All issues > Volume 66(2); 2023
Association between previous abortion history and risk of autism spectrum disorders among offspring: a meta-analysis
- Corresponding author: Erfan Ayubi, PhD, Social Determinants of Health Research Center, Hamadan University of Medical Sciences, Hamadan, Iran Email: aubi65@gmail.com
- Received January 17, 2022 Revised May 11, 2022 Accepted June 13, 2022
- Abstract
-
- Background
- Background
- Previous studies have been showed an association between previous abortion history and risk of Autism spectrum disorders (ASD). However, there is still controversy about true effect estimate of the association.
- Purpose
- Purpose
- This meta-analysis aimed to evaluate the association between previous abortion history and the risk of ASD.
- Methods
- Methods
- A systematic search was performed using PubMed, Scopus, and Web of Sciences databases to identify potential studies published until December 2021. The presence of statistical heterogeneity was determined using the I2 value. In the case of substantial heterogeneity, the random-effects model meta-analysis was used to estimate the pooled relative risks. The publication bias was assessed using the Egger and Begg tests.
- Results
- Results
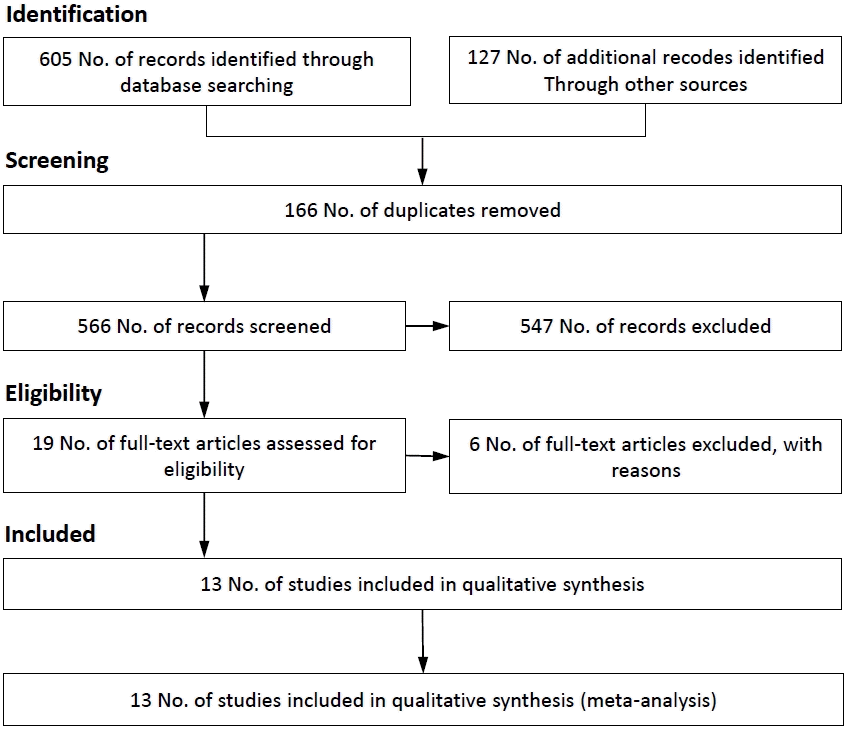
- Thirteen studies with a total of 331,779 children remained in the present meta-analysis. The estimated odds ratio of the risk of ASD associated with previous abortion history was 1.64 (95% confidence interval [CI], 1.28–2.0; I2=61.7%) in adjusted studies and 1.10 (95% CI, 1.01–1.20; I2=0.0%) in crude studies, based on the random effect model. There was moderate heterogeneity in adjusted studies. The p values for Egger and Begg regression among children with ASD were 0.393 and 0.056, respectively.
- Conclusion
- Conclusion
- This meta-analysis suggests that children born from mothers with a history of previous abortion have an increased risk of development of ASD compared to children of mothers without a history of previous abortion.
- Introduction
- Introduction
Statistics have shown that the prevalence of autism spectrum disorder (ASD), a neurological and developmental disorder, among children has increased in recent decades [1]. It is estimated that one of every 160 children has ASD [2]. ASD can manifest as social interaction disorders, communication disorders, and stereotyped behavior patterns [3].A constellation of genetic and environmental factors plays a role in the etiology of ASD; however, the true causal pathways of ASD remain unclear and require further investigation [4]. Regardless of genetic factors, the following risk factors have been introduced as determinants for the development of ASD: cesarean section, preterm labor, low birth weight, maternal infection, small for gestational age, mother’s age and psychological status, toxic exposure, parity, complications during infancy, smoking, obesity, and neonatal icterus [5-9].The effects of a previous abortion history on ASD are controversial. Some studies reported a significant positive association between previous abortion and ASD [10-12], although other studies did not [13-15].To resolve such controversy, it is necessary that the observed results of the association between previous abortion history and ASD be systematically gathered, their findings summarized and combined, and the overall effect measure of this association be derived. Therefore, this study aimed to evaluate the association between abortion history and the risk of ASD through a systematic review and meta-analysis of results of previous observational studies.
- Methods
- Methods
- 1. Database sources and searches
- 1. Database sources and searches
A systematic search was performed of the PubMed, Scopus, and Web of Sciences databases to identify studies published through December 2021. To search these databases, the following MeSH terms and keywords were used: (“spontaneous abortion” or “induced abortion” or “abortion history” or “previous abortion history” or “previous abortion” or “previous miscarriage” or “previous miscarriage history”) and (ASD or “autism spectrum disorder” or “autism spectrum disorders” or autism). We manually searched the reference lists of the included articles to identify additional potential articles. No language restrictions were set.- 2. Inclusion and exclusion criteria
- 2. Inclusion and exclusion criteria
The main inclusion criteria were as follows: observational study design (cross-sectional, case-control, or cohort) evaluating the association between previous abortion history and ASD and providing the relative risks and corresponding confidence intervals (CIs) for this association. Nonoriginal articles, such as reviews, editorials, commentaries, correspondences, notes, and book reviews, were excluded. According to these criteria, 2 authors (EJ and EA) independently screened the titles and abstracts of the articles identified in the initial searches.- 3. Data extraction and quality assessment
- 3. Data extraction and quality assessment
The following data were independently extracted by 2 authors (EJ and EA): first author, year of publication, study design, sample size, diagnosis method for ASD, child age, type of relative risk, and corresponding 95% CI. The 2 authors (EJ and EA) independently assessed the quality of the eligible articles using the Newcastle-Ottawa Scale (NOS) score [17]. The NOS is based on 3 scopes—selection, comparability, and outcome/exposure—that evaluate selection bias, confounding bias, and information bias, respectively. Each study was assigned a score of 0–9, and a score of 6 or higher was indicative of high quality. Any disagreements between the 2 authors (EJ and EA) regarding article screening, data extraction, and quality assessment were resolved through discussion.- 4. Statistical analysis
- 4. Statistical analysis
Statistical heterogeneity was determined using I2 values. The thresholds for substantial heterogeneity were set at I2>50%. In cases of substantial heterogeneity, a random-effects model metaanalysis was used to estimate the pooled relative risks. Otherwise, a fixed-effects model was applied. The results of the meta-analysis were reported using forest plots and relative risks with 95% CI. Egger and Begg tests and funnel plots were used to assess publication bias. Statistical significance was set at P<0.05. All statistical analyses were performed using Stata 14 (StataCorp LLC, College Station, TX, USA).
This systematic review and meta-analysis was performed according to the meta-analyses of observational studies in epidemiology (MOOSE) guideline [16]. The MOOSE checklist is available in the supplementary file (Supplementary Table 1).
- Results
- Results
- 1. Description of studies
- 1. Description of studies
Review of the retrieved studies and their references resulted in the inclusion of 732 studies published through December 7, 2021. Of these, 166 were duplicates and deleted. Thereafter, 547 studies were excluded by the title and abstract review, leaving 19 studies for full-text review. Six studies did not meet the eligibility criteria. Therefore, 13 studies were included in this meta-analysis (Fig. 1). Seven studies [10,11,13,15,18-20] had a case-control design, four [12,14,21,22] had a cohort design, and two [23,24] had a cross-sectional design. Among the included studies, 7 were performed in Europe, 4 in the Americas, and 1 each in Africa and Europe. The total number of children studied was 331,779. All studies were written in English (Table 1).- 2. Effects of exposure
- 2. Effects of exposure
The association between previous abortion history and ASD risk is shown in Fig. 2. The estimated odds ratios (ORs) of the risk of ASD associated with previous abortion history were 1.64 (95% CI, 1.28–2.0; I2=61.7%) in adjusted studies and 1.10 (95% CI, 1.01–1.20; I2=0.0%) in crude studies based on the random-effects model (Fig. 2). There was moderate heterogeneity in the adjusted studies, whereas the results of the crude studies were homogeneous.- 3. Publication bias
- 3. Publication bias
Begg and Egger tests were used to assess publication bias; their P values among children with ASD were 0.393 and 0.056, respectively. Publication bias was not identified among the studies that reported an association between previous abortion history and the risk of ASD (Fig. 3).- 4. Study quality
- 4. Study quality
According to the NOS scale, 12 studies were of high quality and 1 was of low quality (Table 1).
- Discussion
- Discussion
The main objective of this meta-analysis was to estimate the precise effect of the association between a previous abortion history and ASD risk. On stratification of adjusted ORs, our analysis showed that the risk of ASD may increase by 64% among children whose mothers had a history of previous abortions (pooled adjusted OR, 1.64; 95% CI, 1.28–2.00).Our analysis suggests that the offspring of women with a history of abortion were at higher risk of ASD. Regardless of ASD as a neurodevelopmental disorder, previous studies demonstrated a positive association between abortion and other neurodevelopmental disorders such as attention-deficit/hyperactivity disorder [25,26], intellectual disability [27], and cerebral palsy [28]. Since abortion is a common adverse pregnancy outcome, valid and robust evidence of its effect on adverse outcomes in children is a research need that can be addressed using large-scale prospective studies.Some plausible pathways underlie the association between abortion and ASD risk. For example, regardless of the direct effect of abortion on ASD, the relationship between them may occur through intermediate factors, such as preterm birth, low birth weight, and a lower Apgar score, risk factors for ASD [29-32]. In other words, preterm birth may be a step in the pathway between abortion and ASD development.There are data on the association between maternal autoimmunity, such as thyroid antibodies and recurrent pregnancy loss (RPL) [33,34]. Antiphospholipid antibody syndrome is characteristic of autoimmunity, with a fixed autoimmune pathogenesis leading to RPL [35,36]. Other autoimmune disorders, such as systemic lupus erythematosus, inflammatory bowel disease, autoimmune thyroiditis, celiac disease, and elevated autoantibodies isolated, are correlated with higher RPL rates [37]. Adequate management, including systemic steroids, may control abnormal immune processes and prevent RPL [38]. Maternal autoimmunity may also play a role in neurodevelopmental disorders in offspring due to the association between maternal systemic autoimmune diseases and neurological outcomes in their offspring. Maternal systemic lupus erythematous is associated with a range of adverse developmental outcomes, including learning disabilities [39]. Another study examined maternal autoimmune disorders in children with developmental delay and reported that maternal autoimmune disorders were 46% more common than in healthy children [40]. Research has shown that if an autoimmune disease flares during pregnancy, the fetal brain can be exposed to oxidative stress and the direct effects of cytokines on nerve growth. In addition, studies have reported that maternal autoimmune conditions, such as hypothyroxinemia, are associated with adverse neurodevelopmental outcomes. Pop et al. [41] showed a decrease in psychomotor test scores among children born to women with free thyroxine indices in the lowest 10th percentile. Therefore, maternal autoimmunity plays an important role in the development of maternal RPL and fetal neurological disorders.The number of meta-analyses of the determinants of ASD has increased over the last decade. For example, maternal infection during pregnancy [42], perinatal or neonatal factors [30,43], vitamin D status [44], advanced parental age [45], and maternal diabetes [46] were associated with previous abortion history in our meta-analysis. As the number of reviews of autism risk factors increases, it becomes more difficult to determine which factors are important. An umbrella review can resolve this knowledge gap by comparing the risk factors identified through a meta-analysis [47].An important issue worth consideration is that the pooled adjusted OR of 1.64 of the association between previous abortion and ASD may not be generalizable to the association between abortion and autistic disorder or between abortion and Asperger syndrome. However, the pooled OR for abortion and ASD may be modified by abortion number and type. As shown in Fig. 2, the adjusted pooled OR was closer to the true value at 1.64, while the crude (confounded) pooled OR was 1.10. This indicates that the meta-analysis of the crude associations may be biased toward the null, and several negative confounders may exist, such as smoking, alcohol use, gestational diabetes, and toxemia, that were not considered in the studies providing crude associations.This study had several limitations. First, there was a high level of heterogeneity among the pooled studies (I2=61.7%). This indicated that the causes of variability among the included studies were not included in our analysis. Subgroup and meta-regression analyses are needed to quantify this heterogeneity; however, the low number of included studies and the reporting inconsistency among them precluded further analyses. Second, we did not search all databases; thus, the studies included here may not be representative of all potential studies. However, the results from statistical tests and funnel plots (Fig. 3) indicated small bias resulting from ignoring the potential studies on pooled estimates.In conclusion, the results of this meta-analysis suggest that the offspring of mothers with a history of previous abortion are at an increased risk of developing ASD compared to those of mothers without a history of previous abortion. Further large-scale prospective cohort studies with better ascertainment of abortion, ASD spectrum components, and confounders are recommended to increase the validity and robustness of our findings.
Supplementary material
Supplementary material
Supplementary material: Supplementary Table 1 can be found via https://doi.org/10. 3345/cep.2022.00108.Supplementary Table 1.
cep-2022-00108-Supplementary-1.pdfMOOSE (meta-analyses of observational studies in epidemiology) checklist for meta-analyses of observational studies
- Footnotes
-
Conflict of interests The authors have nothing to disclose.
Funding This study was supported and funded by Hamadan University of Medical Sciences with code 140010218787.
Author contribution Conceptualization: EJ, EA; Formal Analysis: EJ, EA; Investigation: EJ, SB, MS, MR; Methodology: EJ, EA, SB, MS, MR; Project Administration: EJ, EA; Writing- Original Draft: EJ, EA; Writing-Review & Editing: EJ, EA, SB, MS, MR
-
Fig. 2.
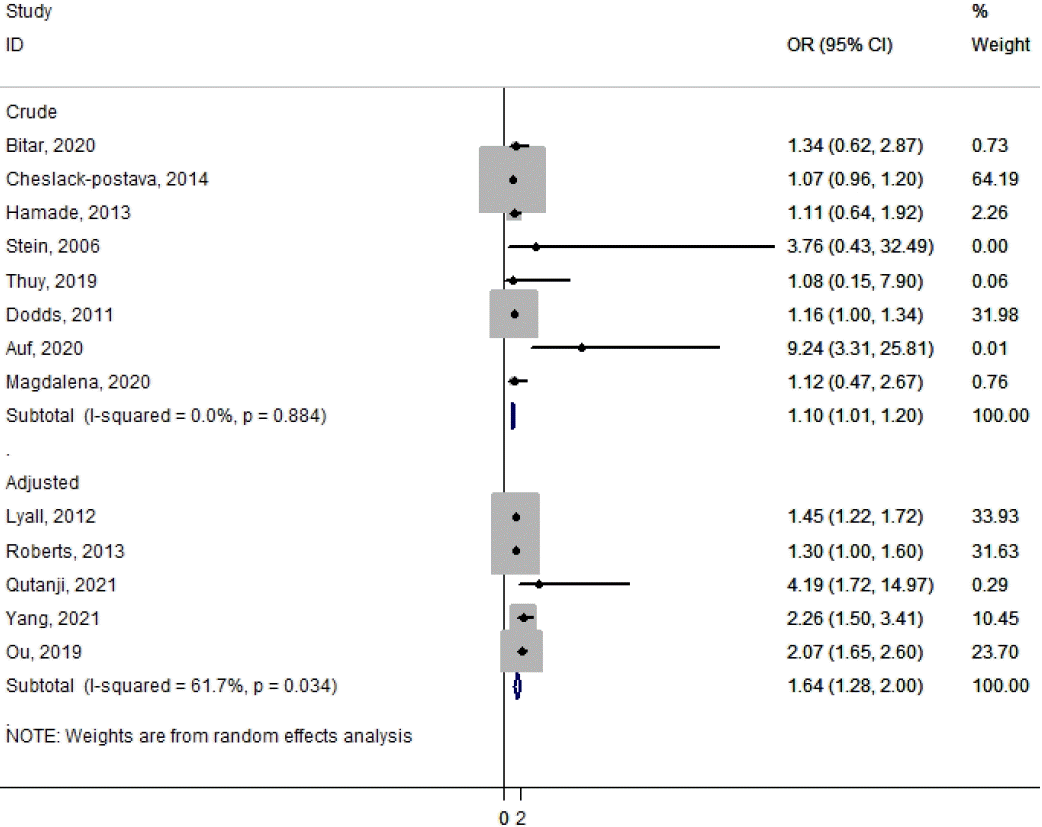
Fig. 3.

Table 1.
| Design | Study | Sample | Diagnose method | Child age (mean/range) | Estimate | Adjustment | Outcome | Quality |
|---|---|---|---|---|---|---|---|---|
| Case-control | Stein et al., 2006 [20] | 358 | DSM-IV | 4–26 Years | OR | Crude | Previous induced abortion | High |
| Cohort | Dodds et al., 2011 [22] | 129,733 | ICD-9, 10 | Not reported | OR | Crude | Previous abortion | High |
| Cohort | Lyall et al., 2012 [12] | 66,445 | PDD-NOS | Not reported | OR | Adjusted | Previous abortion | High |
| Case-control | Hamadé et al., 2013 [15] | 258 | DSM-IV | 9.94 Years | OR | Crude | Previous abortion | High |
| Cohort | Roberts et al., 2013 [21] | 116,430 | ADI-R | <12 Years | RR | Adjust | Previous abortion | High |
| Cohort | Cheslack-Postava et al., 2014 [14] | 7,649 | DSM-IV-TR, ICD-9,10 | Not reported | OR | Crude | Previous abortion | High |
| Case-control | Ou et al., 2019 [11] | 2,306 | DSM-IV-TR | Not reported | OR | Adjusted | Previous induced abortion | High |
| Cross-sectional | Vui et al., 2019 [24] | 5,893 | DSM-IV | 18–30 Months | OR | Crude | Previous abortion | High |
| Case-control | Bitar et al., 2020 [13] | 131 | DSM-IV | 8.61 Years | OR | Crude | Previous abortion | High |
| Case-control | Magdalena et al., 2020 [19] | 221 | ADOS | 3–12 Years | OR | Crude | Previous abortion | High |
| Cross-sectional | Auf et al., 2020 [23] | 500 | CARS | 3–12 Years | OR | Crude | Previous abortion | Low |
| Case-control | Qutranji et al., 2021 [10] | 141 | DSM-IV | <18 Years | OR | Adjusted | Previous abortion | High |
| Case-control | Yang et al., 2021 [18] | 1,714 | DSM-IV | 4.4 Years | OR | Adjusted | Previous abortion | High |
DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Statistical Classification of Diseases; PDD-NOS, pervasive developmental disorder not otherwise specified; ADI-R, autism diagnostic interview-revised; ADOS, autism diagnostic observation schedule; CARS, childhood autism rating scale; OR, odds ratio; RR, risk ratio.
- References
- 1. Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics 2011;127:1034–42.
[Article] [PubMed]2. Arvidsson O, Gillberg C, Lichtenstein P, Lundström S. Secular changes in the symptom level of clinically diagnosed autism. J Child Psychol Psychiatry 2018;59:744–51.
[Article] [PubMed]3. Harper C. Diagnostic and statistical manual of mental disorders. Taylor P, Corteen K, Morley S, editors. A companion to criminal justice, mental health and risk. Bristol (UK): The Policy Press, 2014.4. Siu MT, Weksberg R. Epigenetics of autism spectrum disorder. Adv Exp Med Biol 2017;978:63–90.
[Article] [PubMed]5. Thapar A, Cooper M, Eyre O, Langley K. Practitioner review: what have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013;54:3–16.
[Article] [PubMed] [PMC]6. Jenabi E, Bashirian S, Khazaei S, Basiri Z. The maternal prepregnancy body mass index and the risk of attention deficit hyperactivity disorder among children and adolescents: a systematic review and meta-analysis. Korean J Pediatr 2019;62:374–9.
[Article] [PubMed] [PMC]7. Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124:717–28.
[Article] [PubMed]8. Jenabi E, Bashirian S, Khazaei S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clin Exp Pediatr 2020;63:8–13.
[Article] [PubMed]9. Jenabi E, Bashirian S, Asali Z, Seyedi M. Association between small for gestational age and risk of autism spectrum disorders: a meta-analysis. Clin Exp Pediatr 2021;64:538–42.
[Article] [PubMed] [PMC]10. Qutranji L, Alkayyali T, Alkhateeb W, Sapmaz A, Aleter A, Almoustafa A, et al. Evaluation of the relationship between environmental factors, nutrition, and metabolic changes in children diagnosed with autism in North Cyprus: a case-control study. Cureus 2021;13:e17016.
[Article] [PubMed] [PMC]11. Ou J, Shen Y, Li Y, Xun G, Liu H, He Y, et al. Prenatal environment and perinatal factors associated with autism spectrum disorder. Glob Clin Transl Res 2019;1:100–8.
[Article]12. Lyall K, Pauls DL, Spiegelman D, Ascherio A, Santangelo SL. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses' Health Study II. Autism Res 2012;5:21–30.
[Article] [PubMed]13. Bitar T, Gerges P, Kassab MC, Hallit S, Mattar H, Soufia M, et al. Factors associated with Autism Spectrum Disorder: a case-control study in the Lebanese population. Epidemiol Biostat Public Health 2020;17:e13218.
[Article]14. Cheslack-Postava K, Suominen A, Jokiranta E, Lehti V, McKeague IW, Sourander A, et al. Increased risk of autism spectrum disorders at short and long interpregnancy intervals in Finland. J Am Acad Child Adolesc Psychiatry 2014;53:1074. –81. e4.
[Article] [PubMed] [PMC]15. Hamadé A, Salameh P, Medlej-Hashim M, Hajj-Moussa E, SaadallahZeidan N, Rizk F. Autism in children and correlates in Lebanon: a pilot case-control study. J Res Health Sci 2013;13:119–24.
[PubMed]16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12.
[Article] [PubMed]17. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): Ottawa Hospital Research Institute; c2021 [cited 2021 Dec 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.18. Yang Y, Lin J, Lu X, Xun G, Wu R, Li Y, et al. Anesthesia, sex and miscarriage history may influence the association between cesarean delivery and autism spectrum disorder. BMC Pediatr 2021;21:62.
[Article] [PubMed] [PMC]19. Magdalena H, Beata K, Justyna P, Agnieszka KG, Szczepara-Fabian M, Buczek A, et al. Preconception risk factors for autism spectrum disorder - a pilot study. Brain Sci 2020;10:293.
[Article] [PubMed] [PMC]20. Stein D, Weizman A, Ring A, Barak Y. Obstetric complications in individuals diagnosed with autism and in healthy controls. Compr Psychiatry 2006;47:69–75.
[Article] [PubMed]21. Roberts AL, Lyall K, Rich-Edwards JW, Ascherio A, Weisskopf MG. Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry 2013;70:508–15.
[Article] [PubMed] [PMC]22. Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902.
[Article] [PubMed]23. Auf KAM, Al-Nagar E-SM, Al-Gendy RAR, Abd El-Raouf ER. Screening of autism spectrum disorder by using Gilliam Autism Rating Scale in a sample of Egyptian children attending Bab Al Sha’reya University Hospital. Al-Azhar J Ped 2020;23:1259–73.24. Vui LT, Quynh CTT, Quynh NT, Huong NM, Thuy NTH, Ha BTT, et al. Prevalence of autism spectrum disorders and their relation to selected factors among children aged 18-30 months in Hoa Binh province, 2017. J Health Dev Stud 2019;3:6–17.25. Liu J, He Y, Shen Y, Zhou Y, Meng T, Xiao B, et al. Association of attention deficit/hyperactivity disorder with events occurring during pregnancy and perinatal period. Front Psychol 2021;12:707500.
[Article] [PubMed] [PMC]26. Wang H, Li F, Miao M, Yu Y, Ji H, Liu H, et al. Maternal spontaneous abortion and the risk of attention-deficit/hyperactivity disorder in offspring: a population-based cohort study. Hum Reprod 2020;35:1211–21.
[Article] [PubMed]27. Ji H, Yu Y, Miao M, Qian X, Yuan W, Lin Y, et al. Risk of intellectual disability and maternal history of spontaneous abortion: a nationwide cohort study. Dev Med Child Neurol 2021;63:831–8.
[Article] [PubMed]28. El-Tallawy HN, Farghaly WM, Shehata GA, Rageh TA, Metwally NA, Badry R, et al. Cerebral palsy in Al-Quseir City, Egypt: prevalence, subtypes, and risk factors. Neuropsychiatr Dis Treat 2014;10:1267–72.
[PubMed] [PMC]29. Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparén P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 2009;124:e817–25.
[Article] [PubMed]30. Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011;128:344–55.
[Article] [PubMed] [PMC]31. Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr 2012;161:830–6.
[Article] [PubMed] [PMC]32. Modabbernia A, Sandin S, Gross R, Leonard H, Gissler M, Parner ET, et al. Apgar score and risk of autism. Eur J Epidemiol 2019;34:105–14.
[Article] [PubMed]33. Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010;20:989–93.
[Article] [PubMed]34. Chang DL, Pearce EN. Screening for maternal thyroid dysfunction in pregnancy: a review of the clinical evidence and current guidelines. J Thyroid Res 2013;2013:851326.
[Article] [PubMed] [PMC]35. Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, et al. Pregnancy loss in the antiphospholipid-antibody syndrome—a possible thrombogenic mechanism. N Engl J Med 1997;337:154–60.
[Article] [PubMed]36. Paz Levy D, Wainstock T, Sheiner E, Sergienko R, Landau D, Walfisch A. Maternal recurrent pregnancy loss is associated with an increased risk for long‐term neurological morbidity in offspring. Dev Med Child Neurol 2019;61:91–7.
[Article] [PubMed]37. Grimstad F, Krieg S. Immunogenetic contributions to recurrent pregnancy loss. J Assist Reprod Genet 2016;33:833–47.
[Article] [PubMed] [PMC]38. Quenby S, Kalumbi C, Bates M, Farquharson R, Vince G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril 2005;84:980–4.
[Article] [PubMed]39. Ross G, Sammaritano L, Nass R, Lockshin M. Effects of mothers' autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch Pediatr Adolesc Med 2003;157:397–402.
[Article] [PubMed]40. Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. Maternal immunemediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord 2014;44:1546–55.
[Article] [PubMed] [PMC]41. Pop VJ, Brouwers EP, Vader HL, Vulsma T, Van Baar AL, De Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8.
[Article] [PubMed]42. Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain Behav Immun 2016;58:165–72.
[Article] [PubMed]43. Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 2018;142:e20180134.
[Article] [PubMed]44. Wang Z, Ding R, Wang J. The association between vitamin D status and autism spectrum disorder (ASD): a systematic review and meta-analysis. Nutrients 2020;13:86.
[Article] [PubMed] [PMC]45. Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr Scand 2017;135:29–41.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors