All issues > Volume 65(8); 2022
Effect of cyclic pamidronate administration on osteoporosis in children with β-thalassemia major: a single-center study
- Corresponding author: Mahmoud A. El-Hawy, MD. Pediatrics Department, Faculty of Medicine, Menoufia University, Shebin el Kom, 32511, Menoufia, Egypt Email: mahmodelhawy18@yahoo.com, mahmoud.elhawi@med.menofia.edu.eg
- Received May 18, 2019 Revised August 10, 2020 Accepted August 12, 2020
- Abstract
-
- Background
- Background
- Osteopenia and osteoporosis represent a prominent cause of morbidity in children with thalassemia. Multiple factors are responsible for the pathogenesis of bone loss in thalassemia, including diabetes, hypothyroidism, parathyroid gland dysfunction, accelerated hemopoiesis, direct iron toxicity of osteoblasts, iron chelators, and deficiencies of growth hormone or insulin growth factors.
- Purpose
- Purpose
- To assess the effect of pamidronate administration on β-thalassemia major-induced osteoporosis in children.
- Methods
- Methods
- This study assessed the effects of different treatments (calcium and vitamin D versus calcium, vitamin D, and pamidronate) on patients with β-thalassemia major and osteoporosis. Bone mineral density (BMD) and z scores were measured at baseline and after 1 year of treatment using dual-energy x-ray absorptiometry.
- Results
- Results
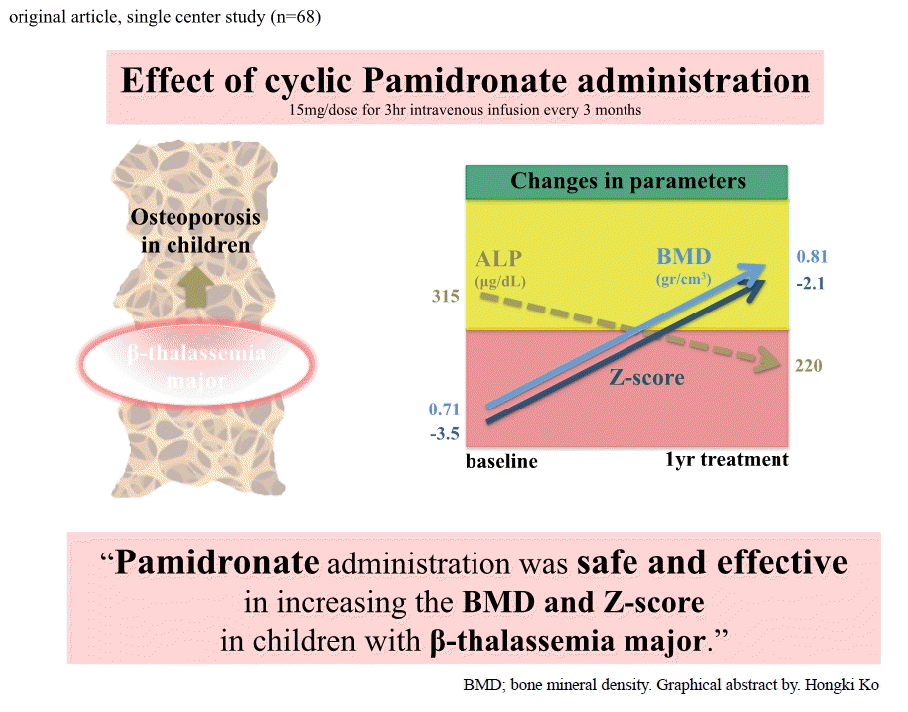
- The mean baseline BMD values of the lumbar spine were 0.71±0.07 (g/cm2) and 0.74±0.07 (g/cm2), respectively, while those at the end of the study were 0.81±0.07 (g/cm2) (P<0.001) and 0.78±0.07 (g/cm2) (P>0.05), respectively. The mean baseline z scores of the lumbar spine were -3.53±0.55 and -3.17±0.61, while those after treatment were -2.1±0.32 (P=0.001) and -3.11±0.67 (P>0.05), respectively. The baseline alkaline phosphatase levels were 351.5±86.07 μg/dL and 357.6±89.7 μg/dL, while those after treatment were 220.4± 59.26.07 μg/dL (P<0.001) and 320.3±83.99 μg/dL (P>0.05), respectively.
- Conclusion
- Conclusion
- Pamidronate administration effectively increased the BMD and z scores of children with β-thalassemia major. Pamidronate had a favorable safety profile with no related serious adverse events during the study period.
Graphical abstract
- Introduction
- Introduction
Thalassemia major is an inherited hemoglobinopathy due to defect in the ability in the synthesis of beta-globin chains [1]. Thalassemia causes a defect in bone metabolism as a chronic hematological disorder due to the disturbance in the balance between osteoblasts and osteoclasts activities [2]. Osteoporosis is a major cause of morbidity in β-thalassemia cases [3]. The pathogenesis of osteoporosis in thalassemia is complicated and several genetic and acquired factors are implicated in the incidence of bone diseases, including hypogonadism, hypothyroidism, growth hormone and insulin growth factor (IGF)-1 deficiency, ineffective haemopoiesis with progressive marrow expansion and direct iron toxicity on osteoblasts [4].Bisphosphonates reported being the major drugs in the therapeutic arsenal of osteoporosis. They have powerful antiresorptive actions. Their diversity in action and antifracture efficacy may be clinically varied depending on the strength of connection and detachment to the bone tissue [5]. Bisphosphonates were effective in increasing bone mineral density (BMD) and prevention of bone fractures in patients with osteoporosis [6]. Pamidronate, a second generation of amino bisphosphonates, was used intravenously with minimal side effects for the management of osteoporosis [7]. The administration of pamidronate in the pediatric patients with β-thalassemia is not widely used and its effects on osteoporosis have not been properly evaluated [8].In this study, BMDs of children with β-thalassemia major were reported. Changes in BMDs due to administration of calcium/vitamin D alone or in combination with pamidronate during a 1-year follow-up were also reported.
- Methods
- Methods
- 1. Study population
- 1. Study population
This study was conducted on 68 Egyptian patients with β-thalassemia major disease during March 2018 to April 2019. Written consent was obtained from each participant and the study was approved by the medical ethics committee of Menoufia University Hospital, Egypt.A full history was taken from all patients regarding demgraphic data and previous medical history including age, sex, height, weight, duration, and frequency of blood transfusion. In addition, all participants were questioned about any symptoms like bone pain and life quality changes during clinical examinations every 2 months. Selected patients were under regular blood transfusion once about every 3 to 4 weeks and all were on chelation therapy by standard protocol. Patients were divided into 2 groups according to treatment protocols:1) Group 1: Patients regularly received a 3-hour intravenous infusion of Pamidronate in a dose of 15 mg/dose, every 3 months for 1 year [9] and also received calcium in dose of 40 mg/kg and vitamin D in dose of 2,000 IU of vitamin D3 daily for 8 weeks supplementary treatment.2) Group 2: Patients received calcium and vitamin D supplementary treatment only.During the entire period of the study, patients were regularly checked by physical examination and also by hematological (complete blood count) and biochemistry (alkaline phosphatase, ferritin, and serum iron) laboratory analysis. For evaluation of treatment efficacy, BMD and z score of patients’ lumber region were measured at the beginning and at the end of the study. The z score is the number of standard deviations above or below the average for age- and sex-matched control subjects where control group consisted of healthy children; nonhospitalized with no pathological findings had been recorded in their physical examinations and being within the same range of age recruited from outpatient general pediatric clinic after being treated for simple infections. They did not receive vitamin D and calcium.- 2. Inclusion and exclusion criteria
- 2. Inclusion and exclusion criteria
Inclusion criteria for the present study were having B- thalassemia major, BMD<-2.5, serum ferritin level>1,000 mg/dL, more than 10 sessions of blood transfusion or having received more than 100 mL/kg of blood up to the study time. Selected patients were under regular blood transfusion once about every 3 to 4 weeks and all were on chelation therapy by standard protocol. Exclusion criteria were a history of bone diseases, leukemia or other neoplastic disorders, gastrointestinal disorders or inflammatory conditions during the study.- 3. Bone mineral density
- 3. Bone mineral density
BMD evaluation was done using dual-energy x-ray absorptiometry (DXA) (Norland XR-46 ver. 3.9.6/2.3.1, Peachtree City, GA, USA) at lumbar spines (LS) (L1–4). The BMD results were converted to age- and sex-specific z scores based on the normative reference data for BMD in Egyptian children. Patients with BMD of less than -2.5, at the beginning of study, were considered osteoporotic.- 4. Growth and puberty
- 4. Growth and puberty
Eleven children of group I were in prepubertal stage (stage 1), 23 children were in pubertal stage where (10 children in stage 2 and 13 children in stage 3). 10 children of group 2 were in prepubertal stage (stage 1), 24 children were in pubertal stage where (11 children in stage 2 and 13 children in stage 3) according to Tanner staging.- 5. Follow-up
- 5. Follow-up
Each patient was examined every 2 months including clinical examination in regard to their weight; height and pubertal staging according to Tanner. They were measured serially and Biochemical measurements were performed at every visit and x-ray. Echocardiography was done every 4 months for cardiac assessment and also abdominal sonography done every 4 months for liver assessment.- 6. Biochemistry
- 6. Biochemistry
Serum total calcium, phosphate, creatinine, and alkaline phosphatase activity were measured using colorimetric methods.- 7. Bone densitometry
- 7. Bone densitometry
BMD evaluation was done using DXA at LS (L1–4) after 1 year from treatment.- 8. Radiographic evaluation
- 8. Radiographic evaluation
For quantitative evaluation of osteoporotic lesions, we compared x-rays on LS that were obtained at the start of treatment to those from the last follow-up visit. Radiographs were screened for lesions that were sufficiently well delimited to allow for measurement of lesion size.- 9. Statistical analyses
- 9. Statistical analyses
Data was collected during the entire period of the study and analyzed by IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Continuous variables were presented as mean±standard deviation, while for categorical variables; numbers (%) were used. Chi-square test was used for comparison of the categorical variables. Student t test and analysis of variance tests were used to compare continuous parametric variables in 2 and more than 2 groups, respectively. Statistical significance was based on 2-sided design-based tests evaluated at the 0.05 level of significance.
- Results
- Results
Thirty-four β-thalassemia patients on monthly pamidronate plus standard treatments (group 1), 34 patients on standard treatments only (group 2), and 34 cases as controls were recruited. Table 1 summarizes their demographic characteristics and controls. Demographic characteristics included age, sex, body weight, height, and body mass index (BMI). There was highly statistical significance (P<0.001) as regards weight, statistical significance (P<0.01) as regards height and no statistical significance as regards age, sex, and BMI.In all patients, the values of hemoglobin, serum calcium, serum phosphorus, alkaline phosphatase, serum 1,25 hydroxy cholecalciferol, ferritin, iron, and total iron-binding capacity were clearly lower than those of the controls (P<0.001) (Table 1). The above parameters did not display any significant differences between the 2 groups. The mean baseline BMD of the LS was 0.71±0.07 (g/cm2). After 1 year of treatment with pamidronate, it reached 0.81/0±0.07 (g/cm2) in group 1 (P<0.001). The baseline mean of the z score for the lumbar region was -3.53±0.55. At the end of the study period it reached -2.1±0.32 (P<0.001) in group 1 (Table 2). The baseline alkaline phosphatase was 351.5±68.07 µg/dL. After treatment, this value decreased to 220.4±59.26 µg/dL (P<0.001) in group 1 (Table 2).The mean baseline BMD of LS in group 2 was 0.74±0.07 (g/cm2). After 1 year of treatment with standard treatment it became 0.78±0.07 (g/cm2) (P>0.05). The baseline mean of the z score for the lumbar region in group 2 was -3.17±0.61. At the end of the study period it became-3.11±0.6 (P>0.05). The baseline alkaline phosphatase in group 2 was 357.6±89.7 µg/dL. After treatment, this value decreased to 320.3±83.99 µg/dL (P>0.05) in group 2.Table 3 illustrates the changes in BMD and z scores at the LS in 2 patients groups. The BMD and z scores significantly improved in patients with standard plus pamidronate treatment (median [interquartile range]: -3.01 [-2.63 to -3.89] at baseline and -2.12 [-2.06 to -3.72] at the end of the study; P=0.018). On the other hand, these changes were not significant in patients with standard treatments (P=0.593).The BMD and z score at the LS were increased in both group but in patients who received the standard treatment; this increase was not significant while a patient who received standard treatment plus pamidronate showed a highly significant increase (Table 4).
- Discussion
- Discussion
The management of thalassemia children by optimized transfusion programs and chelating therapy has improved markedly over the past few years leading to prolongation of the life expectancy. Osteoporosis and osteopenia are a prominent cause of morbidity in children with thalassemia [10]. These changes are due to increased marrow erythropoiesis and excess iron deposition resulting in expansion of bone marrow cavities and reduced trabecular bone volume [11]. Chelation therapy, deficiency of vitamins and minerals like vitamin D and zinc and presence of endocrinopathies like hypoparathyroidism, hypothyroidism and diabetes mellitus also contributes to developing the bone diseases [12].In this study, we evaluated the changes in BMDs due to the administration of calcium/vitamin D alone or in combination with pamidronate during a 1-year follow-up. Few similar studies have assessed the effect of pamidronate on the improvement of osteoporosis in children with thalassemia.In this study, the mean weight and height of patients with thalassemia were significantly lower than controls while BMI of patients was lower though the difference was not statistically significant which is in accordance with previous studies suggesting that chronic illness and endocrinal changes due to iron overload are the main causes [13].Hypocalcemia and vitamin D deficiency were observed in almost all the patients of thalassemia in this study. Also, there was a significant high level of phosphorus in patients with thalassemia. This could be due to hypoparathyroidism due to iron deposition in the parathyroid gland evidenced by elevated bone alkaline phosphatase in these patients [14]. Other factors also play a role including deficient calcium intake, IGF-I deficiency, delayed puberty and hypogonadism and decreased synthesis of 25-OH-D, due to hepatic siderosis [11].This study revealed a highly significant increase in BMD and z score in thalassemic children after administration of pamidronate plus calcium/vitamin D while administration of calcium/vitamin D alone has led to a significant increase in BMD and z score in thalassemic children. Naderi et al. [4] concluded that pamidronate was effective in increasing the bone mineral density and improving the osteoporosis condition in adult patients with β-thalassemia major.Viereck et al. [15] demonstrated that pamidronate can enhance the production of osteoprotegerin (OPG) by primary human osteoblasts, thus antagonizing the osteoclast genetic activity of receptor activator of nuclear factor kappa beta (NFkB ligand) and finally increase the BMD. Voskaridou et al. [16] indicated that pamidronate increased the BMD and diminished the markers of osteoclast function including tartrate-resistant acid phosphatase isoform 5b, N-telopeptides of collagen type I, OPG, and RANKL in thalassemia major-induced osteoporosis and postmenopausal women.In this study, measurement of serum alkaline phosphatase also revealed a highly significant decrease after administration of pamidronate plus calcium/vitamin D and to a significant decrease administration of calcium/vitamin D alone that itself was an indicator of osteoporosis improvement as mentioned by Naderi et al. [4]. In thalassemia major patients had increase in serum alkaline phosphatase as a marker of bone resorption and seemed to account for increased osteoclastic activity, diminished BMD. Consequently, bone diseases were observed [16].Many factors are responsible for increasing bone turnover in patients with thalassemia, but bisphosphonates such as pamidronate, are effective in repairing the BMD plus calcium/vitamin D administration [17].
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Table 1.
Baseline and laboratory characteristics of the studied children
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; Hb, hemoglobin; TIBC, total iron-binding capacity.
Group 1, patients who received pamidronate; group 2, patients who received calcium and vitamin D.
P1: comparison of the 3 groups; P2: comparison of groups 1 and 2.
P>0.05, nonsignificant; P<0.001, highly significant.
Table 2.
Comparison of alkaline phosphatase level and BMD of patients in group 1 as markers of bone remodeling before versus after treatment
Table 3.
Comparison of alkaline phosphatase level and BMD of patients in group 2 as markers of bone remodeling before versus after treatment
| Parameter |
Group 1 |
Change | P value | |
|---|---|---|---|---|
| Before | After | |||
| Alkaline phosphatase (IU/L) | 357.6±89.7 | 320.3±83.99 | 83.3 | >0.05 |
| BMD (g/cm2) | 0.74±0.07 | 0.78±0.07 | -0.04 | >0.05 |
| z score of BMD | -3.17±0.61 | -3.11±0.6 | -0.06 | >0.05 |
Table 4.
Comparison of BMD and z scores at the lumber spine before versus after treatment by group.
- References
- 1. Kowsaryan M, Zafari M. Which pamidronate protocol is the best for treating osteoporosis in beta-thalassemia major? Ann Hematol 2016;95:383–6.
[Article] [PubMed]2. Terpos E, Voskaridou E. Treatment options for thalassemia patients with osteoporosis. Ann N Y Acad Sci 2010;1202:237–43.
[Article] [PubMed]3. Kosaryan M, Vahidshahi K, Jamali AE, Sarparast L. Bone mineral density (BMD) of patients with beta thalassemia, Thalassemia Research Center 2007. J MazandaranUniv Med Sci 2012;21:63–73.4. Naderi M, Zakeri Z, Dorgalaleh A, Alizadeh SH, Tabibian SH, Bamedi T. Effect of pamidronate on osteoporosis in patients with β-thalassemia major. IJBC 2014;6:149–53.5. Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008;19:733–59.
[Article] [PubMed]6. Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 1999;104:1363–74.
[Article] [PubMed] [PMC]7. Bekheirnia MR, Shamshirsaz AA, Kamgar M, Bouzari N, Erfanzadeh G, Pourzahedgilani N, et al. Serum zinc and its relation to bone mineral density in beta-thalassemic adolescents. Biol Trace Elem Res 2004;97:215–24.
[PubMed]8. Morabito N, Lasco A, Gaudio A, Crisafulli A, Di Pietro C, Meo A, et al. Bisphosphonates in the treatment of thalassemia-induced osteoporosis. Osteoporos Int 2002;13:644–9.
[Article] [PubMed]9. Patıroğlu T, Altuner Torun Y, Kula M, Karakükçü M. Treatment of thalassemia-induced osteoporosis with intermittent pamidronate infusions: two-year follow up. Turk J Haematol 2008;25:79–82.
[PubMed]10. Fung EB. Nutritional deficiencies in patients with thalassemia. Ann N Y Acad Sci 2010;1202:188–96.
[Article] [PubMed]11. Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res 2009;24:543–57.
[Article] [PubMed] [PMC]12. Pennisi P, Pizzarelli G, Spina M, Riccobene S, Fiore CE. Quantitative ultrasound of bone and clodronate effects in thalassemia-induced osteoporosis. J Bone Miner Metab 2003;21:402–8.
[Article] [PubMed]13. Meena MC, Hemal A, Satija M, Arora SK, Bano S. Comparison of bone mineral density in thalassemia major patients with healthy controls. Adv Hematol 2015;2015:648349.
[Article] [PubMed] [PMC]14. Tantawy AA, El Kholy M, Moustafa T, Elsedfy HH. Bone mineral density and calcium metabolism in adolescents with beta-thalassemia major. Pediatr Endocrinol Rev 2008;6 Suppl 1:132–5.
[PubMed]15. Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Gründker C, et al. Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 2002;291:680–6.
[Article] [PubMed]16. Voskaridou E, Terpos E, Spina G, Palermos J, Rahemtulla A, Loutradi A, et al. Pamidronate is an effective treatment for osteoporosis in patients with beta-thalassaemia. Br J Haematol 2003;123:730–7.
[Article] [PubMed]17. Izadyar S, Fazeli M, Izadyar M, Salamati P, Gholamrezanezhad A. Bone mineral density in adult patients with major thalassaemia: our experience and a brief review of the literature. Endokrynol Pol 2012;63:264–9.
[PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors