Terramani TT, Salim A, Hood DB, Rowe VL, Weaver FA. Hypoplasia of the descending thoracic and abdominal aorta: a report of two cases and review of the literature. J Vasc Surg 2002;36:844–848.
[Article] [PubMed]9. De Bakey ME, Garrett HE, Howell JF, Morris GC Jr. Coarctation of the abdominal aorta with renal arterial stenosis: surgical considerations. Ann Surg 1967;165:830–843.
[Article] [PubMed] [PMC]11. Cohen JR, Birnbaum E. Coarctation of the abdominal aorta. J Vasc Surg 1988;8:160–164.
[Article] [PubMed]12. Graham LM, Zelenock GB, Erlandson EE, Coran AG, Lindenauer SM, Stanley JC. Abdominal aortic coarctation and segmental hypoplasia. Surgery 1979;86:519–529.
[PubMed]13. Delis KT, Gloviczki P. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther 2005;17:187–203.
[Article] [PubMed]
Fig. 1
Transthoracic echocardiogram. (A) This 2-dimensional image shows a mild left ventricular (LV) hypertrophy (arrow) with increased LV mass index (89 g/m2 or 52 g/m2.7) at 3 years of age. (B) A pulsed Doppler examination reveals increased celiac artery velocity with diastolic tail (arrowhead, peak velocity of greater than 4 m/sec) at 4 years of age.
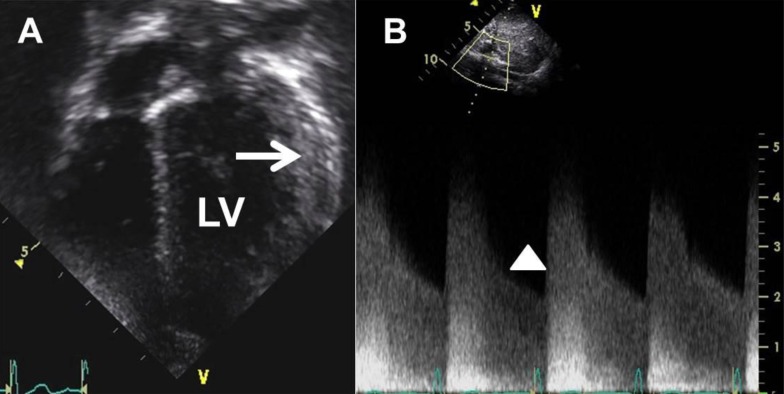
Fig. 2
Chest posteroanterior (PA) radiograph and electrocardiogram at 4 years of age. (A) Chest PA radiogram at admission shows no significant cardiomegaly (cardiothoracic ratio, 0.46). (B) A 12-lead electrocardiogram reveals left ventricular hypertrophy.
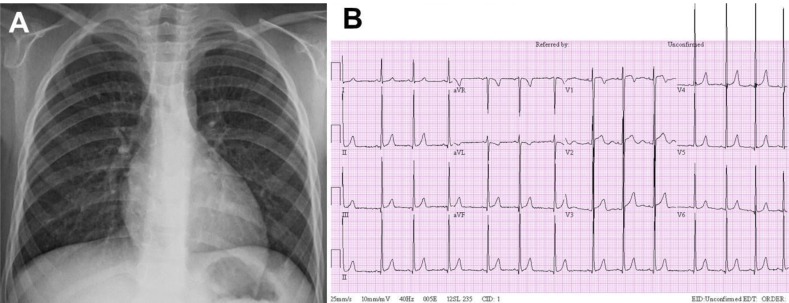
Fig. 3
Renal artery Doppler ultrasonography and computed tomographic (CT) abdominal angiography. (A) Renal artery Doppler ultrasonography shows an intermittent tardus parvus spectrogram with prolonged acceleration time, decreased peak systolic velocity, and elevated diastolic flow in both renal arteries. (B) The 3-dimensional reconstruction image shows diffuse luminal narrowing of the abdominal aorta from the renal artery (arrow) into both femoral arteries with the narrowest diameter of 1.17 mm at the iliac bifurcation (arrowhead).
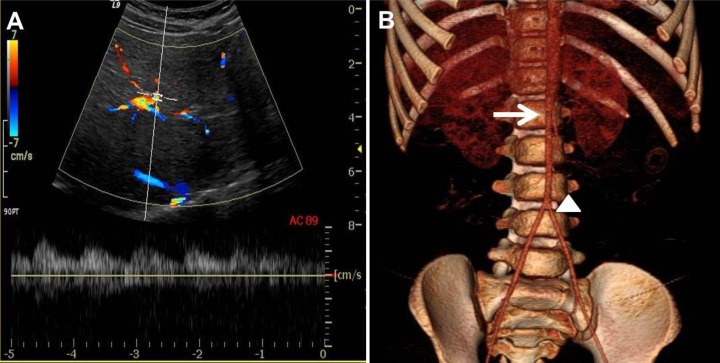
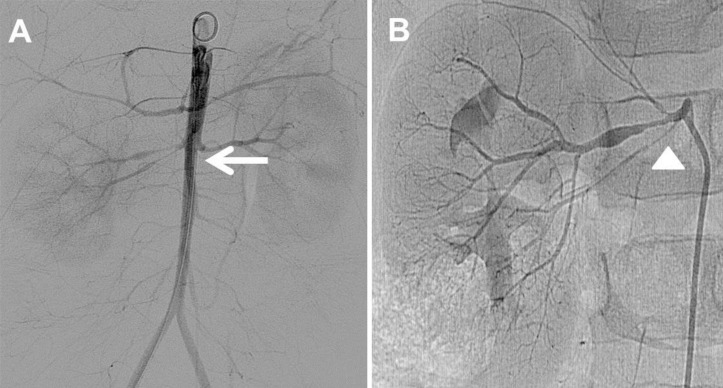
Fig. 4
Abdominal aortography and renal angiography. (A) This abdominal aortography confirms diffuse luminal narrowing of the abdominal aorta from the renal artery origin into the both femoral arteries (arrow). (B) The renal arteriography reveals severe stenosis at the right renal artery with duplication (arrowhead).

 About
About Browse articles
Browse articles For contributors
For contributors
