All issues > Volume 66(7); 2023
Neurodevelopmental outcomes of preterm infants
- Corresponding author: In Gyu Song, MD, MPH, PhD. Department of Pediatrics, Yonsei University College of Medicine, 50-1, Yonsei-Ro, Seodaemun-gu, Seoul 03722, Korea Email: pedigms@yuhs.ac
- Received June 9, 2022 Revised August 31, 2022 Accepted October 24, 2022
- Abstract
-
During the last several decades, the number of preterm infants has increased, and their survival rate has improved owing to advances in perinatal care. As more preterm infants survive, many studies examine their neurodevelopmental outcomes. This study aimed to summarize the neurodevelopmental outcomes of preterm infants according to gestational age at birth using a recently published meta-analysis. The prevalence of neurodevelopmental impairment and behavioral disorders decreased as gestational age and birth weight increased. Recent studies reported that proactive neonatal treatment of periviable preterm infants (gestational age, 22–24 weeks) could improve their prognosis. Moderate and late preterm infants reportedly have less severe disease than very preterm infants; nonetheless, they still experience adverse neurodevelopmental outcomes compared to term infants. Neonatal morbidities such as bronchopulmonary dysplasia and retinopathy of prematurity are associated with poor neurodevelopmental outcomes. Despite improvements in neonatal care, prematurity is still associated with poor neurodevelopmental outcomes. To ensure timely intervention, the establishment of a follow-up system for premature infants is necessary.
- Introduction
- Introduction
Preterm infant is defined as an infant born prior to 37 weeks’ gestation. Such infants are categorized based on gestational age (GA) as extremely preterm (EP; <28 weeks), very preterm (VP; 28–31 weeks), moderate preterm (32–33 weeks), and late preterm (34–36 weeks). During the past few decades, the number of preterm infants has increased, while the survival rate has improved due to advances in care in the neonatal intensive care unit (NICU) worldwide [1-3]. Recently, improved outcomes of infants born during the periviable period (22–23 weeks’ GA) have also been reported in some countries [4]. As more preterm infants survive, increasing studies of neurodevelopmental outcomes are conducted. Most studies focused on EP/VP, and outcomes were worse than those of full-term infants [5-7]. However, debate persists about the outcomes of moderate- to late-preterm (MLPT) infants [8-10]. This study aimed to summarize the neurodevelopmental outcomes of preterm infants according to their GA at birth using recently published meta-analyses. It also reviews recent research into the neurodevelopment of Korean preterm infants.
- Methods
- Methods
A literature search was performed of PubMed using the keywords listed in Table 1. The results were narrowed down to meta-analyses published between April 2017 and March 2022. For a review of Korean studies, “Korea” was added to the keywords and the same period applied. Articles that were not related to the neurodevelopmental outcomes of preterm infants or low birth weight (LBW) infants were excluded. Several widely used assessment tools are reviewed shortly before the systematic reviews are summarized.
- Results
- Results
- 1. Neurodevelopment assessment
- 1. Neurodevelopment assessment
The Bayley Scales of Infant and Toddler Development, Third Edition (BSID III) is among the most common tools used to assess the neurodevelopment of preterm infants. It assesses 5 domains of development in children aged 1–42 months: cognition, language (receptive and expressive communication), motor (gross and fine), social-emotional, and adaptive behavior. The first 3 domains were assessed by a trained examiner, whereas information about the last two was obtained from the child’s main caregiver [11]. The fourth edition was recently updated. In the new edition, the number of items in each domain is decreased, and the examination takes approximately 30% less time than the third edition [12].The Gross Motor Function Classification System (GMFCS) is widely used to assess motor development and functional level in infants and children. This system focuses on self-initiated movements, especially sitting, transfer, and mobility. It consists of 5 levels, and the definition of each was expanded and revised according to patient age. Children with higher GMFCS levels have more movement limitations and may have a more unfavorable prognosis [13].The Wechsler Intelligence Scale for Children is an intelligence test for children aged 6–16 years. The most recent version is the fifth edition published in 2014 [14], which was developed to enable a comprehensive assessment of a child’s general intellectual functioning in various situations, for example, identifying students with learning disabilities, intellectual disability, or giftedness; assessing cognitive processing strengths and weaknesses; and evaluating the impact of brain injuries [14].The Korean Developmental Screening Test for Infants and Children (K-DST) is used as a developmental screening tool in the National Health Screening Program for Infants and Children. The tool is a parent-reported test composed of 6 domains: gross motor, fine motor, cognition, language, social skills, and self-help. It can be used for infants and children aged 4–71 months [15].- 2. Meta-analyses of neurodevelopmental outcomes of preterm infants or LBW infants
- 2. Meta-analyses of neurodevelopmental outcomes of preterm infants or LBW infants
1) Neurodevelopment of preterm infants born at periviable GA
1) Neurodevelopment of preterm infants born at periviable GA
Several meta-analyses examined the neurodevelopmental outcomes of infants born in the periviable period (22 and 23 weeks’ GA). In a meta-analysis of 65 studies, the mean survival rate of infants born at 22 weeks’ GA was nearly 0% of all births, 7.3% of live births, and 24.1% of infants admitted to the NICU. Among survivors, 60.9% of infants born at 22 weeks’ GA had moderate to severe impairments, whereas 50.3% of those born at 23 weeks’ GA and 42.2% born at 24 weeks’ GA had moderate to severe impairment (Fig. 2) [17]. Another study that assessed survivors aged 4–10 years showed that moderate to severe NDI rates were 42.0% at 22 weeks’ GA, 41.0% at 23 weeks’ GA, 32.0% at 24 weeks’ GA, and 23.0% at 25 weeks’ GA [18]. The prognosis was better for infants who were provided proactive neonatal treatment, even when they were born at 22 weeks’ GA. The pooled prevalence of survival was 29.0%; in 5 studies that assessed survival with moderate or severe NDI, the overall rate was 63.0% [19]. As studies have reported that proactive neonatal treatment for periviable preterm infants could improve prognosis, more research is required to establish evidence-based treatment protocols [4]. In addition, the recently published neonatal resuscitation guidelines did not specify GA or birth weights for withholding or withdrawing resuscitation, unlike the previous guidelines [20].2) Neurodevelopment of VP or very LBW infants
2) Neurodevelopment of VP or very LBW infants
The prevalence of NDI during the first 2 years of life decreased as GA or birth weight increased. In a meta-analysis of VP or very LBW (VLBW) infants born after 2006, the pooled prevalence of a moderate to severe cognitive delay was 10.9% for 26–27 weeks’ GA and 5.8% for 28–32 weeks’ GA. Moderate to severe motor delay also showed a similar trend: 12.0% for 26–27 weeks’ GA and 6.3% for 28%–32% weeks’ GA. The prevalence of cerebral palsy assessed using GMFCS in VLBW infants was 6.8%, but the prevalence was higher in extremely LBW (<1,000 g, ELBW) infants (8.4%) than in those with birth weights of 1,000–1,500 g (4.2%) [21]. The prevalence of cerebral palsy in VLBW infants has not notably improved over the last 3 decades [22]. Two meta-analyses found that children or adolescents born with EP or VP also had large deficits in intelligence, and no improvement was observed during the last several decades [23,24]. VP and VLBW infants also have considerable academic difficulties and special educational needs [25]. Studies reported that bronchopulmonary dysplasia (BPD) is an important factor in cognitive outcomes and is suggested a lowering of its incidence [23,25]. Another study that evaluated the outcomes of EP or ELBW in developing countries found that the rate of NDI was 17% (7%–34%) for ELBW and 29% (23%–37%) for EP infants, although the certainty of evidence was very low to low [26].3) Behavioral disorders and mental health of VP infants
3) Behavioral disorders and mental health of VP infants
VP or VLBW infants are at an increased risk of neurodevelopmental disorders such as ASD or ADHD. A meta-analysis of 3,366 preterm infants (mean GA, 28.0 weeks) estimated that the overall prevalence of ASD was 7% (95% confidence interval [CI], 4%–9%) [7]. This was higher than that of the general population, in which 1 of every 44 children (2.3%) aged 8 years has been diagnosed with ASD [27]. A previous study reported that preterm infants were estimated to have an odds of ASD that was 3.3 times higher than general population [28]. VP and VLBW infants are also at higher risk of developing ADHD than term infants [6,29]. The odds ratio (OR) was 3.04 (95% CI, 1.56–3.26) for VP/VLBW and EP/ELBW infants, while EP/ELBW infants had a higher odds (4.05; 95% CI, 2.38–6.87) than VP/VLBW infants (2.25; 95% CI, 1.56–3.26) [6]. Multiple factors can affect causal pathways of neurodevelopmental disorders in premature infants. A nonoptimal intrauterine environment, fetal immune response, exogenous stress during the perinatal period, and genetic factors can affect brain development and interfere with neuronal organization [6,7]. Another meta-analysis reported that preterm-born children also had a higher prevalence of anxiety and specific phobia, but not depressive disorders, than term-born children [30].4) Neurodevelopmental outcomes of MLPT infants
4) Neurodevelopmental outcomes of MLPT infants
MLPT infants are reportedly less severely affected than VP infants but still experience adverse neurodevelopmental outcomes. In a meta-analysis of MLPT infants, no differences were observed in cognitive function between adults born at term and MLPT term. However, they had higher odds for any psychiatric (OR, 1.14; 95% CI, 1.04–1.24), substance use (OR, 1.16; 95% CI, 1.06–1.27), and psychotic disorders (OR, 1.40; 95% CI, 1.07–1.83) [31]. Another study reported that late preterm infants had a lower intelligence quotient (IQ) and higher odds of ADHD (OR, 1.3; 95% CI, 1.1–1.5) [32]. As the number of MLPT infants has increased over the last few decades, more studies are needed, and primary care physicians should cautiously observe their neurodevelopmental outcomes [33].5) Cognitive outcomes of intrauterine growth restriction
5) Cognitive outcomes of intrauterine growth restriction
A study on cognitive outcomes of intrauterine growth restriction and small for GA revealed a significantly increased risk of poorer cognitive outcomes in both preterm and term-born children compared with children who were appropriate for GA [34]. The biological mechanisms are not clearly explained, but the authors speculated that suboptimal trophic inputs due to placental insufficiency or undernutrition may affect in utero brain development [34].
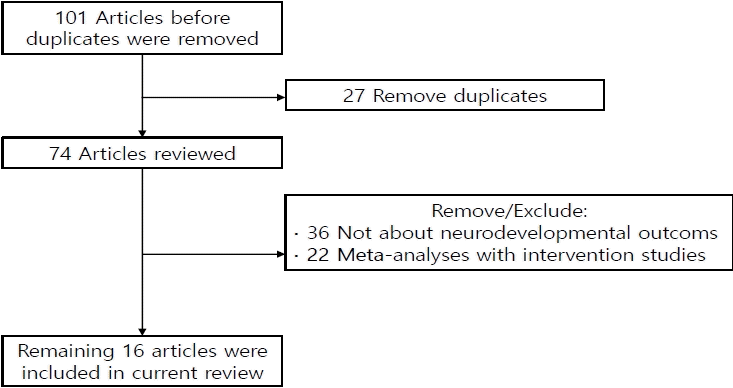
One hundred one studies were identified in the initial search process; of them, 27 duplicated studies were removed, while another 36 studies were manually excluded for irrelevancy. Among the 38 remaining studies, 22 focused on interventional studies improving neurodevelopmental outcomes and 16 focused on the natural outcomes of preterm infants or LBW infants (Fig. 1, Table 2).- 3. Korean studies
- 3. Korean studies
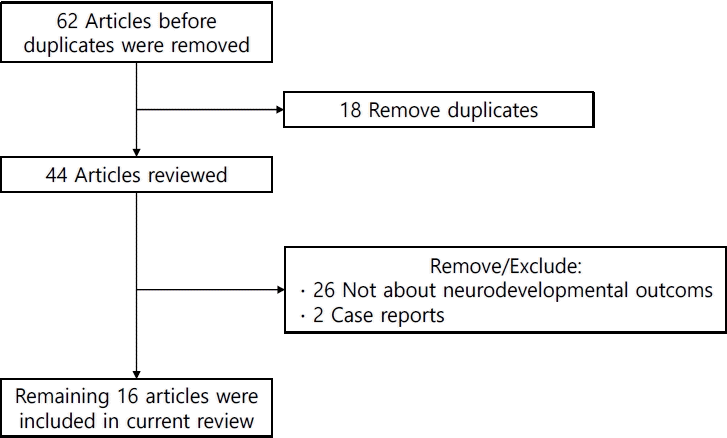
Sixty-two studies were identified in the initial search process. Of them, 18 duplicate studies were removed, while 28 were excluded manually for irrelevancy (Fig. 3). Among the 16 remaining studies (Table 3), 3 used Korean National Health Insurance Service (KNHIS) data and 4 used Korean Neonatal Network (KNN) data. The KNN is a nationwide registry of VLBW infants. The registry was established in 2013; more than 18,000 infants were registered. Healthcare workers report infant demographics and neonatal outcomes (e.g., survival, BPD, intraventricular hemorrhage), and long-term outcomes (growth and developmental outcomes at 18 and 36 months of corrected age [CA]) based on medical record review. The registry uses the K-DST and BSID to monitor developmental outcomes [35].Two studies reported the neurodevelopmental outcomes of late preterm infants or LBW infants (1.5–2.4 kg) using KNHIS data [17,36]. One study revealed that VLBW and LBW are associated with ADHD or ASD. A stronger association between ADHD/ASD and birth weight was observed as birth weight decreased. In a subgroup analysis, LBW infants without a perinatal history showed similar results [17]. Another study of late preterm twins found that they were at increased risk for any neurological or neurodevelopmental delay (hazard ratio, 1.14; 95% CI, 1.07–1.21) compared to term twins. In this study, the hazard ratio of ASD and ADHD was significantly increased (1.17; 95% CI, 1.01–1.36) in preterm twins [37]. A study of MLPT infants born in a single institution showed similar results of a risk of developing borderline intelligence functioning and attention problems [36]. A study of KNN data reported the overall prevalence of cerebral palsy (6.2%–6.6%), blindness (0.2%–0.3%), and hearing loss (0.8%–1.9%) in VLBW infants born in 2013–2014 [38].Several studies assessed the associations between perinatal factors and neurodevelopmental outcomes. A study using KNHIS data reported that periventricular leukomalacia and growth restrictions in head circumference and height were independent risk factors for developmental delay [39]. Others focused on the association between growth and development. Song et al. [40] reported that head circumference growth between a postmenstrual age (PMA) of 35 weeks and 4 months of CA was associated with BSID III scores appropriate for GA preterm infants. Cho et al. [41] revealed that z score changes in head circumference between birth and a PMA of 35 weeks and achieving head growth catchup by 4 months of CA were significantly associated with favorable neurodevelopmental outcomes (BSID III at 18 months) in small GA preterm infants. Another study showed that weight gain in the NICU was positively correlated with full-scale IQ at school age [42].Two studies reported that retinopathy of prematurity (ROP) was associated with unfavorable outcomes of BSID III at 18 months’ CA [43,44]. The authors explain this result using several hypotheses. First, visual impairment can negatively affect synaptic reorganization following brain damage. Second, previous studies reported that severe ROP is associated with delayed white matter maturation and reduced brain volume [43]. Third, as ROP is caused by poor development of the blood vessels in the retina, which is part of the central nervous system (CNS), it may be associated with abnormal CNS function [44]. Shim et al. [45] reported that lateonset sepsis was associated with cognitive delay at 18–24 months’ CA (OR, 1.48; 95% CI, 1.02–2.16), while Kim et al. [46] reported that hypotension in the first week of life increased the OR (3.53; 95% CI, 1.34–9.32) and poor neurodevelopmental outcomes (BSID III) in VLBW infants,.In contrast, exposure to hydrocortisone during the first week in VLBW infants was not associated with adverse developmental outcomes at 18 months’ CA (BSID III) [47]. Two studies examined the association between surgical intervention and development. Sung et al. [48] reported that the risk of NDI (BSID III) increased as the number of surgeries increased. It was impossible to specify the reasons underlying morbidities or anesthetic medications. In contrast, in a single-center study, the surgical ligation of patent ductus arteriosus was not associated with poor neurodevelopmental outcomes (BSID III) at 18months’ CA [49]. An interventional study aimed to improve developmental outcomes. In a multicenter randomized control trial, the authors tried to examine whether a preventive intervention program of 4 home visits by a nurse could improve developmental outcomes, but no significant differences were noted between the control and intervention groups [50].
- Discussion and conclusion
- Discussion and conclusion
In this review of premature infants, the prognosis for survival without NDI was noticeably poorer at a younger GA. MLPT infants also had developmental outcome concerns. Survival free of major impairments among EP infants has increased during the last several decades but remains high among children born at <25 weeks’ GA [51].Several interventions have been attempted to improve neurodevelopmental outcomes. Prophylactic early recombinant human erythropoietin (rhEPO) is expected to provide neuroprotection for preterm infants. Previous studies reported that rhEPO improves the cognitive development of VP but not EP infants. Moreover, there was no significant effect on any secondary outcome, such as psychomotor development, cerebral palsy, or severe visual or hearing impairment [52-55]. The effects of prebiotic and probiotic supplementation and micro- (zinc, iodine) or macronutrient (amino acid) supplementation on the neurodevelopment of preterm infants have been studied, but the evidence remains insufficient [56-60].To identify NDI in time and provide timely interventions, a follow-up system for premature infants must be established. In Korea, the Ministry of Health and Welfare started a pilot program that funds coordinator nurses who promote the regular follow-up of VLBW or VP infants at 4, 8, 18, 24, and 36 months’ CA [61]. Furthermore, the government planned to set a new statistics system using KNN and other national big data (e.g., KNHIS), which would enable efficient monitoring until adulthood [62]. With the sharing of established information with primary care physicians, appropriate monitoring and early intervention can be achieved.
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Table 1.
Table 2.
| Study | Characteristics | Main results |
|---|---|---|
| Myrhaug et al. [17] (2019) | 12 Studies, GA 22–27 wk | Rates of survival without impairment were 1.2% at 22; 9.3% at 24; 40.6% at 25; 64.2% at 27 weeks. |
| Ding et al. [18] (2019) | 15 Studies, 820 GA 22–25 wk | Rates of moderate-to-severe neurodevelopmental disability were 42% at 22; 41% at 23; 32% at 24; 23% at 25 weeks. |
| Backes et al. [19] (2021) | 31 Studies, 2,226 GA 22 wk | Survival without moderate or severe impairment was 37.0%. |
| Pascal et al. [21] (2018) | 30 Studies, 10,293 VLBW or VP | Cognitive delay 16.9%, motor delay 20.6%, CP 6.8% |
| Twilhaar et al. [23] (2018) | 71 Studies, 7,752 EP or VP | 0.86 SD lower IQ compared with controls. |
| Brydges et al. [24] (2018) | 60 Studies, 6,163 VP | 0.82 SD lower on intelligence tests. |
| Twilhaar et al. [25] (2018) | 17 Studies, 2,390 Preterm (mean, 24.5–29.9 wk) | Scored lower on arithmetic, reading, spelling and more needed special education |
| Ramaswamy et al. [26] (2021) | 5 Studies, ELBW, EP | NDI prevalence was 29% for EP. |
| Maenner et al. [27] (2021) | 18 Studies, 3,366 Preterm (median, 25.1–31.3 wk) | Prevalence of ASD was 7% in preterm infants. |
| Laverty et al. [28] (2021) | 52 Studies, preterm | Odds of autism were 3.3 times higher in preterm infants. |
| Franz et al. [6] (2018) | 12 Studies, 1,787 VLBW or VP | Odds of ADHD were 3.04 times higher in preterm infants. |
| Momany et al. [29] (2018) | 88 Studies, 4,645,482 | Birth weight was associated with ADHD (r=-0.15). |
| Fitzallen et al. [30] (2021) | 11 Studies, Preterm 1294 (anxiety), 777 (depressive) | Odds of anxiety were 2.17 times higher in preterm infants. |
| Fernández de Gamarra-Oca et al. [31] (2021) | 16 studies, MLPT | Greater odds for any psychiatric disorders |
| Allotey et al. [32] (2018) | 74 Studies, 64,601 children | Late preterm infants had lower IQ and higher odds of ADHD. |
| Sacchi et al. [34] (2020) | 60 Studies, 52,822 children | IUGR and SGA had significantly poorer cognitive outcomes. |
GA, gestational age; VLBW, very low birth weight; VP, very preterm infants; SD, standard deviation; CP, erebral palsy; EP, extremely preterm; NDI, neurodevelopmental impairment; ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder; ELBW, extremely low birth weight; MLPT, moderate- to late-preterm infants; IQ, intelligent quotient; IUGR, intrauterine growth retardation; SGA, small for gestational age.
Table 3.
| Study | Characteristics | Data | Main results |
|---|---|---|---|
| Song et al. [16] (2022) | 2,143,652 children at age 4–10 years | NHIS | VLBW and LBW had higher odds of ADHD and ASD. |
| Cho et al. [37] (2021) | 17,189 twins ≥34 wk | NHIS | Late preterm twins had a higher risk of neurodevelopmental delay (HR, 1.14; 95% CI, 1.07–1.21). |
| Jin et al. [36] (2020) | 37 MLPT | Hospital | MLPT infants had risks of developing borderline intelligence functioning and attention problems at early school age. |
| Youn et al. [38] (2018) | 2,660 VLBW | KNN | Prevalence of cerebral palsy 6.2%–6.6%, blindness 0.2%–0.3%, and hearing loss 0.8-1.9% in VLBW infants |
| Han et al. [39] (2022) | 2,961 VLBW | NHIS | Periventricular leukomalacia, growth restriction in HC and height were risk factors for developmental delay. |
| Song et al. [40] (2021) | 122 VP or VLBW | Hospital | HC growth between PMA 35 weeks and 4 months CA was associated with BSID III scores. |
| Cho et al. [41] (2021) | 90 SGA VLBW | Hospital | HC growth between birth and PMA 35 weeks and catch-up at 4 months CA were associated with development. |
| Kim et al. [42] (2021) | 56 VP or VLBW | Hospital | IQ was correlated with postnatal weight gain in the NICU. |
| Bae et al. [43] (2021) | 182 VLBW | Hospital | Moderate to severe BPD and severe ROP were associated with adverse neurodevelopmental outcomes of VLBW infants without severe brain injury. |
| Ahn et al. [44] (2022) | 2,132 VLBW | KNN | Motor and cognitive delays were associated with ROP. |
| Shim et al. [45] (2021) | 2,098 VLBW | KNN | Late-onset sepsis was associated with cognitive delay. |
| Kim et al. [46] (2018) | 166 VLBW | Hospital | Hypotension was associated with cognitive and language delay. |
| Youn et al. [47] (2017) | 191 VLBW | Hospital | Early hydrocortisone exposure did not increase the risk for poor developmental outcomes. |
| Sung et al. [48] (2019) | 7,885 VLBW | KNN | Surgeries increased the risk of neurodevelopmental impairment. |
| Youn et al. [49] (2019) | 142 VLBW | Hospital | Surgical ligation of PDA did not increase risk of poor neurodevelopmental outcomes. |
| Youn et al. [50] (2021) | 138 VP | 3 Hospitals | Intervention program showed no positive effect on neurodevelopmental outcomes. |
NHIS, National Health Insurance Service; VLBW, very low birth weight; LBW, low birth weight infants; ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; HR, hazard ratio; CI, confidence interval; MLPT, moderate- to late-preterm infants; KNN, Korean Neonatal Network; HC, head circumference; PMA, postmenstrual age; CA, corrected age; BSID III, The Bayley Scales of Infant and Toddler Development, third edition; SGA, small for gestational age; IQ, intelligent quotient; NICU, neonatal intensive care unit; BPD, bronchopulmonary dysplasia; ROP, retinopathy of prematurity; PDA, patent ductus arteriosus; VP, very preterm.
- References
- 1. World Health Organization. Preterm birth [Internet]. Geneva (Switzerland), World Health Organization. c2022;[cited 2022 Apr 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/preterm-birth.2. World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. Geneva (Switzerland): World Health Organization, 2015.3. Lincetto O, Banerjee A. World Prematurity Day: improving survival and quality of life for millions of babies born preterm around the world. Am J Physiol Lung Cell Mol Physiol 2020;319:L871–4.
[Article] [PubMed]4. Kusuda S. More studies are needed to convince stakeholders of the value of resuscitating periviable infants. Acta Paediatr 2019;108:1182–3.
[Article] [PubMed] [PMC]5. McBryde M, Fitzallen GC, Liley HG, Taylor HG, Bora S. Academic outcomes of school-aged children born preterm: a systematic review and meta-analysis. JAMA Netw Open 2020;3:e202027.
[Article] [PubMed] [PMC]6. Franz AP, Bolat GU, Bolat H, Matijasevich A, Santos IS, Silveira RC, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics 2018;141:e20171645.
[Article] [PubMed]7. Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 2018;142:e20180134.
[Article] [PubMed]8. Townley Flores C, Gerstein A, Phibbs CS, Sanders LM. Short-term and long-term educational outcomes of infants born moderately and late preterm. J Pediatr 2021;232:31–7.e2.
[Article] [PubMed]9. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008;359:262–73.
[Article] [PubMed]10. Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 2009;123:1293–300.
[Article] [PubMed]11. Madaschi V, Mecca TP, Macedo EC, Paula CS. Bayley-III scales of infant and toddler development: transcultural adaptation and psychometric properties. Paidéia (Ribeirão Preto) 2016;26:189–97.
[Article]12. Balasundaram P, Avulakunta ID. Bayley scales of infant and toddler development. 2022 Nov 21. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.13. Palisano RJ. GMFCS-E & R gross motor function classification system: expanded and revised. Hamilton (ON): CanChild, 2007.14. Wechsler D. Wechsler intelligence scale for children. 5th ed. Bloomington: Pearson, 2014.15. Chung HJ, Yang D, Kim GH, Kim SK, Kim SW, Kim YK, et al. Development of the Korean Developmental Screening Test for infants and children (K-DST). Clin Exp Pediatr 2020;63:438–46.
[Article] [PubMed] [PMC]16. Song IG, Kim HS, Cho YM, Lim YN, Moon DS, Shin SH, et al. Association between birth weight and neurodevelopmental disorders assessed using the Korean National Health Insurance Service claims data. Sci Rep 2022;12:2080.
[Article] [PubMed] [PMC]17. Myrhaug HT, Brurberg KG, Hov L, Markestad T. Survival and impairment of extremely premature infants: a meta-analysis. Pediatrics 2019;143:e20180933.
[Article] [PubMed]18. Ding S, Lemyre B, Daboval T, Barrowman N, Moore GP. A meta-analysis of neurodevelopmental outcomes at 4-10 years in children born at 22-25 weeks gestation. Acta Paediatr 2019;108:1237–44.
[Article] [PubMed]19. Backes CH, Rivera BK, Pavlek L, Beer LJ, Ball MK, Zettler ET, et al. Proactive neonatal treatment at 22 weeks of gestation: a systematic review and meta-analysis. Am J Obstet Gynecol 2021;224:158–74.
[Article] [PubMed]20. Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020;142(16_suppl_2): S524–50.
[Article] [PubMed]21. Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol 2018;60:342–55.
[Article] [PubMed]22. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9.
[Article] [PubMed]23. Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr 2018;172:361–7.
[Article] [PubMed] [PMC]24. Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol 2018;60:452–68.
[Article] [PubMed]25. Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CS, van Elburg RM, Oosterlaan J. Academic performance of children born preterm: a meta-analysis and meta-regression. Arch Dis Child Fetal Neonatal Ed 2018;103:F322–30.
[Article] [PubMed] [PMC]26. Ramaswamy VV, Abiramalatha T, Bandyopadhyay T, Shaik NB, Bandiya P, Nanda D, et al. ELBW and ELGAN outcomes in developing nations-Systematic review and meta-analysis. PLoS One 2021;16:e0255352.
[Article] [PubMed] [PMC]27. Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ 2021;70:1–16.28. Laverty C, Surtees A, O'Sullivan R, Sutherland D, Jones C, Richards C. The prevalence and profile of autism in individuals born preterm: a systematic review and meta-analysis. J Neurodev Disord 2021;13:41.
[Article] [PubMed] [PMC]29. Momany AM, Kamradt JM, Nikolas MA. A meta-analysis of the association between birth weight and attention deficit hyperactivity disorder. J Abnorm Child Psychol 2018;46:1409–26.
[Article] [PubMed] [PMC]30. Fitzallen GC, Sagar YK, Taylor HG, Bora S. Anxiety and depressive disorders in children born preterm: a meta-analysis. J Dev Behav Pediatr 2021;42:154–62.
[Article] [PubMed]31. Fernández de Gamarra-Oca L, Ojeda N, Gómez-Gastiasoro A, Peña J, Ibarretxe-Bilbao N, García-Guerrero MA, et al. Long-term neurodevelopmental outcomes after moderate and late preterm birth: a systematic review. J Pediatr 2021;237:168–76.e11.
[Article] [PubMed]32. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG 2018;125:16–25.
[Article] [PubMed]33. Korean Statistical Information Service. Vital statistics of Korea [Internet]. Daejeon (Korea), Statistics Korea. c2021;[cited 2022 Apr 1]. Available from: https://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_0_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&statId=1962004&themaId=A&outLink=Y&entrType=#A_3.2.34. Sacchi C, Marino C, Nosarti C, Vieno A, Visentin S, Simonelli A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: a systematic review and meta-analysis. JAMA Pediatr 2020;174:772–81.
[Article] [PubMed] [PMC]35. Lee JH, Noh OK, Chang YS; Korean Neonatal Network. Neonatal outcomes of very low birth weight infants in Korean neonatal network from 2013 to 2016. J Korean Med Sci 2019;34:e40.
[Article] [PubMed] [PMC]36. Jin JH, Yoon SW, Song J, Kim SW, Chung HJ. Long-term cognitive, executive, and behavioral outcomes of moderate and late preterm at school age. Clin Exp Pediatr 2020;63:219–25.
[Article] [PubMed] [PMC]37. Cho GJ, Cho KD, Kim HY, Ha S, Oh MJ, Won HS, et al. Short-term neonatal and long-term infant outcome of late-preterm twins: nationwide population-based study. Ultrasound Obstet Gynecol 2022;59:763–70.
[Article] [PubMed]38. Youn Y, Lee SM, Hwang JH, Cho SJ, Kim EK, Kim EA, et al. National registry data from Korean neonatal network: two-year outcomes of korean very low birth weight infants born in 2013-2014. J Korean Med Sci 2018;33:e309.
[Article] [PubMed] [PMC]39. Han JH, Yoon SJ, Lim JH, Shin JE, Eun HS, Park MS, et al. The impact of neonatal morbidities on child growth and developmental outcomes in very low birth weight infants: a nationwide cohort study. Eur J Pediatr 2022;181:197–205.
[Article] [PubMed]40. Song IG, Kim EK, Cho H, Shin SH, Sohn JA, Kim HS. Differential effect of growth on development between AGA and SGA preterm infants. Int J Environ Res Public Health 2020;17:3022.
[Article] [PubMed] [PMC]41. Cho H, Kim EK, Song IG, Heo JS, Shin SH, Kim HS. Head growth during neonatal intensive care unit stay is related to the neurodevelopmental outcomes of preterm small for gestational age infants. Pediatr Neonatol 2021;62:606–11.
[Article] [PubMed]42. Kim YJ, Shin SH, Lee ES, Jung YH, Lee YA, Shin CH, et al. Impact of size at birth and postnatal growth on metabolic and neurocognitive outcomes in prematurely born school-age children. Sci Rep 2021;11:6836.
[Article] [PubMed] [PMC]43. Bae SP, Shin SH, Yoon YM, Kim EK, Kim HS. Association of severe retinopathy of prematurity and bronchopulmonary dysplasia with adverse neurodevelopmental outcomes in preterm infants without severe brain injury. Brain Sci 2021;11:699.
[Article] [PubMed] [PMC]44. Ahn JH, Lee KM, Kim MJ, Park HK, Kim YJ, Ahn SJ, et al. Neurodevelopmental outcomes in very low birthweight infants with retinopathy of prematurity in a nationwide cohort study. Sci Rep 2022;12:5053.
[Article] [PubMed] [PMC]45. Shim SY, Cho SJ, Park EA. Neurodevelopmental outcomes at 18-24 months of corrected age in very low birth weight infants with late-onset sepsis. J Korean Med Sci 2021;36:e205.
[Article] [PubMed] [PMC]46. Kim TH, Moon CJ, Sung IK, Youn YA. Hypotension within 1 week of life associated with poor short- and long-term outcomes in very low birth weight infants. Cardiol Young 2018;28:1037–41.
[Article] [PubMed]47. Youn Y, Moon CJ, Sung IK. Long-term postnatal steroid effect in very low birth weight infants. Steroids 2017;125:33–6.
[Article] [PubMed]48. Sung SI, Lee NH, Kim HH, Kim HS, Han YS, Yang M, et al. The impact of surgical intervention on neurodevelopmental outcomes in very low birth weight infants: a nationwide cohort study in Korea. J Korean Med Sci 2019;34:e271.
[Article] [PubMed] [PMC]49. Youn Y, Seo YM, Yum SK, Sung IK. Patent ductus arteriosus ligation on neurodevelopmental outcomes at corrected 2years. Ital J Pediatr 2019;45:110.
[Article] [PubMed] [PMC]50. Youn YA, Shin SH, Kim EK, Jin HJ, Jung YH, Heo JS, et al. Preventive intervention program on the outcomes of very preterm infants and caregivers: a multicenter randomized controlled trial. Brain Sci 2021;11:575.
[Article] [PubMed] [PMC]51. Cheong JLY, Olsen JE, Lee KJ, Spittle AJ, Opie GF, Clark M, et al. Temporal trends in neurodevelopmental outcomes to 2 years after extremely preterm birth. JAMA Pediatr 2021;175:1035–42.
[Article] [PubMed] [PMC]52. Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics 2017;139:e20164317.
[Article] [PubMed]53. Liang L, Yu J, Xiao L, Wang G. Sustained low-dose prophylactic early erythropoietin for improvement of neurological outcomes in preterm infants: a systematic review and meta-analysis. J Affect Disord 2021;282:1187–92.
[Article] [PubMed]54. Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic erythropoietin for neuroprotection in very preterm infants: a meta-analysis update. Front Pediatr 2021;9:657228.
[Article] [PubMed] [PMC]55. Qin N, Qin H. Efficacy and safety of high and low dose recombinant human erythropoietin on neurodevelopment of premature infants: a meta-analysis. Medicine (Baltimore) 2021;100:e25805.
[PubMed] [PMC]56. Upadhyay RP, Taneja S, Chowdhury R, Strand TA, Bhandari N. Effect of prebiotic and probiotic supplementation on neurodevelopment in preterm very low birth weight infants: findings from a meta-analysis. Pediatr Res 2020;87:811–22.
[Article] [PubMed]57. Alshaikh B, Abo Zeed M, Yusuf K, Guin M, Fenton T. Effect of enteral zinc supplementation on growth and neurodevelopment of preterm infants: a systematic review and meta-analysis. J Perinatol 2022;42:430–9.
[Article] [PubMed]58. Walsh V, Brown JVE, McGuire W. Iodine supplementation for the prevention of mortality and adverse neurodevelopmental outcomes in preterm infants. Cochrane Database Syst Rev 2019;2:CD005253.
[Article] [PubMed] [PMC]59. Osborn DA, Schindler T, Jones LJ, Sinn JK, Bolisetty S. Higher versus lower amino acid intake in parenteral nutrition for newborn infants. Cochrane Database Syst Rev 2018;3:CD005949.
[Article] [PubMed] [PMC]60. Lin L, Amissah E, Gamble GD, Crowther CA, Harding JE. Impact of macronutrient supplements for children born preterm or small for gestational age on developmental and metabolic outcomes: A systematic review and meta-analysis. PLoS Med 2019;16:e1002952.
[Article] [PubMed] [PMC]61. Ministry of Health and Welfare. Follow-up System for preterm and low birth weight children. Sejong (Korea): Ministry of Health and Welfare, 2021.62. Choi E, Lee KH, Lim JW, Lee S, Song IG, Chung SH, et al. Statistics management system for premature newborns. Sejong (Korea): Ministry of Health and Welfare, 2021.

 About
About Browse articles
Browse articles For contributors
For contributors