All issues > Volume 66(7); 2023
Seroprevalence of maternal peripartum human T-cell lymphotropic virus type-1 infection: a systematic review and meta-analysis of the Nigerian literature
- Corresponding author: Usman Abdulrasheed, PhD. Department of Pharmacology and Therapeutics, Faculty of Pharmaceutical Science Ahmadu Bello University, Zaria, Nigeria Email: myola4real@gmail.com
- Received May 15, 2022 Revised December 1, 2022 Accepted December 10, 2022
- Abstract
-
- Background
- Background
- The peripartum period is both a highly vulnerable stage and a significant indicator of a population’s health status. Interest is increasing in human T-cell lymphotropic virus type-1 (HTLV-1) transmission due to its adverse health impacts. However, nationally representative data on HTLV-1 that are important for health planning are unavailable for this subpopulation.
- Purpose
- Purpose
- This study aimed to conduct a pooled estimate of HTLV-1 prevalence among pregnant women in Nigeria to quantify its clinical burden and public health implications.
- Methods
- Methods
- This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 statement.
- Results
- Results
- After a systematic review of the Nigerian literature, 12 studies (2,821 pregnant or postnatal women) were included in the final evidence synthesis. The estimated HTLV-1 prevalence in Nigerian peripartum women following a positive screening test by enzyme-linked immunosorbent assay was 5.44% (95% confidence interval [CI], 3.16%–9.20%). A subgroup analysis of the 2 major regions showed a slightly higher prevalence in the Western versus Southern region (5.55% [95% CI, 2.49%–11.87%]; and 4.91% [95% CI, 2.11%–11.02%]; P=0.84). However, a subgroup analysis by geopolitical zone revealed that Southwestern and Northwestern Nigeria had the highest prevalence (9.23% [95% CI, 4.35%–18.55%; I2=93%] and 7.15% [95% CI, 1.54%–27.54%]; I2=92%). Our decade-old subgroup analysis found inconsistencies in the HTLV-1 prevalence. Furthermore, our literature review revealed a prevalence of HTLV infection among patients with various clinical types of lymphomas/leukemias and myelopathy of 2%–22%.
- Conclusion
- Conclusion
- These findings have important implications in defining the epidemiological patterns of HTLV-1 infection in Nigeria. They also suggest the presence of HTLV-endemic clusters near low-endemic areas, even within the same geopolitical zones.
Graphical abstract
- Introduction
- Introduction
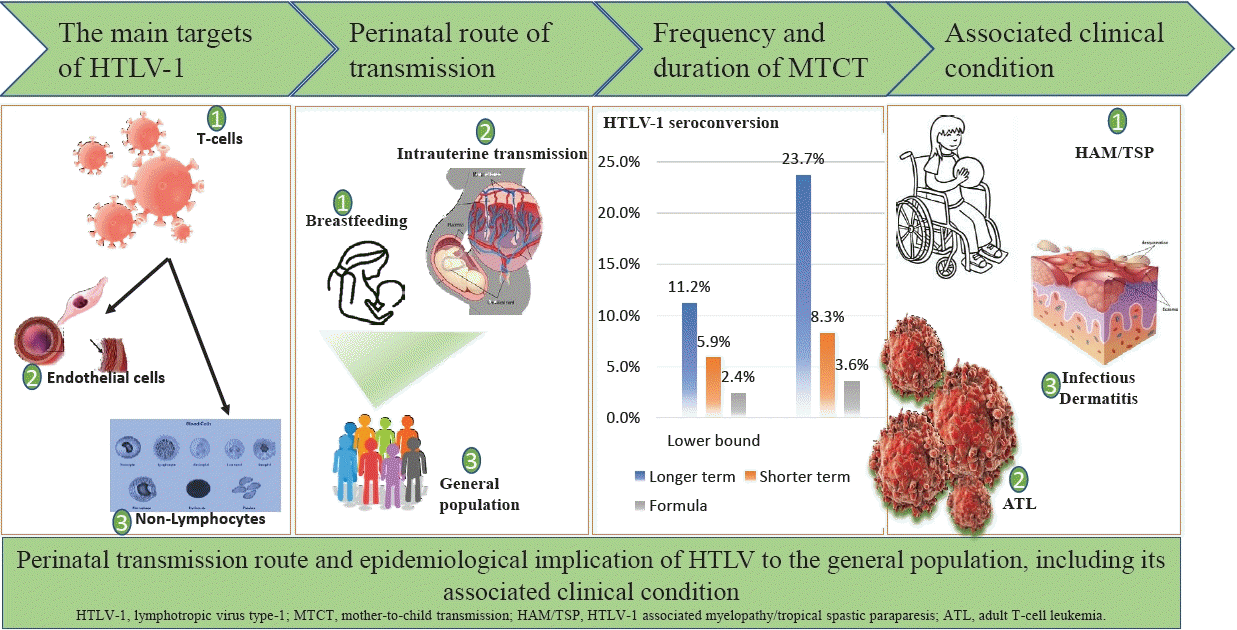
Human T-cell lymphotropic virus (HTLV) and its subtypes were estimated to have infected 5–10 million people worldwide [1-3]. Although HTLV belongs to the primate Retroviridae Family and subfamily of Oncovirinae, unlike other retroviruses, HTLV-1 subtype infection has a latency period of about 20 years. However, a few infected individuals may live with several hematopoietic malignancies and disabling neuroinvasive diseases [4]. The primary disease condition caused by HTLV-1 is adult T-cell leukemia (ATL) and HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [4-6]. Other disease outcomes are arthritis, uveitis, dermatological lesions, pneumonitis, urinary tract disorders, and increased venerability to common infectious diseases [7-9]. These later conditions are themselves secondary to ATL or HAM/TSP development [9]. Although the development of these conditions is relatively rare, their clinical implication makes them particularly important. HTLV-1 carriers are estimated to have a 2%–7% lifetime risk of developing ATL and 0.25%–3.8% for HAM/TSP [10]. HTLV subtypes have also been isolated these include HTLV-II and more recently HTLV-II and HTLV-IV [11]; however, their pathogenesis and public health implication remain unclear. In a Nigerian study, Williams et al. [12] reported a 100% association with ATL and an 18% association with non-Hodgkin lymphoma in HTLV-1 carriers. Although, the full clinical spectrum of HTLV-causally associated diseases is unknown [9]. Currently, available treatment option for TSP/HAM is largely disappointing, and symptomatic treatment remains the mainstay of therapy. The perinatal period represents an important route for HTLV transmission to the general population. Retrovirally infected mothers can transmit to their babies at any stage during pregnancy, childbirth, or breastfeeding [1]. Therefore, mother-to-child transmission (MTCT) is an important route for the spread of HTLV. One study in Iran found a prevalence rate of 1.5% in pregnant women and a vertical transmission rate of 16.6% in children born to their carrier mothers [7]. Breastfeeding is reported to be the most imported transmission route [7], unlike other retroviruses such as human immunodeficiency virus (HIV), which transmits infectivity even via various acellular varion media, effective viral transmission of HTLV-I majorly occurs through cell-to-cell interaction, hence intuitive that high level of HTLV-1 proviral load in milk and in blood cells as well as long duration of breastfeeding for more than 6 months after effective depletion of maternal protective immunity are a major representative risk factor for HTLV-1 MTCT transmission [3,7,13]. The transmission rate of this route can be as high as 25% in endemic areas and may reach 31% in mothers co-infected with sexually transmitted diseases [1,3]. A low level of education and vulnerable socioeconomic status is an additional risk factor in this route of transmission [12-14]. However, there are no representative data regarding the prevalence of HTLV in the general Nigerian population and there are few geographical subgroups studied. One recent meta-analysis [15] reported 3% of a pooled sample size of 2,383 of the general population including pregnant women, however, this study included only published articles using molecular signatures thereby increasing the chances of publication bias. Moreover, the included primary studies in their meta-analysis used polymerase chain reaction (PCR) only after initial positive enzyme-linked immunosorbent assay (ELISA) screening; the most common screening method known to have high sensitivity but poor specificity to the virus subtype. However, samples could escape a more rigorous assay for HTLV presence using this method [16].Although the study of HTLV-1 prevalence in the general population is considered more representative data, outcomes from HTLV-1 prevalence among pregnant women could help guide the implementation of measures to prevent MTCT [8,17]. Hence, early diagnosis and proper management during pregnancy and after childbirth may reduce MTCT of the virus. Therefore, obtaining a national estimate of HTLV-1 prevalence in pregnant women which represents an important epidemiological link in the transmission cycle of the infection is needed to assess the possible benefits of implementing such a wide screening program. In this paper, we estimated HTLV-1 national prevalence during the maternal perinatal period generated through a systematic review and meta-analysis of primary studies. This study is expected to inform policymakers on the contribution of maternal healthcare to the clinical and public health burden of HTLV infection.
- Methods
- Methods
- 1. Literature search
- 1. Literature search
We conducted searches in the following databases: PubMed, Advanced Google scholar, and Africa journals online databases. The search was not restricted by date or publication type. Search includes relevant terms in the title, abstract, and text, such as human T-cell lymphotropic virus, Pregnant Women, and Nigeria. We searched the reference lists (‘Snowball’) of the studies included in our review to identify additional publications. Additionally, we searched gray literature employing multiple strategies.After the exclusion of duplicate citations, all authors separately reviewed the titles and abstracts of selected articles. Relevant fulltext studies obtained were assessed for eligibility and risk of bias. All original articles from peer-reviewed scientific journals with a cross-sectional or survey design that estimated the prevalence of HTLV infection were considered potentially eligible for inclusion in this review. Relevant studies whose full text was not available were obtained by contacting the corresponding author via email.- 2. Inclusion and exclusion criteria
- 2. Inclusion and exclusion criteria
Women eligible for inclusion in the study were expectant mothers and mothers assessing postnatal care or mother-child pair. Studies that used either ELISA and/or Western blot assay technique for HTLV were included, in the case where their subtype was discriminated, only data for HTLV-1 was included. Data for HIV mothers and other comorbid conditions were excluded. Studies outside of Nigeria were also excluded- 3. Study selection and data extraction
- 3. Study selection and data extraction
Titles and abstracts of identified publications were screened independently by 2 reviewers (AB and BIS), and any publication deemed potentially relevant by either reviewer was carried forward to full-text evaluation. Any discordance in data interpretation between the authors was resolved through collective discussion and subsequent consensus.- 4. Risk of bias assessment
- 4. Risk of bias assessment
The risk of bias assessment established was assessed with an adapted version of the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies [17]. The overall strength of the body of evidence was assessed using the “Grading of Recommendations Assessment, Development, and Evaluation” (GRADE) tool, using the principles and domains applied in the quality assessment for prognostic studies and as modified by Vieira et al. [18] and Salie et al. [19]. Using a quantitative scoring system, studies were characterized as being of a low (7– 6), moderate (5–4), or high (≤3) risk of bias. Therefore, studies were characterized as being of a low, moderate, or high risk of bias [20-22].- 5. Data analysis
- 5. Data analysis
The population-based HTLV prevalence from 12 studies was extracted from their raw proportions, and 95% confidence intervals (CIs) were calculated using the Wilson method. To calculate the pooled prevalence, Logit transformation was performed in advance for the prevalence range as most of our data reported less than 0.2 or greater than 0.8 effect size estimate, hence they have become less likely to be normally distributed [23-26]. For pooled data, the I2 statistic was used to estimate heterogeneity and risk of bias, specifically publication bias, based on the Egger test result. I2 statistic values of 50% or more were considered to indicate substantial heterogeneity, and the random effects model using the DerSimonian-Laird estimator was used for the between variance estimator. A cumulative meta-analysis was done to evaluate the possible impact of publication year on the prevalence of HTLV by entering the older studies at the top and adding the newer studies at the bottom. The true summary proportion was later transformed back for our final output. Analyses were conducted using the functions for proportion and summary meta-analysis in R ver. 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Where there were no observed HTLV cases (zero seroprevalence), 0.5 was added to frequencies for the meta-analysis. Due to the high variability between studies; therefore, they all received similar weights in random effects meta-analysis. Forest plots were created from the results of the meta-analysis: squares represent the prevalence reported for the individual studies, square areas being proportional to the weight the study received in the meta-analysis. Horizontal lines show the 95% CIs for the prevalence [22,27-31]. The diamond shape represents the combined prevalence estimates and the width of the diamond represents the 95% CI. The CIs were calculated on the log odds scale and transformed back to the proportion scale, so they are not symmetrical [20]. The data presented here were incorporated into the final analyses to give both national and subnational HTLV seroprevalence estimates.
This systematic review was prepared in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) 2020 statement [18].
- Results
- Results
- 1. Study selection and characteristics
- 1. Study selection and characteristics
Twelve cross-sectional studies satisfied the criteria for inclusion in the qualitative and quantitative analysis of the primary studies (Supplementary Fig. 1). The main characteristics of the studies selected for the final analysis are shown in Table 1. The studies cover 5 of the total 6 geopolitical zones in Nigeria (Southsouth-1, Southwest-5, Southeast-1, Northcentral-3, and Northwest-2). The total number of maternal subjects in the 12 studies was 2,821 out of whom 1,510 (54%) were recruited in the Southwest followed by 620 (22%) recruited in the northcentral geopolitical zone.- 2. Publication quality and risk of bias
- 2. Publication quality and risk of bias
The overall quality of evidence for HTLV prevalence was rated according to the NIH Quality Assessment Tool for Observational Cohort and Cross-sectional Studies [21,32] (Supplementary Table 1). In general, the quality of evidence is good. In the point “Was a sample size justification, power description, or variance and effect estimates provided?” the majority of the answers were ‘no’ (Supplementary Table 1), due to the fact that the included studies were observational and not designed to investigate the association, although they were more exploratory. In the point “Was the participation rate of eligible persons at least 50%?” and “Were the outcome assessors blinded to the exposure status of participants?” most studies did not report any explanation.- 3. Overall prevalence
- 3. Overall prevalence
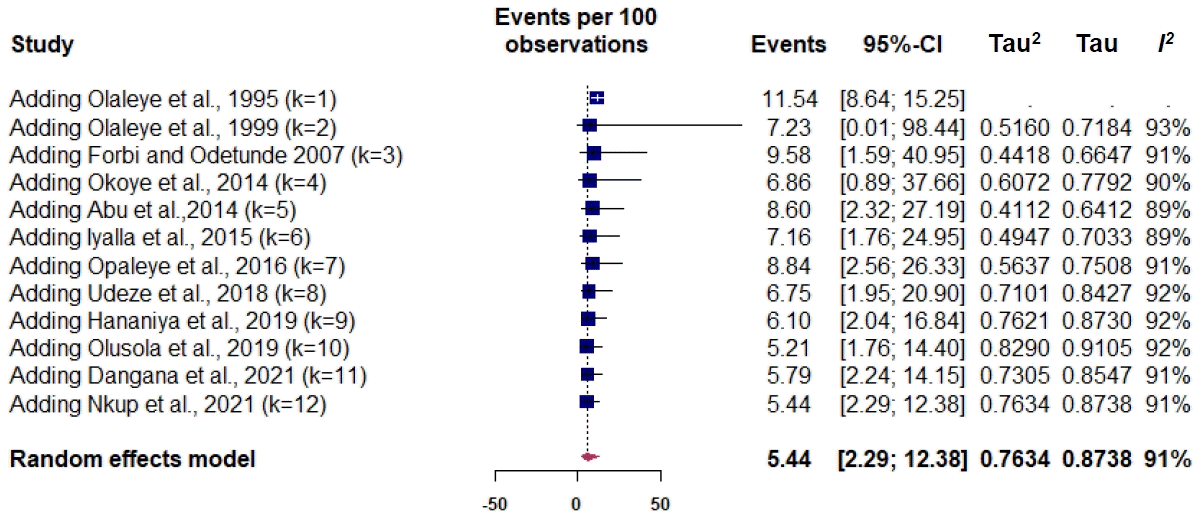
The prevalence of maternal HTLV infection among the included studies varied from 0% to 24.18%. The pooled prevalence estimate was 5.44% (95% CI, 3.16%–9.20%) (Fig. 1). The measure of the pooled heterogeneity I2 was 91% (P<0.01). The prediction interval for HTLV prevalence ranged from 0.73% to 30.90% (Fig. 1). Here the prediction interval represents the range of expected maternal HTLV prevalence in healthy maternal subjects in 95% of settings.Publication bias was obtained on Egger testing (Egger test, b=-4.91; 95% CI, -7.37 to -2.45; P=0.003) (Supplementary Fig. 2). The asymmetric funnel plot corroborated this result (Fig. 2). In addition, the cumulative meta-analysis did not display any major change in prevalence over time (Fig. 3). Hence, the publication year did not appear to impact the estimated pooled prevalence of HTLV.- 4. Subgroup analysis
- 4. Subgroup analysis
Given the heterogeneity observed in this study, a subgroup analysis along the geopolitical zones of Nigeria showed a higher prevalence of HTLV in the Southwest: 9.23%: (95% CI, 4.35%–18.55%) and 7.15% (95% CI, 1.54%–27.54%) in Northwestern Nigeria. However, the lowest prevalence was reported in the Northcentral and Southsouth geopolitical zones (Northcentral: 3.60% [95% CI, 0.99%–12.25%]; Southsouth: 0% [95% CI, 0.00%–1.66%] and Southeast: 0.5% [95% CI, 0.07%–3.46%], Table 2). The prevalence of HTLV subgroup analysis using the old subregion due to their cultural similarities and less diversity in their political struggle was further explored. Results show Southern and Northern Nigeria to have a similar prevalence rate (P=0.89). However, the pool prevalence of HTLV in southern states was highest (South: 5.55% [95% CI, 2.49%–11.87%]; North: was 4.91% (95% CI, 2.11%–11.02%; Table 2). Although Western blot as a specificity tool and the more recent robust PCR in the analysis of HTLV are available in Nigeria, its usage is still very limited. However, a subgroup analysis of ELISA and Western blot showed no statistical significance (P=0.72) with the method having a significant heterogeneity score (I2=91%).Further subgroup analysis by decade-old differences showed high variability in the prevalence of HTLV with 2010–2020 having the lowest pooled estimate of 2.90% and the highest before 2010 and after 2020 (9.58% and 6.01%, respectively). Although, studies conducted after 2020 had less heterogeneity.- 5. Prevalence of HTLV-1 infection in lymphoproliferative malignancy and myelopathy
- 5. Prevalence of HTLV-1 infection in lymphoproliferative malignancy and myelopathy
Although evidence shows that HTLV is still endemic in Nigeria, there exists a lack of recent epidemiological incidence and prevalence data on the net clinical impact of HTLV infection. Few studies with wide heterogeneity in the diagnosis of lymphomas/leukemia and the same study centers for the prevalence site prevented us from implementing a meta-analysis on the net clinical impact of HTLV infection. Of the 6 studies that met the inclusion criteria of adult lymphoproliferative malignancy and myelopathy, only one study was done on the prevalence of HTLV-1 in patients with myelopathy. The purposive study or case report was carried out at the individual state’s university teaching hospital and reported a prevalence of HTLV infection in patients with various clinical types of lymphomas/leukemias and myelopathy to range from 2%–22% with variable sample size and screening method (Table 3).
- Discussion
- Discussion
This meta-analysis describes the magnitude and distribution of HTLV prevalence in Nigerian maternal mothers. Overall, using data from a total of 12 studies representing 2,821 participants we found the prevalence of HTLV on a national scale to be arguably one of the highest in the world with important differences between regions. The prevalence of HTLV was higher in the Southwest and Northwest compared to other regions. Although, the studies included in the systematic review show restrictions to specific cities or regions and presented a high level of heterogeneity.The pooled prevalence of HTLV was synthesized by including studies of pioneering efforts of different investigators that either used sensitivity (ELISA) or specificity/confirmatory analysis (Western blot) in detecting HTLV. Although similar analyses [15,33-36] have been attempted they included only 2 studies of maternal subjects receiving antenatal care which inadequately characterized the pool prevalence rate of HTLV in Nigerian pregnant women. Thereby underestimating the actual global public health burden of maternal HTLV prevalence in Nigeria. The reported pooled estimate of this study is similar to that reported by Olaleye et al. [37], arguably the largest retrospective seroprevalence study done to date in the general population which included blood samples from 25 different locations in Nigeria taken from 1985–1991 totaling 4,153 sera. This study reported 5.6% with a similar rate in both males and females. Although this large study was done 3 decades ago. Nonetheless, the finding from this meta-analysis suggests a steady prevalence rate of the infection. However, these findings corroborate the conclusions by Ngoma et al. [38], that prevalence in pregnant women could be a convenient sentinel and a better reflection of the general population when attempting to estimate the prevalence of viral infections. Probably due to their representative characteristics of a given region and the comparable similarities in their reproductive mean ages.The clinical etiological implications of where HTLV-1 infection will be a necessary diagnostic criterion remain largely unclear. Characterized neurological syndromes in the tropics have been classified as tropical myeloneuropathies and grouped into tropical ataxic neuropathy or TSP [39]. However, our brief review of the literature shows the involvement of HTLV-1 infection although the route of transmission could not be verified. Our included studies show a higher prevalence of leukemia with a variability of 2%–5% among lymphoblastic leukemia and non-Hodgkin lymphoma to a higher 22% in other malignant lymphoproliferative disorders such as Burkitt lymphoma. Interesting findings from this brief literature review show 100% of patients with clinical characteristics of ATL/TSP/HAM had the virus. Furthermore, the findings from this review also show the predominance of infected males as against females widely reported elsewhere. This is consistent with epidemiological studies that show the prevalence of HTLV to vary along sex and reported a higher infection rate in African men or men of African descent [39-41]. This could suggest an etiological factor such as perinatal infectivity as against the transfusion transmission route. It is imperative to state here that excessive consumption of cassava containing cyanogenic glycosides is a major etiological factor associated with myelopathy, especially tropical ataxic neuropathies in southern states of Nigeria with the highest consumption as compared to the northern regions where zero studies have been reported [39]. Thus, this could complicate the course of disease progression and the incidence of myelopathy.However, subgroup analysis according to geopolitical zones indicates that Southwestern Nigeria has the highest prevalence rate. Our findings are contrary to an earlier report by Fleming et al. [42] who showed a higher prevalence in the Northwest as compared to Southsouth and an intermediary prevalence rate in the Southwest. However, the absence of studies from the Northeast, further compounded by the 0% prevalence rate reported in Southsouth, and 0.5% of the Southsouth states of Nigeria contributed to the significant heterogeneity observed in these studies. Although a similar prevalence rate in the general population has been reported [15,43]. these observations might be an absence of proof due to limited studies and sample size rather than proof of absence. This subnational variation in HTLV prevalence has important implications for efforts to bring the infection under control, especially as it relates to the prevention of the number of new cases and future treatment options. Nevertheless, this meta-analysis demonstrates the need for adequate characterization of HTLV geographic distribution across the states in Nigeria.Two plausible reasons could have accounted for differences in the gradient of seropositivity along the calendar year. Firstly, the incidence of HTLV has been associated with other infectious diseases such as HIV, and that screening of blood replacement donation by family members; a veritable channel of infectivity was not implemented until the early ‘90s, hence transfusion transmission has been common. Secondly, interspecies transmission from non-human primates to humans due to their close interaction and practices in the southern part of Nigeria, and pathological similarities in the context of disease manifestations seen in simian T-cell leukemia virus type 1 (STLV-1)-infected gorillas, baboons, macaques, and African green monkeys have been described [4,44,45], suggesting zoonotic and/or evolutionary transmission. Phylogenetic studies have also reported HTLV-1 concordance with STLV-1 [44].The risk of hyperendemicity in these epidemiologically unique populations of perinatal carrier mothers and transmission to entire populations reinforces the importance of public health interventions for HTLV control. The results obtained from this study will contribute to the existing data and body of knowledge on the prevalence of HTLV as screening mothers before and after delivery could serve as an intermediate intervention strategy with long time benefits.Most epidemiologic data are based on serologic studies rather than on molecular typing due to the low replicating nature of the HTLV virus [4]. However, Nasir et al. [17], have shown the number of positivity to HTLV virus to be higher when PCR is used alone as compared to ELISA and then PCR for confirmation/discrimination. Similar systematic reviews and meta-analyses of HTLV prevalence studies have used confirmatory tests mostly done in high-resource settings with access to more advanced Line Immunoassay and PCR [17]. It is worth noting that confirmatory PCR tests started to be utilized in 2,014 according to our subgroup analysis by test type. Nevertheless, the use of PCR as a method to confirm and discriminate HTLV-1 and -2 might lead to higher prevalence estimates, due to an increase in sensitivity/specificity [16,17]. The positivity rates of HTLV using the confirmatory method is now ranged to be 0.067% to 0.663% in Japan, the once highest HTLV-endemic region of the world [46]. Furthermore, a recent meta-analysis estimated the prevalence in pregnant Brazilian mothers to be 0.32% and 0.04% for HTLV1 and -2 respectively [17]. While prevalence is decreasing in once endemic areas of the world with several government measures, it remains stable in long-known endemic regions such as Nigeria. Possible reasons include: HTLV known to be transmitted from their simian reservoir (STLV), could have been acquired in addition to known route through non-human primates (bites/hunting/butchering pets), through the use of unsterile instrumentation during female genital mutilation and tattooing/piercing/ tribal mark. Others include bush-meat hunting which is widely practiced across Nigerian regions, though to a varying degree [42]. Therefore, a well design mechanistic study will be required to elucidate the predominant mode of transmission responsible for the stable transmission rate and the variations we observed in the studied area.The fact that these prevalence estimates were calculated through meta-analysis of individual primary studies and not direct products of proper national or regional surveillance systems raises the possibility of a significant underestimation of the true burden of HTLV infection in Nigeria.Compared to other infections among pregnant women, HTLV-1 appears to have an intermediary prevalence rate to HIV (1.4%) [47] and hepatitis B virus infection (9.5%) [48]. While there is an established national surveillance program for the last 2, there is no national screening for HTLV. The similarity in prevalence could reflect either true differences in estimates or a lack of nationwide information.It is, however, worthy of note that this study does not, in any way, underestimate the fact that only a large representative rigorous national epidemiological study conducted at the same time can give a more reliable overall prevalence of HTLV in Nigeria. However, in the absence of such a national survey, a meta-analysis of all the observational studies cutting across most, if not all, the geopolitical zones of Nigeria, provides the best evidence and, hence, could be of use in developing prevention strategies and planning future treatment options in Nigeria.The included studies did not cover all the states in the country, offering limited estimates of HTLV for each region. Moreover, regions also differed in the number and sample size of the included studies, leading to high within and between-group heterogeneity.In conclusion, owing to the selective maintenance of certain clones subsequent to initiation of leukemogenesis, majorly caused by the stable nature of long-term carriers infected during infancy, interventions such as screening, counseling, and educating high-risk individuals and populations at large are morally compelling and have the potential to decrease the spread of this burdensome, potentially deadly, blood-borne virus.
Supplementary material
Supplementary material
Supplementary Table 1 and Figs. 1 and 2 can be found via https://doi.org/10.3345/cep.2022.00710.Supplementary Fig. 1.
cep-2022-00710-Supplementary-Fig-1.pdfPRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart of article selection for systematic review and meta-analysis of human T-cell lymphotropic virus prevalence. The figure is modeled after the reference.
- Footnotes
-
Conflict of interests No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution Conceptualization: AB, MHM; Formal Analysis: AB; Investigation: AB, MHM, BIS; Methodology: AB, BIS; Project Administration: AB, MHM, BIS; Writing – Original Draft: AB, MHM, BIS; Writing – Review & Editing: AB, MHM, BIS, OB, MA
-
Fig. 1.
Prevalence of HTLV-1/-2 in perinatal healthy women in Nigeria was determined through a meta-analysis of the included studies. The squares represent each individual study’s prevalence with its area being proportional to the weight the study received in the meta-analysis. The horizontal lines show the 95% confidence interval (CI). The diamonds represent the combined estimates for each region, with their widths representing the 95% CI. HTLV-1/-2, human T-cell lymphotropic virus type-1/-2.
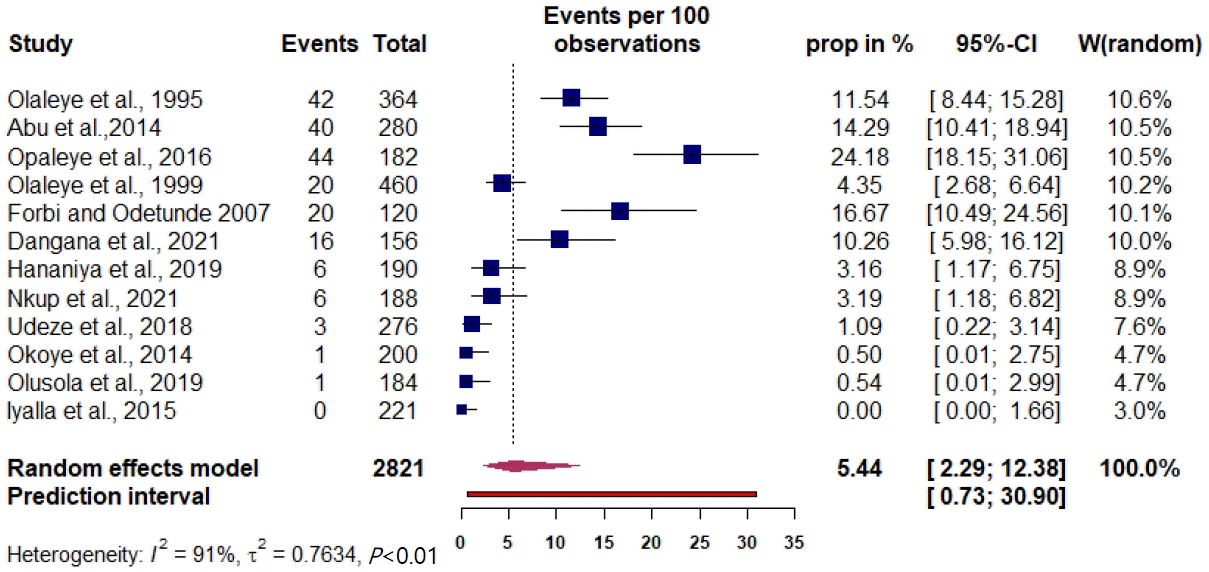
Table 1.
Characteristics of the included studies
| Study | Subregion | Study population | Sample size | Age (yr), mean | Age (yr), range | HTLV-1/ 2+ | ScrMethod | ConMethod |
|---|---|---|---|---|---|---|---|---|
| Olaleye et al., [49] 1995 | Southwest | Prevalence of HIV-1, HIV-2, HTLV-1, and HTLV-2 antibodies in sera of pregnant women attending an antenatal clinic in Ibadan, Nigeria | 364 | - | 17–42 | 42 | ELISA | Western blot |
| Olaleye et al., [50] 1999 | Southwest | Postpartum women in Southwestern Nigeria identified using blood samples collected in 1993 | 460 | - | 20–40 | 20 | ELISA | Western blot |
| Forbi and Odetunde [51] 2007 | Southwest | Prevalence of HTLV infection amongst 2 highly sexually active groups, pregnant women and CSWs in Southwestern Nigeria | 120 | 26 | 14–30 | 20 | ELISA | - |
| Okoye et al., [52] 2014 | Southwest | Seroprevalence of HTLV-1 antibodies and asso- ciated risk factors for HTLV-1 infection among pregnant women in University of Nigeria Teach- ing Hospital, Enugu, Southeast Nigeria | 200 | 28.94 | 26–30 | 1 | ELISA | Western blot |
| Abu et al., [53] 2014 | Northwest | Pregnant women attending antenatal clinic in | 280 | - | - | 40 | ELISA | - |
| Iyalla et al., [54] 2015 | Southsouth | Pregnant women attending the Braithwaite Me- morial Specialist Hospital and University of Port Harcourt Teaching Hospital | 221 | - | - | 0 | ELISA | - |
| Opaleye et al., [55] 2016 | Southwest | Pregnant women attending antenatal clinic, in Ladoke Akintola University Teaching Hospital, Osogbo, and Southwestern Nigeria | 182 | - | 15–49 | 44 | ELISA | - |
| Udeze et al., [56] 2018 | Northcentral | Serological detection of HTLV types I and II among pregnant women in Ilorin, Nigeria | 276 | 28.2 | 16–40 | 3 | ELISA | - |
| Hananiya et al., [57] 2019 | Northwest | Prevalence of HTLV antibodies as well as the sociodemographic and risk factors associated with HTLV among women attending postnatal clinics in Zaria | 190 | - | 15–45 | 6 | ELISA | - |
| Olusola et al., [43] 2019 | Southwest | Prevalence of HTLV-1 and 2 infection amongst 3 sexually active populations comprising of pregnant women, adults and teenagers as well as sexually transmitted infections clinic attendees | 184 | - | - | 1 | ELISA | - |
| Dangana et al., [58] 2021 | Northcentral | Seroprevalence and risk factors of HTLV-1/-2 infections among pregnant women attending the University of Abuja Teaching Hospital, Abuja, Nigeria | 156 | - | 18–48 | 16 | ELISA | - |
| Nkup et al., [34] 2021 | Northcentral | Detecting antibodies to HTLV-I/II among pregnant women on antenatal visits to selected hospitals in Jos, Nigeria | 188 | 27 | - | 6 | ELISA | - |
Table 2.
Subgroup analysis of the pooled prevalence of HTLV infection in Nigeria
Table 3.
Cohort studies on prevalence of HTLV-1 infection in lymphoproliferative malignancy and myelopathy
| Study | Study population | HTLV-associated diagnosis | State | HTLV-1/2+ | Sample size | Age, mean | ScrMethod |
|---|---|---|---|---|---|---|---|
| Williams et al., [59] 1984 | Human T-lymphotropic virusassociatedlymphoprolifera- tive disease: report of 2 cases in Nigeria | Malignant lymphoproliferative disorder (1 adult T-cell leukemia, 1 chronic lymphocytic leukemia) | Ibadan | 2 | 9 | ELISA | |
| Fleming et al., [42] 1986 | Antibodies to HTLV-I in Nige- rian blood-donors, their rela- tives and patients with leuke- mias, lymphomas and other diseases | 4 of 20 patients (20.0%) with chronic lymphatic leukemia, 3 (10.0%) of 30 with non-Hodgkin lymphoma, one of 12 with Burkitt lymphoma and one of 7 with acute lymphoblastic leukemia | Zaria-Kaduna/Maiduguri/Calabar/Lagos | 9 | 69 | 45 | ELISA |
| Williams et al., [40] 1993 | Frequency of adult T-cell leu- kemia/lymphoma and HTLV- 1 in Ibadan | Non-Burkitt non-Hodgkin lym- phoma patients (acute lympho- blastic leukemia-II morphology) | Ibadan | 6 | 30 | ELISA/confirmatory Western blot test | |
| Olaleye et al., [60] 1996 | HTLV types I and II infections in patients with leukemia/lym- phoma and in subjects with sexually transmitted diseases in Nigeria | Leukemia/lymphoma (acute lym- phoblastic leukemia-II mor- phology) | Ibadan | 1 | 65 | 29.3 | Commercial enzyme immunoassays (EIA)/polymerase chain reaction technique |
| Olaleye et al., [41] 1998 | Detection of HTLV-1 antibodies and DNA in blood sample of a patient with myelopathy in Nigeria | Progressive loss of tone to the 2 lower limbs and later inability to walk | Ibadan | 1 | 5 | 25–50 | Abbott HTLV-1 EIA and Coulter SELECT-HTLV 1/2 |
| Akinbami et al., [39] 2014 | Seroprevalence of human T- lymphotropic virus antibodies among patients with lym- phoid malignancies at a ter- tiary center in Lagos, Nigeria | One of the patients was a case of non-Hodgkin lymphoma, while the other was a case of chronic lymphocytic leukemia | Lagos | 2 | 39 | 51.9 | ELISA/Western blot confirmatory testing |
- References
- 1. Percher F, Jeannin P, Martin-Latil S, Gessain A, Afonso PV, Vidy-Roche A, et al. Mother-to-child transmission of HTLV-1 epidemiological aspects, mechanisms and determinants of mother-to-child transmission. Viruses 2016;8:40.
[Article] [PubMed] [PMC]2. Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005;24:6058–8.
[Article] [PubMed]3. Kazuo I, Miyazawa T. Mother-to-child transmission of human T-cell leukemia virus type 1: mechanisms and nutritional strategies for prevention. Cancers 2021;13:4100.
[Article] [PubMed] [PMC]4. Magalhães T, Mota-Miranda AC, Alcantara LC, Olavarria V, Galvão-Castro B, Rios-Grassi MF. Phylogenetic and molecular analysis of HTLV-1 isolates from a medium sized town in northern of Brazil: tracing a common origin of the virus from the most endemic city in the country. J Med Virol 2008;80:204–45.5. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012;3:388.
[Article] [PubMed] [PMC]6. Tanaka G, Okayama A, Watanabe T, Aizawa S, Stuver S, Mueller N, et al. The clonal expansion of human T lymphotropic virus type 1-infected T cells: a comparison between seroconverters and long-term carriers. J Infect Dis 2005;191:1140–7.
[Article] [PubMed]7. Boostani R, Sadeghi R, Sabouri A, Ghabeli-Juibary A. Human T-lymphotropic virus type I and breastfeeding; systematic review and meta-analysis of the literature. Iran J Neurol 2018;17:174–9.
[Article] [PubMed] [PMC]8. Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor JM. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis 2019;vv:pp. Lancet Infect Dis 2020;20:133–43.
[PubMed]9. Martin F, Taylor GP, Jacobson S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol 2014;10:1531–46.
[Article] [PubMed]10. Wyld PJ, Tosswill JH, Mortimer PP, Weber JN. Sporadic HTLV-I associated adult T-cell leukaemia (ATL) in the U.K. Br J Haematol 1990;76:149–50.
[Article] [PubMed]11. Hinuma Y. Preleukemia and typical adult T-cell leukemia (ATL) etiologically associated with a retrovirus (HTLV/ATLV). Haematologica 1987;72:72–4.
[PubMed]12. Williams CK, Dada A, Blattner WA. Some epidemiological features of the human T-cell lymphotropic virus type I (HTLV-I) and ATL in Nigerians. Leukemia 1994;8 Suppl 1:S77–82.
[PubMed]13. Bittencourt AL. Vertical transmission of HTLV-I/II: a review. Rev Inst Med Trop Sao Paulo 1998;40:245–51.
[Article] [PubMed]14. Sodré Barmpas DB, Monteiro DLM, Taquette SR, Rodrigues NCP, Trajano AJB, Cunha JC, et al. Pregnancy outcomes and mother-to-child transmission rate in HTLV-1/2 infected women attending two public hospitals in the metropolitan area of Rio de Janeiro. PLoS Negl Trop Dis 2019;13:e0007404.
[Article] [PubMed] [PMC]15. Rosadas C, Malik B, Taylor GP, Puccioni-Sohler M. Estimation of HTLV-1 vertical transmission cases in Brazil per annum. PLoS Neglect Trop Dis 2018;12:e0006913.
[Article] [PubMed] [PMC]16. Anyanwu N, Ella EE, Aminu M, Azam M, Ajmal M, Kazeem HM. Prevalence of human T-lymphotropic virus 1/2 in Nigeria's capital territory and meta-analysis of Nigerian studies. SAGE Open Med 2019;7:2050312119843706.
[Article] [PubMed] [PMC]17. Nasir IA, Ahmad AE, Emeribe AU, Shehu MS, Medugu JT, Babayo A. Molecular detection and clinical implications of HTLV-1 infections among antiretroviral therapy-naïve HIV-1-infected individuals in Abuja, Nigeria. Virology Res Treat 2015;6:17–23.
[Article] [PubMed] [PMC]18. Vieira BA, Bidinotto AB, Dartora WJ, Pedrotti LG, de Oliveira VM, Wendland EM. Prevalence of human T-lymphotropic virus type 1 and 2 (HTLV-1/-2) infection in pregnant women in Brazil: a systematic review and meta-analysis. Sci Rep 2021;11:15367.
[Article] [PubMed] [PMC]19. Salie T, Engel K, Moloi A, Muhamed B, Dale JB, Engel ME. Systematic review and meta-analysis of the prevalence of Group A streptococcal emm clusters in Africa to inform vaccine development. mSphere 2020;5:e00429. –20.
[Article] [PubMed] [PMC]20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
[Article] [PubMed] [PMC]21. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9.
[Article] [PubMed]22. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft 2010;36:1–48.23. Wang N. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial. ResearchGate [Preprint], 2018;Available from: http://dx.doi.org/10.13140/RG.2.2.27199.00161.24. Afonso PV, Cassar O, Gessain A. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology 2019;16:39.
[Article] [PubMed] [PMC]25. Paiva A, Casseb J. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop Sao Paulo 2015;57:1–13.
[Article] [PubMed] [PMC]26. Ramos-Rincón JM, Ortiz-Martínez S, Vásquez-Chasnamote ME, de-Miguel-Balsa E, Gamboa-Paredes ON, et al. Screening for human T-cell lymphotropic virus (HTLV) in pregnant women in the Peruvian Amazon and systematic review with meta-analysis of HTLV infection in Peru. Pathogens 2021;10:260.
[Article] [PubMed] [PMC]27. Okorie PN, Ademowo GO, Saka Y, Davies E, Okoronkwo C, Bockarie MJ, et al. Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis 2013;7:e2416.
[Article] [PubMed] [PMC]28. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ Clin Res 2016;355:i4919.
[Article] [PubMed] [PMC]29. Fisher Z, Tipton E, Zhipeng H. Robumeta: robust variance metaregression. R package version 2.0 [Internet]. Vienna (Austria): The R Project for Statistical Computing, 2017;Available from: https://CRAN.R-project.org/package=robumeta.30. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Soft 2019;4:1686.
[Article]31. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: R package and ShinyApp for producing PRISMA 2020 compliant flow diagrams [Internet]. Genève (Switzerland): European Organization for Nuclear Research, 2023;Available from: http://doi.org/10.5281/zenodo.4287834.32. Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870.
[Article] [PubMed]33. McGuinness LA. Risk of bias plots. In: Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide (online version). Available from: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/rob-plots.html.34. Nkup JY, Aminu M, Inabo HI, Katnap RS, Chundusu DY, Diyong JJ, et al. Detection of antibodies to human T-cell lymphotrophic virus 1/2 among pregnant women on antenatal visits to selected hospitals in Jos, Nigeria. Adv Microbiol Biotechnol Res J 2021;1:1–6.35. Carneiro-Proietti AB, Amaranto-Damasio MS, Leal-Horiguchi CF, Bastos RH, Seabra-Freitas G, Borowiak DR, et al. Mother-to-child transmission of human T-cell lymphotropic viruses-1/2: what we know, and what are the gaps in understanding and preventing this route of infection. J Pediatr Infect Dis Soc 2014;3 Suppl 1(Suppl 1): S24–9.
[Article] [PubMed] [PMC]36. Dumas M, Houinato D, Verdier M, Zohoun T, Josse R, Bonis J, et al. Seroepidemiology of human T-cell lymphotropic virus type I/II in Benin (West Africa). AIDS Res Hum Retrovirus 1991;7:447–51.
[Article]37. Olaleye DO, Bernstein L, Sheng Z, Ekweozor CC, Li XY, Sullivan-Halley J, et al. Type-specific immune response to human T cell lymphotropic virus (HTLV) type I and type II infections in Nigeria. Am J Trop Med Hygiene 1994;50:479–86.
[Article]38. Ngoma AM, Omokoko MD, Mutombo PB, Mvika ES, Muwonga JM, Nollet KE, et al. Population-based prevalence of human T-lymphotropic virus type 1 in sub-Saharan Africa: a systematic review and meta-analysis. Trop Med Int Health 2019;24:1277–90.
[Article] [PubMed]39. Akinbami A, Durojaiye I, Dosunmu A, John-Olabode S, Adediran A, Oshinaike O, et al. Seroprevalence of human T-lymphotropic virus antibodies among patients with lymphoid malignancies at a tertiary center in Lagos, Nigeria. J Blood Med 2014;5:169–74.
[PubMed] [PMC]40. Williams CK, Alexander SS, Bodner A, Levine A, Saxinger C, Gallo RC, et al. Frequency of adult T-cell leukaemia/lymphoma and HTLV-I in Ibadan, Nigeria. Br J Cancer 1993;67:783–6.
[Article] [PubMed] [PMC]41. Olaleye OD, Ogunniyi A, Sheng ZJ, Li Z, Rasheed S. Detection of HTLV-I antibodies and DNA in blood sample of a patient with myelopathy in Nigeria. Rev Inst Med Trop Sao Paulo 1998;40:55–7.
[Article] [PubMed]42. Fleming AF, Maharajan R, Abraham M, Kulkarni AG, Bhusnurmath SR, Okpara RA, et al. Antibodies to HTLV-I in Nigerian blood-donors, their relatives and patients with leukaemias, lymphomas and other diseases. Int J Cancer 1986;38:809–13.
[Article] [PubMed]43. Olusola B, Faneye A, Nejo Y, Opayele A, Bakarey S. Human T cell lymphotrophic virus infection among sexually active individuals in Nigeria: a cross sectional study. Sci Afr 2019;3:e00072.
[Article]44. Fox JM, Mutalima N, Molyneux E, Carpenter LM, Taylor GP, Bland M, et al. Seroprevalence of HTLV-1 and HTLV-2 amongst mothers and children in Malawi within the context of a systematic review and meta-analysis of HTLV seroprevalence in Africa. Trop Med Int Health 2016;21:312–24.
[Article] [PubMed] [PMC]45. Quintana DS. From pre-registration to publication: a non-technical primer for conducting a meta-analysis to synthesize correlational data. Front Psychol 2015;6:1549.
[Article] [PubMed] [PMC]46. Gbadebo BM, Salawu AT, Afolabi RF, Salawu MM, Fagbamigbe AF, Adebowale AS. Cohort analysis of the state of female genital cutting in Nigeria: prevalence, daughter circumcision and attitude towards its discontinuation. BMC Women Health 2021;21:182.
[Article] [PubMed] [PMC]47. Itabashi K, Miyazawa T, Sekizawa A, Tokita A, Saito S, Moriuchi H, et al. A nationwide antenatal human T-cell leukemia virus type-1 antibody screening in Japan. Front Microbiol 2020;11:595.
[Article] [PubMed] [PMC]48. Ajuwon BI, Yujuico I, Roper K, Richardson A, Sheel M, Lidbury BA. Hepatitis B virus infection in Nigeria: a systematic review and meta-analysis of data published between 2010 and 2019. BMC Infect Dis 2021;21:1120.
[Article] [PubMed] [PMC]49. Olaleye DO, Ekweozor CC, Sheng Z, Rasheed S. Evidence of serological cross-reactivities with human immunodeficiency virus types 1 and 2 and human T-lymphotropic virus types I and II in sera of pregnant women in Ibadan, Nigeria. Int J Epidemiol 1995;24:198–203.
[Article] [PubMed]50. Olaleye DO, Omotade OO, Sheng Z, Adeyemo AA, Odaibo GN. Human T-cell lymphotropic virus types I and II infections in mother-child pairs in Nigeria. J Trop Pediatr 1999;45:66–70.
[Article] [PubMed]51. Forbi JC, Odetunde AB. Human T-cell lymphotropic virus in a population of pregnant women and commercial sex workers in South Western Nigeria. Afr Health Sci 2007;7:129–32.
[PubMed] [PMC]52. Okoye AE, Ibegbulam OG, Onoh RC, Ezeonu PO, Ugwu NI, Lawani LO, et al. Seroprevalence and correlates of human T-cell lymphoma/leukemia virus type 1 antibodies among pregnant women at the University of Nigeria Teaching Hospital, Enugu, Nigeria. Int J Women Health 2014;6:849–55.53. Abu RJ, Olonitola OS, Whong CM, Ella EE. Seroprevalence of human T-cell leukaemia virus antibodies among pregnant women attending selected hospitals in Zaria, Kaduna state. Biol Environ Sci J 2014;11:179–84.54. Iyalla C, Ejele AO, Okoh DA, Igbigbi E. Seroprevalence study of HTLV-1 and 2 in prospective blood donors and pregnant women in Port Harcourt, Nigeria. Afr J Infect Dis 2015;9:57–60.
[Article]55. Opaleye OO, Igboama MC, Ojo JA, Odewale G. Seroprevalence of HIV, HBV, HCV, and HTLV among Pregnant Women in Southwestern Nigeria. J Immunoassay Immunochem 2016;37:29–42.
[Article] [PubMed]56. Udeze AO, Odebisi-Omokanye MB, Faneye A, Olusola BA, Ogunsemowo O, Iwuoha C, et al. Serological detection of human T-cell lymphotropic virus types I and II among pregnant women in Ilorin, Nigeria. J Immunoassay Immunochem 2018;39:428–38.
[Article] [PubMed]57. Hananiya HS, Ella EE, Aminu M, Anyanwu N. Prevalence of human T-cell lymphotropic virus and the socio-demographic and risk factors associated with the infection among post-natal clinics women in Zaria, Nigeria. J Immunoassay Immunochem 2019;40:485–94.
[Article] [PubMed]58. Dangana A, Abdullahi IN, Billyrose O, Emeribe AU, Abu JM, Anka AU, et al. Sero-epidemiology of human T-cell lymphotropic viruses-1 and -2 infection among pregnant women attending Abuja Teaching Hospital, Nigeria. Human Antibodies 2021;29:101–8.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors