All issues > Volume 66(8); 2023
Association between dyslipidemia and asthma in children: a systematic review and multicenter cohort study using a common data model
- Corresponding author: Hey Sung Baek, MD, PhD. Department of Pediatrics, Kandong Sacred Heart Hospital, Hallym University College of Medicine, 150, Seongan-ro, Gangdong-gu, Seoul, 035355, Korea Email: paviola7@gmail.comCo-corresponding author: Ju Hee Kim, MD. Department of Pediatrics, Kandong Sacred Heart Hospital, Hallym University College of Medicine, 150, Seongan-ro, Gangdong-gu, Seoul, 035355, Korea Email: 2004052@gmail.com
- Received February 6, 2023 Revised April 27, 2023 Accepted June 7, 2023
- Abstract
-
- Background
- Background
- The association between dyslipidemia and asthma in children remains unclear.
- Purpose
- Purpose
- This study investigated the association between dyslipidemia and cholesterol levels in children.
- Methods
- Methods
- A systematic literature review was performed to identify studies investigating the association between dyslipidemia and asthma in children. The PubMed database was searched for articles published from January 2000–March 2022. Data from a cohort study using electronic health records from 5 hospitals, converted to the Observational Medical Outcomes Partnership Common Data Model (OMOP-CDM), were used to identify the association between total cholesterol (TC) levels and asthma in children. This cohort study used the Cox proportional hazards model to examine hazard ratio (HR) of asthma after propensity score matching, and included an aggregate meta-analysis of HR.
- Results
- Results
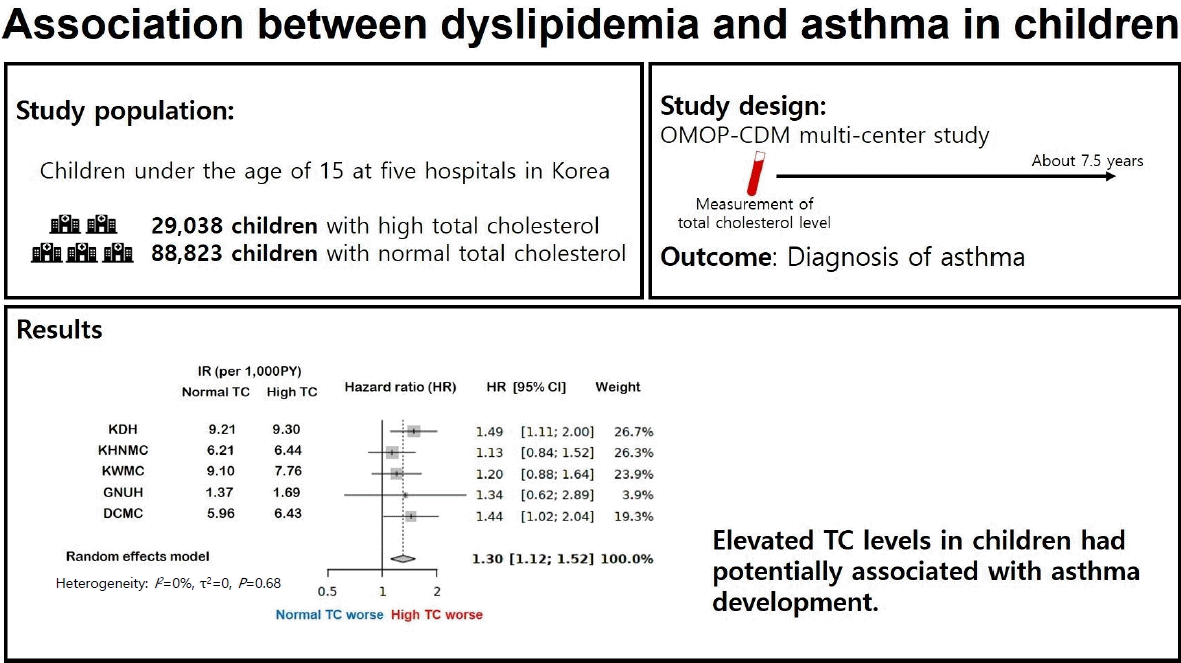
- We examined 11 studies reporting an association between dyslipidemia and asthma in children. Most were cross-sectional; however, their results were inconsistent. In OMOP-CDM multicenter analysis, the high TC (>170 mg/dL) group included 29,038 children, while the normal TC (≤170 mg/dL) group included 88,823 children including all hospital datasets. In a meta-analysis of this multicenter cohort, a significant association was found between high TC levels and later development of asthma in children <15 years of age (pooled HR, 1.30; 95% confidence interval, 1.12–1.52).
- Conclusion
- Conclusion
- Elevated TC levels in children may be associated with asthma.
- Graphical abstract. OMOP-CDM, Observational Medical Outcomes Partnership Common Data Model; R, incidence rate; PY, patient-years; TC, total cholesterol; KDH, Kangdong Sacred Heart Hospital; KHNMC, Kyung Hee University Hospital at Gandong; KWMC, Kangwon National University Hospital; GNUH, Gyeongsang National University Hospital; DCMC, Deagu Catholic University Hospital.
- Introduction
- Introduction
Asthma is a chronic inflammatory airway disorder and is regarded as a multifactorial disease. The prevalence of dyslipidemia in children has increased in recent years, and it is present in approximately 20% of adolescents [1,2]. In recent decades, researchers have found that dyslipidemia is one of the proinflammatory host factors of asthma [3,4]. Elevated levels of cholesterol can trigger proinflammatory cellular responses and induce the release of inflammatory cytokines from the endothelium, which in turn leads to atherosclerotic plaque formation. However, the associations between asthma and dyslipidemia were found to be inconsistent, studies in children or adolescents were limited, and the results were different from those in adults [5]. A study to assess the risk of asthma with dyslipidemia through blood sampling for lipid profiles and long-term follow-up in children is practically difficult and has many limitations. There have been few longitudinal follow-up cohort studies assessing the causative relationship between dyslipidemia and asthma development.This study aimed to determine the association between dyslipidemia and asthma in children. We reviewed previous studies reporting an association between dyslipidemia and asthma in children. Furthermore, since total cholesterol (TC) is often part of common blood tests at clinics, we used a multicenter electronic health record (EHR) database converted to the Observational Medical Outcomes Partnership Common Data Model (OMOP-CDM) to assess the association between serum levels of TC and asthma with long-term follow-up in a large sample population.
- Methods
- Methods
- 1. Systemic review
- 1. Systemic review
1) Search strategy
1) Search strategy
Studies on the association between dyslipidemia and asthma in children reported between January 2000 and May 2022 were searched using PubMed (Table 1). The search was performed using the terms dyslipidemia, TC, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol, asthma, and children. Eligible studies had to be published in English and included randomized controlled trials and prospective followup, retrospective, and cross-sectional studies. Letters, editorials, reviews, commentaries, case reports, and personal communication were not included. The population of included studies comprised those with children or adolescents under the age of 18 years who could have asthma and control groups without asthma. In addition, studies had to assess at least one part of the lipid profile of their study population, such as TC, HDL-C, LDL-C, or triglyceride (TG). Additionally, eligible studies had to include quantitative results regarding the outcomes of interest. Candidate studies were screened using a 2-step process. First, by reviewing the titles and abstracts of each study, studies that did not meet the inclusion criteria were excluded. Second, the full texts of the remaining studies were reviewed according to inclusion and exclusion criteria. Two reviewers identified the eligible studies. A third reviewer was consulted in the case of any uncertainty regarding eligibility. Among 226 studies, 11 were included.2) Data extraction
2) Data extraction
We extracted the following relevant data from the included studies: name of first author, publication years, study design, participation age and number, exposure, and outcome of interest, and summarized the results.
- 2. OMOP-CDM multicenter analysis
- 2. OMOP-CDM multicenter analysis
1) Data source
1) Data source
The present study used 8 hospital-based cohorts that were converted to the OMOP-CDM format using the FEEDERNET platform, which provides EHR data without patients’ personal information. The Observational Health Data Sciences and Informatics (OHDSI) organization is an international collaboration that works to create high-quality evidence by creating and using open-source data analytics solutions on a large network of health databases from different countries [6]. This allows for the systematic analysis of disparate observational databases. The concept behind this approach is to transform the data in those databases into a common format and representation (terminologies, vocabularies, coding schemes) and then use a library of standard analytical routines that have been written based on the common format to do systematic analyses. A key infrastructure requirement for large-scale distributed comparative effectiveness research is that all healthcare systems use CDM [7]. Once a database has been converted to the OMOP-CDM, evidence can be generated using standardized analytics tools. The CDM contains 18 data tables: person, drug exposure, drug era, condition occurrence, condition error, observation period, observation, procedure occurrence, visit occurrence, death, drug cost, procedure cost, location, provider, organization, care site, payment plan period, and cohort [6].The 5 secondary or tertiary hospitals included Kangdong Sacred Heart Hospital in Seoul (KDH), Kyung Hee University Hospital at Gangdong in Seoul (KHNMC), Kangwon National University Hospital in Chuncheon (KWMC), Gyeongsang National University Hospital in Changwon (GNUH), and Deagu Catholic University Hospital in Deagu (DCMC). All hospitals signed a memorandum of understanding for research in border-free zones. The enrollment period and total number of patients were 1986 to 2018 and 1,689,604 in KDH, 2006 to 2017 and 822,183 in KHNMC, 2003 to 2018 and 519,700 in KWMC, 2009 to 2022 and 618,246 in GNUH, and 2005 to 2018 and 1,688,980 in DCMC, respectively. The total number of enrolled patients was 5,338,713 (Fig. 1). The study protocol was approved by the Institutional Review Board of Hallym University (IRB 2019-09-005) without approval from the institutional review boards of other institutions in accordance with the Memorandum of Understanding on the Research Border-Free Zone.2) Study design and cohort definition
2) Study design and cohort definition
This was a retrospective cohort study. A flowchart of the study is shown in Fig. 1. The index date was the date when the blood was drawn for TC measurements. Children under 15 years of age who underwent blood tests for the measurement of TC were identified. Children diagnosed with asthma before the index date were excluded. The target group was the high TC group, defined as a TC level greater than 170 mg/dL [8]. The comparator group was the normal TC group, defined as a TC level of 170 mg/dL or less. In both groups, participants were censored either at the time of outcome identification or at the end of the observation period in the database. Children were excluded if they belonged to either group by performing the TC level test several times. Finally, there were 88,823 children in the normal TC group and 29,038 in the high TC group.3) Outcomes
3) Outcomes
The primary outcome was the first diagnosis of asthma. Asthma was defined as one or more principal diagnoses of the International Classification of Diseases, Tenth Edition (ICD-10) codes for asthma (J45.X) and 2 or more prescriptions for asthma treatment drugs, such as inhaled corticosteroids (ICS), combination ICS and long-acting beta-agonists, and leukotriene modifiers [9-11].4) Covariates
4) Covariates
To balance the baseline characteristics between the high TC and normal TC groups, the demographic and clinical variables were considered covariates. Age at the index date and sex were regarded as demographic characteristics. In addition, the diagnosed diseases and medications during the 365 days before the index date were regarded as clinical characteristics. Diagnosed diseases were identified using ICD-10. Medications were prescribed at the hospital visit. The covariates in each hospital are shown in Supplementary Table 1 to 5.
- 3. Statistical analysis
- 3. Statistical analysis
To adjust for covariates, we performed propensity matching score analysis. A 4:1 propensity score (PS) matching with oneto-one greedy matching and a caliper of 0.2 on the standardized logit scale was performed. Standardized differences were used to compare differences in covariates between groups in both the unmatched and matched samples (differences >10% were considered significant) [12].A Cox proportional hazards model was then fitted to the matched cohorts using the Cohort Method R package (https://github.com/OHDSI/CohortMethod). For the outcomes of interest, hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The incidence rate was determined per 1,000 person-years. Using the Kaplan-Meier plot, the survival probability for asthma during the follow-up period was calculated, and the log-rank test was used to compare each cohort.In addition, negative outcomes that were assumed to not be associated with the target or comparative cohorts were established (Supplementary Table 6). The empirical correction of P values was performed by applying the empirical null distribution to the point estimates of the negative control outcomes. It was assumed that the true relative risk of negative control outcomes between the target and control cohorts was 1.A random-effects meta-analysis was performed without aggregating the data from each hospital. Study heterogeneity was assessed using Cochran Q test and I2 statistics. Heterogeneity was considered statistically significant when the P value was less than 0.1 in the Cochran Q test, and the I2 statistic value was greater than 50%. All analyses were performed using R statistical software (Version 3.6.1; R Foundation for Statistical Com puting, Vienna, Austria) and the R meta-package.
- Results
- Results
- 1. Systemic review
- 1. Systemic review
The 11 included studies (January 2000–May 2022) are summarized in Table 2. There were 7 cross-sectional studies, 1 casecontrol study, 2 prospective cohort studies, and 1 retrospective study. In studies using the United States National Health and Nutrition Examination Survey database by Lu et al. [13], reduced HDL-C and elevated LDL-C, TC, TG, and glucose levels were not significantly associated with the presence of current asthma in approximately 23,000 children and adolescents. On the other hand, 2 cross-sectional studies by Chanachon et al. [14,15] reported that asthmatic children with dyslipidemia had significant associations between parameters of lung function tests, including impulse oscillometry, and spirometry. Three other cross-sectional studies [16-18] have described significant associations between serum levels of lipid panels and asthma. In particular, Chen et al. [19] discovered not only an association between serum levels of TC and LDL-C and asthma but also an interactive effect of obesity and asthma on high LDL-C levels in boys (P=0.03).In a case-control study [20], adolescents with asthma aged 16– 18 years had a lower HDL-C level at 11–12 and 16–18 years of age than those without asthma. In addition, low HDL-C levels at 16–18 years of age had a positive association with asthma even after adjusting for HDL-C levels at 11–12 years of age.Two longitudinal studies reported conflicting results. A prospective community-based cohort study from 14–20 years of age by Rasmussen et al. [21] showed that the level of lipid profiles at 14 and 20 years of age had no association with airway hyperresponsiveness measured at 20 years of age. In another longitudinal study of 3,982 adolescents aged 11–12 to 15–17 years [22], low HDL level at 11–12 years of age was associated with an increased risk of asthma at 15–17 years of age.- 2. OMOP-CDM multicenter analysis
- 2. OMOP-CDM multicenter analysis
1) Study characteristics
1) Study characteristics
In all hospital datasets after PS matching, the high TC group as a target group included 29,038 children, and the normal TC group as a comparator group included 88,823 children. Table 3 shows the baseline characteristics of the matched cohort. The baseline demographic and clinical data of the unmatched and matched cohorts in each hospital are described in Supplementary Table 1 to 5. Before PS matching, age group distribution; sex ratio; medical history, such as acute respiratory disease and urinary tract infection; and medication history, such as antibiotics and anti-inflammatory drugs, differed between the high TC and normal TC groups. However, after PS matching, the age group distribution, sex ratio, medical history, and medication history were balanced between the high TC and normal TC groups. Each hospital had slightly different characteristics, but the age group of 5–9 years accounted for the largest proportion, and the male-to-female ratio was comparable in all hospitals.2) Association between TC and asthma in children using EHR CDM database
2) Association between TC and asthma in children using EHR CDM database
Table 4 and Fig. 2 show the association between total levels and asthma in children. The asthma incidence rate (per 1,000 patient-years) of the high TC group tended to be higher than that of the normal TC group, except for KWMC. The meta-analysis showed that the high TC group was significantly associated with an increased risk of asthma (pooled HR, 1.30; 95% CI, 1.12–1.52). There was no significant heterogeneity across the databases (I2=0%, P=0.68). The survival curves for asthma in each hospital are shown in Fig. 3.
- Discussion
- Discussion
Using multicenter EHR record in Korea, this study found that hypercholesterolemia in children had a potential association with an increased risk of asthma development. It also summarized the reported associations between dyslipidemia and asthma in children in the last 20 years. Most of the previous studies were cross-sectional studies, and the results of the association between dyslipidemia and asthma in children were inconclusive.Cholesterol is an essential and major molecule in the body for the construction of the cell membrane and the synthesis of steroid hormones, bile acids, and fat-soluble vitamins. However, dyslipidemia, defined as abnormal plasma levels of TC, HDL-C, LDL-C, TG, or other lipoproteins, adversely affects human health. Elevated serum cholesterol levels enhance proinflammatory genes, cellular adhesion molecules, and proinflammatory cytokines [23]. The serum level of HDL-C had a negative correlation with CRP level, which is a biomarker of systemic inflammation [14]. Dyslipidemia could activate innate and acquired immunity, then amplify airway inflammation pathways. This consequently increased bronchial smooth muscle tone, airway inflammation, and hyperreactivity [24]. In asthmatic children, there was an association between dyslipidemia and airway resistance measured by forced oscillation technique [14]. Furthermore, it has been reported in an animal study that dyslipidemia was associated with a switch from Th1 to Th2 response [25]. Dyslipidemia increased the release of Th2 and Th17 cytokines including IL-1, IL-4, IL 6, and IL 17, and decreased the release of IL-10 [26].Obesity is a well-established risk factor for asthma in children, and dyslipidemia, which commonly co-occurs with obesity, has been suggested as a potential mechanism by which obesity increases the risk of asthma [24]. However, a retrospective study with children found that hypercholesterolemia and obesity each independently increased the likelihood of asthma. This suggests that dyslipidemia may have a direct influence on asthma risk, in addition to its association with obesity [27]. Moreover, dyslipidemia appears to be a factor that affects pulmonary function and sensitization, even in nonobese patients [28]. Unfortunately, due to limitations in the data available from the CDM database used in the study, information on the subjects' body weight or body mass index was not accessible. Therefore, caution is needed when interpreting our results, and further confirmation of the associations through well-designed prospective cohort studies will be necessary.This study recapitulated the reported associations between dyslipidemia and asthma in children in the last 20 years by reviewing previous studies. Compared with adults, studies on the association between dyslipidemia and asthma in children have been limited. In previous studies over the past 20 years, most of them were cross-sectional studies [13-20], which made it difficult to determine the causal relationship and showed only simple associations. Moreover, 2 longitudinal observational studies showed conflicting results [21,22]. In addition, all previous studies considered the onset of asthma as an outcome limited to children or adolescents [13-22, 27].The present large-scale study included 5,338,713 Korean patients to assess the associations between hypercholesterolemia in children and asthma using multicenter databases converted to the OMOP-CDM, which allowed PS matching with covariates including age, sex, and clinical conditions such as diagnosed diseases and prescribed medications. In addition, the OMOP-CDM database is useful for pediatric studies in which randomized controlled trials are practically limited. Our results could help guide further large-scale cohort studies aimed at revealing an association between dyslipidemia and asthma development.However, this study has several limitations as well. First, because this was an observational study, residual confounding factors may have affected the study results despite applying PS matching. As mentioned earlier, information on the subjects' anthropometric index, family history of allergies, lifestyle habits, and dietary habits was lacking in this study. Second, the definition of asthma was based on ICD-10 diagnostic and prescription codes. Third, it was not possible to distinguish between fasting and not fasting when measuring the cholesterol levels. However, except for TG, nonfasting lipid panel levels can be used to screen for dyslipidemia in children [29]. Furthermore, we were unable to demonstrate associations with HDL-C, LDL-C, and TG levels, except for TC. TC is often included in routine pediatric laboratory tests, whereas HDL-C, LDL-C, and TG levels are typically measured as additional tests in cases of obesity or other clinical conditions. As a result, the number of results available for HDL-C, LDL-C, and TG in the CDM database was small, and there was concern about selection bias, so we were unable to analyze them.In conclusion, elevated serum TC levels were associated with an increased risk of asthma in multicenter EHR databases using PS matching. Our results suggest that asthma should be considered a systemic disorder that shares certain characteristics with other chronic inflammatory disorders.
Supplementary materials
Supplementary materials
Supplementary Tables 1-6 can be found via https://doi.org/10.3345/cep.2023.00290.Supplementary Table 1.
cep-2023-00290-Supplementary-Table-1.pdfBaseline characteristics of KDH participantsSupplementary Table 2.
cep-2023-00290-Supplementary-Table-2.pdfBaseline characteristics of KHNMC participantsSupplementary Table 3.
cep-2023-00290-Supplementary-Table-3.pdfBaseline characteristics of KWMC participantsSupplementary Table 4.
cep-2023-00290-Supplementary-Table-4.pdfBaseline characteristics of GNUH participantsSupplementary Table 5.
cep-2023-00290-Supplementary-Table-5.pdfBaseline characteristics of participants with DCMC
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution Conceptualization: HSB, JHK, MYH; Data curation: JHK; Formal analysis: JHK; Funding acquisition: none; Methodology: HSB, JHK; Project administration: JEL, HMK; Visualization: JEL, HMK; Writing-original draft: JEL, HMK; Writing-review & editing: HSB, JHK, and MYH
-
Fig. 1.

Fig. 2.

Fig. 3.

Table 1.
Table 2.
| Study | Study design | No. and age of participants | Exposure | Outcome | Results |
|---|---|---|---|---|---|
| Chanachon et al., [14] 2022 | Cross-sectional study | 141 Asthmatic children aged 0–19 years | TC, LDL-C, and TG | IOS parameter (R5, R20, Fres, ALX) | Asthmatic children with high LDL-C had significantly higher expiratory phase R5, whole breath R20, and expiratory phase R20 than did children with normal LDL-C concentrations, irrespective of their obesity status. |
| Chanachon et al., [15] 2022 | Cross-sectional study | 150 Asthmatic children aged 5–18 years | TC, LDL, HDL-C, TG, non-HDL, TG/HDL-C, LDL-C/HDL-C | Spirometry parameter | TG/HDL-C ratio was associated with airway obstruction (% FEV1/FVC ratio <90) after adjusting with other blood lipids, body weight, BMI z score, and obesity status. |
| aOR, 2.78; 95% CI, 1.5–5.15, P=0.001. | |||||
| Lu et al., [13] 2019 | Cross-sectional study | 11,662 Children aged 3–11 years and 12,179 adolescents aged 12–19 years | Glucose, TC, HDL, LDL- C, TG, HOMA-IRa) | Current pre- sence of asthma | Elevated fasting plasma glucose, reduced HDL-C, elevated LDL-C, TC, TG, and HOMA-IR had no association with the presence of current asthma in children or adolescents. |
| Ko et al., [16] 2018 | Cross-sectional study | 123 Adolescents with asthma and 2,718 adolescents with- out asthma, aged 11–18 years | TC, LDL-C, HDL-C, TG | Asthma pre- valence | Asthma prevalence was greater in adolescents with a high TC level (aOR 1.69; 95% CI 1.01–2.82) and TG/HDL-C ratio (aOR 1.67; 95% CI 1.01–2.76). |
| Yiallouros et al., [20] 2014 | Case-control study | 68 Children with asthma, 123 children with current wheez- er only, and 660 control children for their ages 11–12 to 16–18 years. | Asthma, current wheez- er only | HDL-C | Adolescent asthma is associated with low serum HDL-C independent levels of previous HDL-C levels in childhood. |
| Chen et al., [19] 2013 | Cross-sectional study | 237 Adolescents with asthma and 225 control adolescents aged 10–15 years | Nonobese controls, obese controls, non- obese asthmatics, and obese asthma- tics | TC, LDL-C | TC and LDL-C levels increased progressively in the group of obese asthmatics >nonobese asthmatics >obese controls >nonobese controls. |
| There was an interactive effect of obesity and asthma on hyperlipidemia in boys (P for interaction=0.03). | |||||
| Rasmussen et al., [21] 2013 | longitudinal follow-up study | 272 Participants were tracked from 14 to 20 years of age. | BMI, TC, LDL-C, HDL-C, LDL-C/HDL-C ratio | AHR | After adjusting for sex, lung function, smoking and asthma, BMI at age 14 or 20 years had positive associations with increased AHR at age 20, while neither LDL-C, HDL-C, LDL-C /HDL-C ratio, nor total cholesterol were significantly associated with AHR. |
| Yiallouros et al., [22] 2012 | longitudinal follow-up study | 3,982 Adolescents were tracked from 11–12 years to 15–17 years. | TC, LDL-C, HDL-C, TG | Ever having asthma | Low HDL-C level (<40 mg/dL) in 11–12-year-olds was associated with an increased risk of asthma in 15-17-years-olds. |
| OR, 1.89; 95% CI, 1.19–3.00 for ever having asthma | |||||
| OR, 1.89; 95% CI, 1.02–3.53 for active asthma | |||||
| Cottrell et al., [17] 2011 | Cross-sectional study | 17,994 Children aged 4–12 years | TC, HDL-C, LDL-C, TG | Asthma | Regardless of BMI, children with asthma have higher TG levels than children without asthma |
| β=0.04, P=0.006 | |||||
| Fessler et al., [18] 2009 | Cross-sectional study | 7,005 Children aged 6 years or over | TC, HDL-C, non-HDL-C | Asthma/wheeze | TC and non-HDL-C levels had an inverse association with asthma. |
| OR, 0.92; 95% CI, 0.86–0.98, per 1–SD increased TC for current asthma | |||||
| OR, 0.91; 95% CI, 0.85–0.98, per 1–SD increased non-HDL-C for current asthma | |||||
| AI-Shawwa et al., [28] 2006 | Retrospective study | 188 Children and adolescents aged 4–20 years | TC | Asthma | TC levels had a positive association with asthma. |
| OR, 7.54; 95% CI, 1.13–50.7 | |||||
| Obese patients had a higher risk of asthma than nonobese patients. | |||||
| OR, 2.29; 95% CI, 1.13–4.63 | |||||
| Obesity and hypercholesterolemia increased the likelihood of asthma without interaction effects between both (P=0.6). |
TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; IOS, impulse oscillometry; R5, resistance at 5 Hz; R20, resistance at 20 Hz; ALX, reactance area; HDL-C, high-density lipoprotein cholesterol; FEV1, forced expiratory volume in 1 second; Fres, frequency response; FVC, forced vital capacity; BMI, body mass index; aOR, adjusted odds ratio; AHR, airway hyperresponsiveness; HOMA-IR, homeostatic model assessment-insulin resistance; CI, confidence interval; OR, odds ratio; SD, standard deviation.
Table 3.
Table 4.
TC, total cholesterol; HR, hazard ratio; CI, confidence interval; KDH, Kangdong Sacred Heart Hospital; KHNMC, Kyung Hee University Hospital at Gandong; KWMC, Kangwon National University Hospital; GNUH, Gyeongsang National University Hospital; DCMC, Deagu Catholic University Hospital; PY, patient-years.
- References
- 1. Kim EY. Clinical and diagnostic importance of dyslipidemia in children and adolescents during the coronavirus disease 2019 pandemic. Clin Exp Pediatr 2022;65:129–30.
[Article] [PubMed] [PMC]2. Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999-2012. JAMA Pediatr 2015;169:272–9.
[Article] [PubMed] [PMC]3. Rastogi D, Holguin F. Metabolic dysregulation, systemic inflammation, and pediatric obesity-related asthma. Ann Am Thorac Soc 2017;14(Supplement_5): S363–7.
[Article] [PubMed] [PMC]4. Su X, Ren Y, Li M, Zhao X, Kong L, Kang J. Association between lipid profile and the prevalence of asthma: a meta-analysis and systemic review. Curr Med Res Opin 2018;34:423–33.
[Article] [PubMed]5. Peng J, Huang Y. Meta-analysis of the association between asthma and serum levels of high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. Ann Allergy Asthma Immunol 2017;118:61–5.
[Article] [PubMed]6. Yoon D, Ahn EK, Park MY, Cho SY, Ryan P, Schuemie MJ, et al. Conversion and data quality assessment of electronic health record data at a Korean Tertiary Teaching Hospital to a Common Data Model for Distributed Network Research. Healthc Inform Res 2016;22:54–8.
[Article] [PubMed] [PMC]7. Rijnbeek PR. Converting to a common data model: what is lost in translation? : Commentary on "fidelity assessment of a clinical practice research datalink conversion to the OMOP common data model". Drug Saf 2014;37:893–6.
[Article] [PubMed]8. Lim JS, Kim EY, Kim JH, Yoo JH, Yi KH, Chae HW, et al. 2017 Clinical practice guidelines for dyslipidemia of Korean children and adolescents. Clin Exp Pediatr 2020;63:454–62.
[Article] [PubMed] [PMC]9. To T, Dell S, Dick PT, Cicutto L, Harris JK, MacLusky IB, et al. Case verification of children with asthma in Ontario. Pediatr Allergy Immunol 2006;17:69–76.
[Article] [PubMed]10. Yousif A, Dault R, Courteau M, Blais L, Cloutier AM, Lacasse A, et al. The validity of diagnostic algorithms to identify asthma patients in healthcare administrative databases: a systematic literature review. J Asthma 2022;59:152–68.
[Article] [PubMed]11. Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J 2009;16:183–8.
[Article] [PubMed] [PMC]12. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79.
[Article] [PubMed] [PMC]13. Lu M, Wu B, Qiao R, Gu H, Din Y, Dong X. No associations between serum lipid levels or HOMA-IR and asthma in children and adolescents: a NHANES analysis. J Clin Res Pediatr Endocrinol 2019;11:270–7.
[Article] [PubMed] [PMC]14. Chanachon PN, Jotikasthira W, Kiewngam P, Sawatchai A, Kanchongkittiphon W, Manuyakorn W. Association of dyslipidemia and respiratory resistance assessed by the forced oscillation technique in asthmatic children. Lung 2022;200:73–82.
[Article] [PubMed]15. Chanachon PN, Jotikasthira W, Kiewngam P, Sawatchai A, Kanchongkittiphon W, Manuyakorn W. TG/HDL-C ratio independent of obesity associates with airflow obstruction in children with asthma. Indian J Pediatr 2022;89:92.
[Article] [PubMed]16. Ko SH, Jeong J, Baeg MK, Han KD, Kim HS, Yoon JS, et al. Lipid profiles in adolescents with and without asthma: Korea National Health and nutrition examination survey data. Lipids Health Dis 2018;17:158.
[Article] [PubMed] [PMC]17. Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med 2011;183:441–8.
[Article] [PubMed]18. Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, et al. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J Allergy Clin Immunol 2009;124:967–74.e1-15.
[Article] [PubMed] [PMC]19. Chen YC, Tung KY, Tsai CH, Su MW, Wang PC, Chen CH, et al. Lipid profiles in children with and without asthma: interaction of asthma and obesity on hyperlipidemia. Diabetes Metab Syndr 2013;7:20–5.
[Article] [PubMed]20. Yiallouros PK, Savva SC, Kolokotroni O, Dima K, Zerva A, Kouis P, et al. Asthma: the role of low high-density-lipoprotein cholesterol in childhood and adolescence. Int Arch Allergy Immunol 2014;165:91–9.
[Article] [PubMed]21. Rasmussen F, Hancox RJ, Nair P, Hansen HS, Siersted HC, Nybo M. Associations between airway hyperresponsiveness, obesity and lipoproteins in a longitudinal cohort. Clin Respir J 2013;7:268–75.
[Article] [PubMed]22. Yiallouros PK, Savva SC, Kolokotroni O, Behbod B, Zeniou M, Economou M, et al. Low serum high-density lipoprotein cholesterol in childhood is associated with adolescent asthma. Clin Exp Allergy 2012;42:423–32.
[Article] [PubMed]23. Stokes KY, Cooper D, Tailor A, Granger DN. Hypercholesterolemia promotes inflammation and microvascular dysfunction: role of nitric oxide and superoxide. Free Radic Biol Med 2002;33:1026–36.
[PubMed]24. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol 2008;121:1087. –93. quiz 1094-5.
[Article] [PubMed]25. Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand J Immunol 2004;59:285–93.
[Article] [PubMed]26. Sheha DS, El-Korashi LA, AbdAllah AM, El-Begermy MM, Elmahdi AR. Dyslipidemia among allergic rhinitis patients: frequency and risk factors. World Allergy Organ J 2021;14:100523.
[Article] [PubMed] [PMC]27. Al-Shawwa B, Al-Huniti N, Titus G, Abu-Hasan M. Hypercholesterolemia is a potential risk factor for asthma. J Asthma 2006;43:231–3.
[Article] [PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors