All issues > Volume 66(4); 2023
High-resolution esophageal manometry in children
- Corresponding author: Yogesh Waikar, Superspeciality GI Clinics & Endoscopy Center, Dhantoli, Nagpur, Maharashtra, India Email: pedgihep@yahoo.com
- Received May 31, 2022 Revised August 30, 2022 Accepted September 14, 2022
- Abstract
-
Functional disorders of the esophagus are known as esophageal dysmotility disorders. Esophageal manometry can be used to study swallowing disorders, feeding problems, nonobstructive dysphagia, and lower esophageal dysfunction. This paper discusses the recent advances in and reviews the use of high-resolution esophageal manometry in children. The Chicago 4.0 classification should be used judiciously in children. Manometric studies can guide the proper management of dysmotility in children.
Graphical abstract
- Introduction
- Introduction
Structural disorders of the esophagus are well-managed endoscopically. Functional and motor disorders of the esophagus, known as esophageal dysmotility disorders, can be assessed using manometry in children. Esophageal manometry can be used to study swallowing disorders, infantile feeding problems, nonobstructive dysphagia, and lower esophageal dysfunction. Lower esophageal dysfunction-induced reflux disease and esophageal gastric junction outlet obstruction syndrome can be evaluated using esophageal manometry. Normal metric values are currently available. This paper discusses recent advances in and reviews the use of high-resolution esophageal manometry (HREM) in children.
- Indications for esophageal manometry [1]
- Indications for esophageal manometry [1]
HREM can be used to diagnose and treat children with swallowing disorders, vomiting, noncardiac chest pain, refractory heartburn, recurrent water brash, and hiatal hernias. It is also used prior to fundoplication to assess the esophageal motility peristalsis reserve. Post fundoplication, it is used to treat persistent dysphagia. It is best used to diagnose rumination and supra-gastric belching. Esophageal function can be evaluated using HREM in patients with neurological issues.
- HREM and age
- HREM and age
In children younger than 4 years, HREM is difficult [2] to perform and analyze, as patient cooperation is required. However, in children younger than 2 years, a swallow study can be performed [3]. Normal manometry biometrics are available for 9–16-year-old patients [4]. A normal 5th–95th percentile range are published for children <18 years of age [5]. Esophageal manometry parameters are also available for preterm and term infants [6].
- HREM and catheters
- HREM and catheters
HREM can be performed safely in children with solid-state and water-perfused systems [1]. Solid-state and water-perfused system values are comparable except for small differences in outcome measures [7]. High-resolution manometry using either system is the gold standard for diagnosing esophageal motility disorders. The standard esophageal manometry catheter has 36 transducers spaced 1 cm apart. Manufacturer recommendations must be followed during the analysis [1].
- Procedure
- Procedure
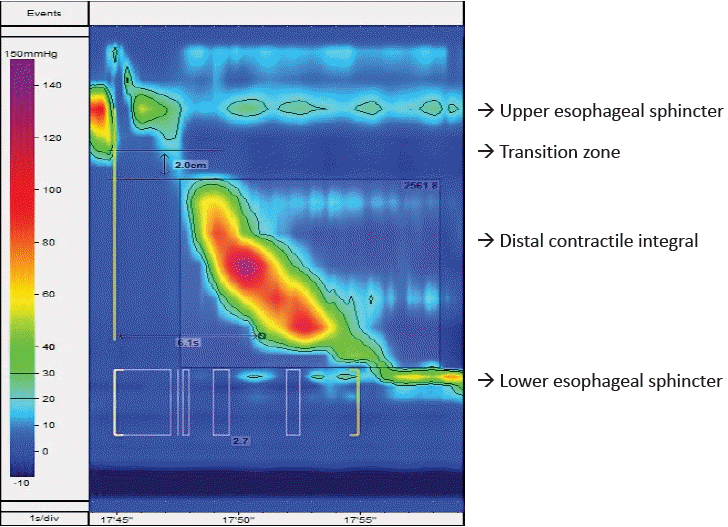
Prokinetics and anticholinergic agents must be discontinued 2 days prior to the study. In particular, baclofen, antipsychotic drugs, and attention-deficit disorder medicines may affect these values. The child should fast for at least 4 hours prior to the procedure, while those with achalasia or suspected significant motility disorders should fast for 8 hours. The HREM catheter is placed transnasally and advanced into the stomach. In children with craniofacial syndrome, an HREM catheter can be placed transorally. Topical 1–2 mL of 2% lidocaine jelly can help in transnasal catheter intubation. Oral midazolam/intranasal midazolam can be anxiolytic. In a study published by Fung et al. [8]. IV midazolam 0.5 mg/kg showed no effect on lower esophageal biometrics. Ketamine do not affect esophageal motility [9]. Propofol 0.3 mg/kg does not generally affect lower esophageal sphincter (LES) pressure, but it may reduce normal upper esophageal sphincter (UES) pressure [10,11]. Opiates should not be used to study esophageal dysmotility. If other anesthetic drugs are used for catheter placement, their use should be recorded in the analysis.Esophageal manometry is generally performed with the patient in the supine position. The child should ideally fast 4 hours prior. Informed consent is obtained from each patient’s parent or guardian. A manometry catheter is passed transnasally. The Chicago 4.0 protocol is followed. A 60-second postplacement adaptation period is allowed. Catheter position is confirmed using 3 deep inspirations. After 30 seconds, anatomical landmarks including the UES, LES, and respiratory inversion point are identified. Ten wet swallows (5 mL each) at normal room temperature water are given to each patient at 30-second intervals. multiple rapid swallow (MRS) sequences are elicited using 5 wet swallows (2 mL each) with a 10-mL syringe 2–3 seconds apart. The patient’s position is changed to upright and the catheter position confirmed on deep inspiration; thereafter, 5 wet swallows (5 mL each) are performed. A rapid drinking challenge using 200 mL of water is then performed and the patient’s response noted. A solid swallowing test, solid test meal, or pharmacological stimulation is performed in cases of inconclusive results. Patient cooperation is also confirmed throughout the procedure.The integrated relaxation pressure (IRP) of the UES underscores its tone. Esophageal peristalsis is assessed at the distal end of the UES following swallowing at a transition zone that signifies division between the striated esophageal and smooth muscle. The distal contractile integral (DCI) measures peristalsis of the distal esophagus. Distal latency (DL) measures the interval from UES relaxation to the contractile deceleration point just proximal to the LES. The IRP of the LES denotes its tone.
- HREM biometric analysis
- HREM biometric analysis
The esophagus is evaluated manually to define the motor function of the UES, esophageal body, and LES. Fig. 1 shows topographical plot of high-resolution esophageal manometry in children. Table 1 depicts the studied biometrics in esophageal manometry. The UES and LES are manometrically localized. A deep breathing maneuver is used to identify the pressure inversion point. The biometrics of esophageal function are analyzed based on the Chicago classification 4.0 [12]. The Chicago classification of esophageal motility disorders should be used for the esophageal function assessment. However, this has been poorly validated in children. The IRP correlated with lower esophageal pressure can vary by age and size [13]. The DCI, DL, and esophageal break size in the transition zone can vary by age [14]. Before considering treatment, patients should be screened and the results clinically correlated with those of other investigations such as a barium swallow test. Younger patients had a higher IRP, shorter DL, and smaller break segments [15]. Age-adjusted values reduce the ability to classify abnormal motility disorders according to the Chicago classification by up to 50% [15]. Similarly, esophageal length affects biometrics; the mean esophageal length in children is 16.8±2.8 cm. A shorter esophageal length correlates with a higher LES pressure (IRP) [15].Another important point of piecemeal deglutition requires focus when analyzing HREM topographical plots. Piecemeal deglutition is defined as the swallowing of a single bolus in 2 or more portions in the empty oral cavity. Hence, swallow markers on manometry may not represent dominant swallowing, which should be identified in children and used for the analysis [16].
- Upper esophageal biometrics in HREM
- Upper esophageal biometrics in HREM
The UES is the high-pressure area between the pharynx and cervical esophagus. UES pressure correlates with the cricopharyngeal muscle, which relaxes transiently during swallowing, belching, and vomiting [17]. UES metrics, which can be evaluated using manometry, include IRP, URP, upper esophageal resting pressure, upper esophageal nadir pressure (UNP), and peak pharyngeal pressure. The UES – IRP value was lower in children than in adults. The URP is lower in preterm and term infants than in older children and adults. The UNP is lower in children than in neonates but similar to that in adults. The peak pharyngeal pressure is higher in children than in neonates and adults. Thus, the median URP increases with age. The UES-IRP 0.2 second and UES-IRP 0.8-second median values were 1 mmHg and 18 mmHg, respectively [5].The 5th–95th percentile UES-IRP 0.2 second and UES-IRP 0.8 second are -7.9 to 19.48 and 4.05–44.45, respectively. The 5th–95th percentiles of URP [5] in children are 25–108. The 5th–95th percentile of UNP [5] in children is -9 to 15.95 mmHg, that in preterm infants is 32–36 mmHg, and that in term infants is approximately 26 mmHg [6,18]. The peak velopharyngeal pressure in children [5] is approximately 210 mmHg, while the peak mesopharyngeal pressure [5] is approximately 144 mmHg.Children with cerebral palsy and neurogenic dysphagia have poor LES relaxation or higher UES-IRP and UNP for their age. Poor pharyngeal pressure has also been correlated with dysphagia. Both an abnormally high IRP and low pharyngeal pressure can relate sequentially to swallowing difficulty [5]. The velopharyngeal region is the superior portion that participates in swallowing. The mesopharynx is the pressure region between the velopharynx and the UES, which lies below the mesopharynx and is tightly closed. UES hypertonicity confirms the presence of cricopharyngeal achalasia. UES can be normal to a hypertonic globus sensation. UES can be hypotonic in the early stages of neurogenic diseases, such as stroke and Parkinson disease, and progresses further to impaired relaxation. In Zenker diverticulum, the UES has impaired relaxation [19].Preterm infants have fewer pharyngeal contractions, lower pharyngeal activity, and a higher pharyngeal contractile integral (PhCI) per contraction than full-term infants. PhCI (mmHg.s.cm) is a 3-dimensional measure of contractile amplitude >20 mmHg over the length of the pharynx and the duration of each contraction. PhCI denotes the pharyngeal contractile effort required to swallow. Preterm infants with a higher PhCI are more prone to fatigue [20].Impendence manometry can help predict the risk of aspiration using the swallow risk index. A swallow risk index of <17±5 indicates a low risk of aspiration [21]. Bradycardia associated with pharyngeal stimulation suggests aberrant pharyngoesophageal motility in preterm infants. This subgroup is at risk of clinically significant cardiorespiratory events [22]. It is also noted that children who have higher UES-IRP 0.2 second with feeding difficulties are more likely to undergo gastrojejunostomy tube [5] or gastrostomy tube [23] placement. Cardiorespiratory symptoms, such as apnea on pharyngeal stimulation and decreased distal esophageal contractility, carry a high likelihood of aspiration [24].
- Biometrics in Chicago 4.0 classification and pediatric alterations
- Biometrics in Chicago 4.0 classification and pediatric alterations
The IRP assesses the relaxation pressure across the esophagogastric junction (EGJ) in response to deglutition. The DCI measures esophageal peristalsis. The DL is measured as the interval between the start of relaxation of the UES and the contractile deceleration point. An isobaric contour pressurization greater than 30 mmHg is considered important.UES pressure and relaxation are affected by subject position, bolus composition, and catheter size. Failed swallows are rare among children. The mean swallow break in children was approximately 0.5 cm, while the median UES resting pressure was 41 mmHg. The median DCI in children was 1,426 mmHg.s.cm, median DL was 6.6 seconds, and mean LES resting pressure was 27 mmHg. The Chicago 4.0 classification uses adult-guided normal volumes to define esophageal dysmotility. The IRP in the upright position was lower than that in the supine position. Normal contraction in the esophageal body has a DCI of 450–8,000 mmHg.s.cm. A weak contraction is defined as a DCI of 100–450 mmHg.s.cm. Failed peristalsis was defined as a DCI <100 mmHg.s.cm. Hypercontractile swallowing is defined as DCI > 8,000 mmHg.s.cm. Premature contraction is defined as a DL < 4.5 second with a DCI > 450 mmHg.s.cm.The increased median IRP with failed peristalsis suggests achalasia type I, while an increased median IRP with failed peristalsis and a PEP ≥20% of swallows suggests achalasia type II (Fig. 2). The increased median IRP with ≥20% of swallows with a DL <4.5 second in a setting of DCI ≥450 mmHg.s.cm (premature contraction) suggests achalasia type III without peristalsis (Fig. 3). Table 2 describes the different types of esophageal motility disorders which are diagnosed by esophageal manometry.An increased median IRP with ≥20% swallow elevated intrabolus pressure not meeting the criteria for achalasia is defined as an EGJ outflow obstruction (EGJOO). The manometric diagnosis of EGJOO requires an elevated IRP in primary and secondary positions.A normal IRP and the presence of at least 20% premature contractions are defined as diffuse esophageal spasm (DL <4.5 seconds). A normal IRP and ≥20% hypercontractile swallows (DCI >8,000) are defined as a hypercontractile esophagus. A normal IRP and ≥50% failed swallows or ≥70% ineffective swallows are called ineffective esophageal motility.In the MRS test, the patient performs 5 swallows (2 mL each) at 2-second intervals. The absence of esophageal body contractility with complete deglutitive inhibition of the LES during MRS and the presence of post-MRS contraction augmentation, that is, the DCI of the MRS is more than single swallow mean DCI suggest normal response. Normal MRS findings suggest a good esophageal peristaltic reserve.In a rapid drinking challenge, 200 mL of liquid is rapidly consumed. Absent esophageal contractility with complete deglutitive inhibition of the LES during the test is normally noted. If inconclusive manometry findings are noted on a solid test swallow or with pharmacological provocation, a timed barium esophagogram or endoFLIP test can be used to diagnose motility disease.In addition to acid exposure time, esophageal peristaltic reflexes play a major role in the phenotypic expression of gastroesophageal reflux disease (GERD) as evaluated by manometry [25]. Prior to fundoplication for refractory GERD, HREM is indicated, please delete highlighted part [3]. HREM sleep can be useful for evaluating post-fundoplication dysphagia [26]. Intraoperative HREM may optimize pressure changes during laparoscopic fundoplication, help align the LES and diaphragmatic pillar, and improve LES pressure by 40% [27]. HREM can also help differentiate between infective esophageal motility and GERD. In patients with infective esophageal motility, unnecessary proton pump inhibitor and antidepressant treatment and functional diagnoses can be avoided [28].Patients with tracheoesophageal fistula/atresia (TOF/EA) have associated esophageal dysmotility. HREM can diagnose these as a peristaltic subtype, isolated distal contraction, and pressurization subtype [3]. Chronic lower esophageal stasis after corrective surgery can lead to microscopic esophagitis [29]. Esophageal bolus transport is delayed in patients after TOF/EA repair. Hence, DCI would be helpful in post-TOF/EA repair cases for the calculation of residual distal contractions.In children with eosinophilic esophagitis, HREM reveals early pan-esophageal pressurization and a normal IRP [30]. These children can also experience effective esophageal peristaltic waves, higher-amplitude peristalsis, or isolated contractions with abnormal bolus pressurization. As the disease progresses, the prevalence of motility disorder increases in children with eosinophilic esophagitis [31].HREM is the gold standard test for diagnosing achalasia and its subtypes. Patients with a higher DCI after Heller’s cardiomyotomy may not require fundoplication. Even in patients with a higher UES pressure, fundoplication can be avoided [32].Simultaneous high-amplitude spikes in pressure seen in both the stomach and esophagus in the absence of a cough are defined as the R-wave of rumination. Pediatric rumination disorders can be diagnosed using HREM. The 3 subtypes of rumination are L-R-F: LES relaxation prior to R-wave and bolus flow; R-L-E: LES relaxation after R-wave; and N-R-F: waves with no LES relaxation that can be differentiated on manometry [33].
- Newer biometrics for esophageal function
- Newer biometrics for esophageal function
The DCI to esophageal impedance integral (DCIIR) ratio at an impedance cutoff of 1,500 Ω can be used to diagnose patients with ineffective esophageal motility. A DCIIR of 1500 <0.009 suggests ineffective esophageal motility [34].The use of a functional luminal imaging probe (EndoFLIP) has been studied in children with pediatric esophageal disorders. The EndoFLIP is safe for use in children with achalasia, post-Heller’s myotomy dysphagia, EGJOO, stricture, and esophageal stenosis. The EndoFLIP tests esophageal distensibility. The EndoFLIP balloon can be inflated to 30 mL. A distensibility index >2.8 mm2/mmHg suggest no major obstruction, while that <2.9 mm2/mmHg suggest persistent dysphagia symptoms [35].Laryngopharyngeal reflux disease can be diagnosed using the UES impedance integral (UESII). A low UESII (UES <2,900 Ω.s.cm) indicates poor bolus transit and is significantly associated with prominent pharyngeal reflux [36].
- Conclusion
- Conclusion
The use of HREM is a safe and important tool for diagnosing esophageal dysmotility disorders in children. Normal values are available for children. Standard and new biometrics can help its classification. The Chicago 4.0 classification provides a framework and should be used judiciously in children with age-appropriate adjustment. Manometry with impedance studies can guide the proper management of esophageal dysmotility. HREM can be used pre-, intra-, and postoperatively to differentiate and classify dysphagia for optimal results. HREM can help with the diagnosis and localization of feeding problems in children. Its use can enable the avoidance of the unnecessary use of prokinetics, proton pump inhibitors, and antidepressants. Motility disorders of the esophagus can be secondary to underlying systemic diseases or drugs. A clinical diagnosis, radiological interpretative correlations, and documentation of the patient’s personal drug history is must prior to the HREM analysis.
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Fig. 2.
Topographical plot suggesting achalasia type 3 with an increased integrated relaxation pressure (IRP). The green column indicates panesophageal pressurization. The red color indicates increased lower esophageal sphincter (LES) pressure. DCI, distal contractile integral; DL, distal latency.
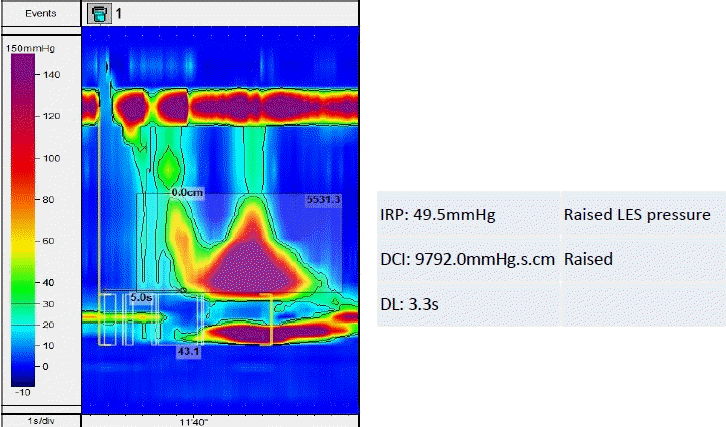
Fig. 3.
Topographical plot suggesting achalasia type 2 with an increased integrated relaxation pressure (IRP). The red color indicates increased lower esophageal sphincter (LES) pressure. DCI, distal contractile integral; DL, distal latency.
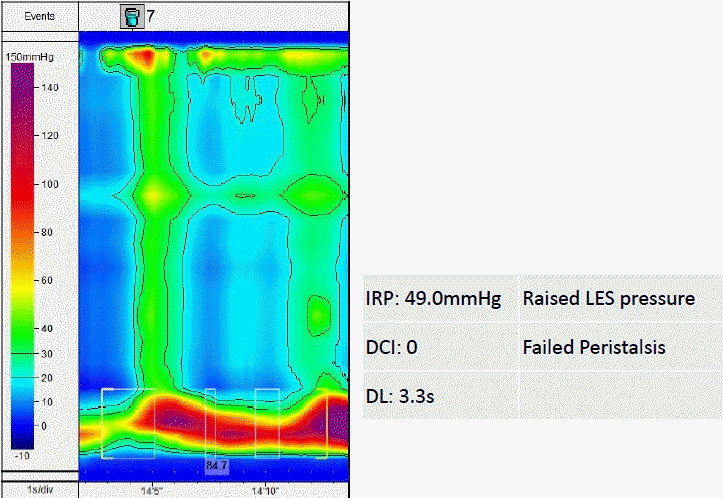
Table 1.
Important esophageal biometrics
| Biometrics | Details |
|---|---|
| IRP | Integrated relaxation pressure |
| DCI | Vigor of esophageal body contraction |
| DL | Measures latency of deglutitive inhibition |
| PEP | Pan-esophageal pressurization isobaric contour |
Table 2.
Major esophageal motility disorders
- References
- 1. Rosen R, Garza JM, Tipnis N, Nurko S. An ANMS-NASPGHAN consensus document on esophageal and antroduodenal manometry in children. Neurogastroenterol Motil 2018;30:10.1111/nmo.13239.
[Article] [PubMed] [PMC]2. van Lennep M, Leijdekkers ML, Oors JM, Benninga MA, van Wijk MP, Singendonk MMJ. Clinical experience with performing esophageal function testing in children. J Pediatr Gastroenterol Nutr 2021;72:226–31.
[Article] [PubMed] [PMC]3. Omari TI, Krishnan U. What is the role of high-resolution oesophageal manometry in paediatrics? J Paediatr Child Health 2020;56:1754–9.
[Article] [PubMed]4. Kovacic K, Kern M, Pawela L, Shaker R, Sood MR. Characteristics of high-resolution esophageal manometry in children without dysphagia. Neurogastroenterol Motil 2022;34:e14184.
[Article] [PubMed]5. Damrongmanee A, El-Chammas K, Fei L, Zang H, Santucci N, Kaul A. Pharyngeal and upper esophageal sphincter motor dynamics during swallow in children. Neurogastroenterol Motil 2021;33:e13962.
[Article] [PubMed]6. Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, et al. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil 2011;23:e401–8.
[Article] [PubMed]7. Kessing BF, Weijenborg PW, Smout AJ, Hillenius S, Bredenoord AJ. Water-perfused esophageal high-resolution manometry: normal values and validation. Am J Physiol Gastrointest Liver Physiol 2014;306:G491–5.
[Article] [PubMed]8. Fung KP, Math MV, Ho CO, Yap KM. Midazolam as a sedative in esophageal manometry: a study of the effect on esophageal motility. J Pediatr Gastroenterol Nutr 1992;15:85–8.
[Article] [PubMed]9. Chen KZ, Pan JH, Ji XA. The effects of three kinds of anesthesia on lower esophageal sphincter pressure. Can J Anaesth 1990;37(4 Pt 2): S59.
[PubMed]10. Turan A, Wo J, Kasuya Y, Govinda R, Akça O, Dalton JE, et al. Effects of dexmedetomidine and propofol on lower esophageal sphincter and gastroesophageal pressure gradient in healthy volunteers. Anesthesiology 2010;112:19–24.
[Article] [PubMed]11. de Leon A, Ahlstrand R, Thorn SE, Wattwil M. Effects of propofol on oesophageal sphincters: a study on young and elderly volunteers using high-resolution solid-state manometry. Eur J Anaesthesiol 2011;28:273–8.
[Article] [PubMed]12. Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil 2021;33:e14058.
[PubMed] [PMC]13. Singendonk MM, Kritas S, Cock C, Ferris L, McCall L, Rommel N, et al. Applying the Chicago Classification criteria of esophageal motility to a pediatric cohort: effects of patient age and size. Neurogastroenterol Motil 2014;26:1333–41.
[Article] [PubMed]14. Goldani HA, Staiano A, Borrelli O, Thapar N, Lindley KJ. Pediatric esophageal high-resolution manometry: utility of a standardized protocol and size-adjusted pressure topography parameters. Am J Gastroenterol 2010;105:460–7.
[Article] [PubMed]15. Singendonk MMJ, Ferris LF, McCall L, Seiboth G, Lowe K, Moore D, et al. High-resolution esophageal manometry in pediatrics: effect of esophageal length on diagnostic measures. Neurogastroenterol Motil 2020;32:e13721.
[Article] [PubMed] [PMC]16. Ferris L, King S, McCall L, Rommel N, Scholten I, Teague W, et al. Piecemeal deglutition and the implications for pressure impedance dysphagia assessment in pediatrics. J Pediatr Gastroenterol Nutr 2018;67:713–9.
[Article] [PubMed]17. Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med 2000;108 Suppl 4a:27S–37S.
[Article] [PubMed]18. Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil 2005;17:663–70.
[Article] [PubMed]19. Norton P, Herbella FAM, Schlottmann F, Patti MG. The upper esophageal sphincter in the high-resolution manometry era. Langenbecks Arch Surg 2021;406:2611–9.
[Article] [PubMed]20. Prabhakar V, Hasenstab KA, Osborn E, Wei L, Jadcherla SR. Pharyngeal contractile and regulatory characteristics are distinct during nutritive oral stimulus in preterm-born infants: implications for clinical and research applications. Neurogastroenterol Motil 2019;31:e13650.
[Article] [PubMed] [PMC]21. Rommel N, Selleslagh M, Hoffman I, Smet MH, Davidson G, Tack J, et al. Objective assessment of swallow function in children with suspected aspiration using pharyngeal automated impedance manometry. J Pediatr Gastroenterol Nutr 2014;58:789–94.
[Article] [PubMed]22. Hasenstab KA, Nawaz S, Lang IM, Shaker R, Jadcherla SR. Pharyngoesophageal and cardiorespiratory interactions: potential implications for premature infants at risk of clinically significant cardiorespiratory events. Am J Physiol Gastrointest Liver Physiol 2019;316:G304–12.
[Article] [PubMed] [PMC]23. Swiader N, Hasenstab KA, Yildiz VO, Jadcherla SR. Characterization of esophageal and sphincter reflexes across maturation in dysphagic infants with oral feeding success vs infants requiring gastrostomy. Dysphagia 2022;37:148–57.
[Article] [PubMed]24. Jadcherla SR, Hasenstab KA, Osborn EK, Levy DS, Ipek H, Helmick R, et al. Mechanisms and management considerations of parentchosen feeding approaches to infants with swallowing difficulties: an observational study. Sci Rep 2021;11:19934.
[Article] [PubMed] [PMC]25. Collins CR, Hasenstab KA, Nawaz S, Jadcherla SR. Mechanisms of aerodigestive symptoms in infants with varying acid reflux index determined by esophageal manometry. J Pediatr 2019;206:240–7.
[Article] [PubMed] [PMC]26. Omari T. Addendum to A study of dysphagia symptoms and esophageal body function in children undergoing anti-reflux surgery. United European Gastroenterol J 2018;6:1274–5.
[Article] [PubMed] [PMC]27. Caruso AM, Milazzo M, Tulone V, Acierno C, Girgenti V, Amoroso S, et al. High resolution manometry guidance during laparoscopic fundoplication in pediatric surgically "fragile" patients: preliminary report. Children (Basel) 2020;7:215.
[Article] [PubMed] [PMC]28. Hsing TY, Tsai IJ, Hsu CT, Wu JF. Role of esophageal manometry in children with refractory gastroesophageal reflux symptoms. Pediatr Int 2019;61:807–11.
[Article] [PubMed]29. Tambucci R, Isoldi S, Angelino G, Torroni F, Faraci S, Rea F, et al. Evaluation of gastroesophageal reflux disease 1 year after esophageal atresia repair: paradigms lost from a single snapshot? J Pediatr 2021;228:155–63.e1.
[Article] [PubMed]30. Chai C, Krishnan U. Dysmotility in eosinophilic esophagitis. Front Pediatr 2022;10:853754.
[Article] [PubMed] [PMC]31. van Rhijn BD, Oors JM, Smout AJ, Bredenoord AJ. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil 2014;26:1349–55.
[Article] [PubMed]32. Vandewalle RJ, Frye CC, Landman MP, Croffie JM, Rescorla FJ. Clinical factors and high-resolution manometry predicting response to surgery for achalasia in children. J Surg Res 2018;229:345–50.
[Article] [PubMed]33. Rosen R, Rodriguez L, Nurko S. Pediatric rumination subtypes: a study using high-resolution esophageal manometry with impedance. Neurogastroenterol Motil 2017;29:10.1111/nmo.12998.
[Article] [PubMed] [PMC]34. Wu JF, Chung C, Tseng PH, Tsai IJ, Lin YC, Yang CH. Distal contractile to impedance integral ratio assist the diagnosis of pediatric ineffective esophageal motility disorder. Pediatr Res 2018;84:849–53.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors