All issues > Volume 66(8); 2023
Trends of vitamin D in asthma in the pediatric population for two decades: a systematic review
- Corresponding author: Myongsoon Sung, MD, PhD. Department of Pediatrics, Soonchunhyang University Gumi Hospital, 179 1(il)gongdan-ro, Gumi 39371, Korea Email: myong47@hanmail.net
- Received September 2, 2022 Revised December 28, 2022 Accepted January 17, 2023
- Abstract
-
Vitamin D exhibits anti-inflammatory properties through multiple mechanisms. Vitamin D deficiency is associated with increased inflammation, exacerbations, and overall worse outcomes in pediatric asthma and is observed in asthmatic children with obesity. In addition, given the increase in the prevalence of asthma over the last few decades, there has been enormous interest in vitamin D supplementation as a potential therapeutic option. However, recent studies have suggested no strong association between vitamin D levels or supplementation and childhood asthma. Recent studies have reported that obesity and vitamin D deficiency are associated with increased asthma symptoms. Thus, this review summarizes the findings of clinical trials regarding the role of vitamin D in pediatric asthma and analyzes the study trends of vitamin D over the past 2 decades.
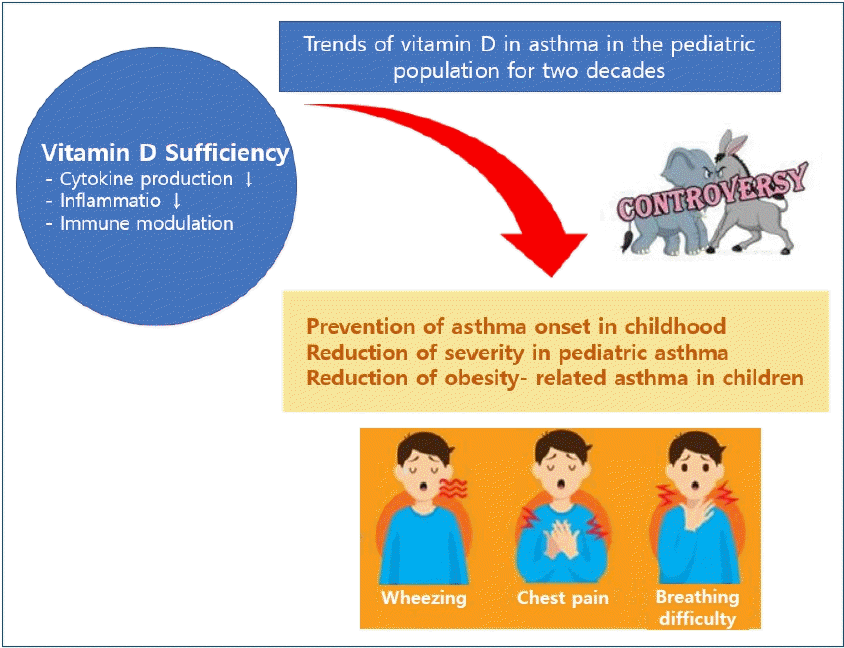
Graphical abstract
- Introduction
- Introduction
Asthma is a chronic disorder of the conducting airways characterized by reversible airway obstruction and airway inflammation [1]. Pediatric asthma is a significant concern because it increases the number of hospital visits and economic burden more than asthma in adulthood [1]. So far in the 2000s, asthma prevalence has increased globally over a short period due to the impact of environmental and genetic factors [2]. Previous epidemiological Korean studies reported a 10%–14% prevalence of asthma in children [3,4].Vitamin D is essential for calcium and bone metabolism and immunomodulation [5]. Vitamin D may affect asthma and allergy risk via multiple mechanisms. Vitamin D deficiency, one of the increasing causes of asthma, has become more severe globally over the past few decades [5,6]. Recent studies in adults and children have shown a higher prevalence of low vitamin D levels in asthmatics than in the general population [6,7]. Moreover, low vitamin D levels are associated with higher severity of asthma and impaired pulmonary function [6,7]. Asthmatic patients with vitamin D deficiency have shown increased airway hyperresponsiveness and corticosteroid requirements, and vitamin D might increase their response to glucocorticoids [8]. In a prospective study, maternal vitamin D intake during pregnancy was inversely associated with the risk of recurrent wheezing in childhood [9]. However, although many studies have been conducted in Korea and abroad of the relationship between asthma and vitamin D deficiency or insufficiency, the condition has increased worldwide, especially in adolescents and young adults [10,11].Recent contradictory results suggest no strong association between serum vitamin D levels or vitamin D supplements and childhood asthma. Thus, this review aimed to summarize published clinical research investigating the effects of vitamin D on the development and aggravation of asthma in children. Here, we critically review the findings of clinical trials regarding the role of vitamin D in pediatric asthma and analyzed the study trends of vitamin D over the past 2 decades.
- Study selection and characteristics
- Study selection and characteristics
PubMed was searched by combining the terms (asthma, pediatric asthma), (vitamin D, 25-hydroxyvitamin D [25(OH) D]), and duration (January 2002 to August 2022) to identify studies reporting research trends in vitamin D and asthma in children. The latest search was performed on August 15, 2022. The search revealed 133 results after the removal of duplicates and elimination of patients aged >19 years. The title and abstract screening excluded 92 records, while the full-text screening eliminated another 11. Finally, 31 articles were included in this systematic review. The included articles are summarized in Tables 1–3.
- Effects of vitamin D prevention on asthma onset in childhood
- Effects of vitamin D prevention on asthma onset in childhood
Several well-established studies have been conducted on this topic (Table 1). Some birth cohort studies demonstrated that serum vitamin D levels at birth and maternal vitamin D status or exposure during pregnancy may affect the incidence of childhood asthma. The association between vitamin D and asthma in children will be summarized from 2 main aspects: serum vitamin D levels before delivery; and cord blood and early infancy supplementation.
- Effects of vitamin D levels on asthma onset in childhood
- Effects of vitamin D levels on asthma onset in childhood
According to Hollams et al. [12], serum vitamin D levels were assayed in 989 (6-year-olds) and 1,380 (14-year-olds) children from an unselected community birth cohort; of them, 689 were assessed at both ages. Children (particularly males) with inadequate vitamin D levels were at increased risk of developing atopy, bronchial hyperresponsiveness, and asthma.Owing to the early onset of childhood asthma, we hypothesized that maternal vitamin D status and exposure during pregnancy might play a role in the development of asthma. The subjects’ 25(OH)D levels were measured at midgestation and at birth, and airway resistance was measured of the offspring at 6 years of age to clarify the association of maternal and fetal 25(OH)D levels with lung function and childhood asthma [13]. The maternal 25(OH)D levels were not associated with airway resistance in offspring at 6 years of age, but low levels of 25(OH)D at birth were associated with higher airway resistance in childhood [13].In a Taiwanese birth cohort study [14], children aged 0–4 years underwent serum 25(OH)D level measurements 6 times: maternal blood (before delivery), cord blood, and ages 1.5, 3, and 4 years. Specific immunoglobulin E antibodies against food and inhalant allergens were measured 6 times in children (at 6 months and at 1, 1.5, 2, 3, and 4 years). A significant correlation was found between maternal and cord blood 25(OH)D levels and persistently lower vitamin D serum levels in children born to mothers with vitamin D deficiency. Vitamin D deficiency in mothers (<20 ng/mL) appears to be associated with a higher prevalence of allergen sensitization before 2 years of age. Higher maternal 25(OH)D levels were significantly associated with a lower risk of asthma at age 4 years [14]. Additionally, in the Generation R Study [15], 4,951 mother-child pairs comprised a population-based prospective cohort and underwent blood sampling of maternal (in midgestation) and umbilical cord (at birth). After additional adjustment for the child’s 25(OH)D concentrations at 6 years of age, only the associations of 25(OH)D concentrations in midgestation with forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) and forced expiratory flow at 75% at 10 years of age remained.
- Vitamin D supplementation during pregnancy and infancy
- Vitamin D supplementation during pregnancy and infancy
Vitamin D intake by infants or mothers during pregnancy might play a role in the development of asthma in children (Table 1). In a prospective birth cohort study up to 6 years of age, prevention through modified vitamin D3 supplementation in infancy could reduce the prevalence of allergic diseases [16]. However, in the 31-year Northern Finland Birth Cohort, vitamin D supplementation in infancy did not appear to influence the development of asthma or wheezing in children [17].Three randomized controlled trial studies ascertained the role of maternal diet in the risk of asthma development in their offspring. In one trial [18], 180 pregnant women at 27 weeks’ gestation were allocated to receive no vitamin D, 800 IU of ergocalciferol daily until delivery, or a single oral bolus of 200,000 IU of cholecalciferol. Researchers blinded to the allocation assessed the offspring at 3 years of age using a questionnaire, impulse oscillometry, and exhaled nitric oxide. However, no significant difference was found in atopy, lung function, or exhaled nitric oxide between supplemented groups and controls [19]. In another trial [19], 881 mothers and infants received vitamin D (440 women administered 4,000 IU of vitamin D daily plus a prenatal vitamin containing 400 IU of vitamin D; 436 women administered placebo plus a prenatal vitamin containing 400 IU of vitamin D). The incidence of asthma and recurrent wheezing in children at 3 years of age was 6.1% lower; however, this result was insignificant. In the other trial [20], mothers and infants had lower or higher doses of vitamin D (400 IU/800 IU in infants and 1,000 IU/2,000 IU in mothers). Vitamin D supplementation during pregnancy and infancy reduces the proportion of children sensitized to house mites at 18 months of age, and there were study group differences in the proportion of children with primary care visits described by doctors as being for asthma [20].In summary, vitamin D supplementation during pregnancy and infancy may prevent asthma onset in childhood, but this remains controversial.
- Effects of vitamin D and asthma severity in pediatric asthma
- Effects of vitamin D and asthma severity in pediatric asthma
Over the past 2 decades, several research groups have focused on the role of vitamin D in asthma pathogenesis. Subsequent studies identified a link between vitamin D deficiency and an overall worse outcome of lung function, symptom control, and exacerbation in children with asthma (Table 2).
- Vitamin D levels and pediatric asthma
- Vitamin D levels and pediatric asthma
Brehm et al. [7] assayed serum vitamin D levels in 616 children with asthma; lower vitamin D levels were associated with increased asthma severity. In addition, vitamin D deficiency was higher in asthmatics than in controls, while vitamin D sufficiency was lower in asthmatics than in controls [21,22]. In asthmatic children, 25(OH)D was positively correlated with FEV1 and FEV1/FVC in the 2 studies [21,23], but it remained controversial whether asthmatics had a much lower 25(OH)D level than healthy controls [23]. In the age-, sex-, and ethnicity-matched case-control studies, 25(OH)D levels were significantly lower, while immunoglobulin E levels were significantly higher in cases than in controls with a negative correlation evident [24]. According to the National Health and Nutrition Examination Survey in the United States, from 2001 to 2010, there was a positive correlation between vitamin D insufficiency (<30 ng/mL) and current asthma or wheezing in children (Table 2) [25].However, in a study of nonobese asthmatic children currently not receiving anti-inflammatory treatment [26], although the mean vitamin D was 23 ng/mL, no correlation was found between vitamin D level, airway reactivity, and airway inflammation [26]. Furthermore, in a population-based cohort of adults, serum 25(OH)D level was positively associated with FEV1 and FVC and negatively associated with fraction of exhaled nitric oxide (FeNO); these associations disappeared after the adjustment for confounders, including body mass index (BMI) [27]. Thus, 25(OH)D levels were not associated with lung function or airway inflammation in nonobese adults [27].
- Vitamin D level and pediatric asthma exacerbation
- Vitamin D level and pediatric asthma exacerbation
Several studies have emphasized a link between vitamin D levels and asthma exacerbation in children [28-30]. In the analysis of 25(OH)D levels in 36 children with steroid-resistant asthma, 26 with moderate asthma, and 24 healthy controls, 25(OH)D levels were significantly lower in the steroid-resistant asthma group than the other 2 groups [28]. Low 25(OH)D levels were correlated with asthma exacerbation and medication usage [28]. In another study [29], no significant difference in 25(OH)D levels was observed between 287 asthmatic children and 273 controls, but a lower 25(OH)D level was correlated with severe asthma exacerbation. Additionally, an analysis of 25(OH)D levels in preschoolers with asthma versus healthy controls showed a significant decrease in serum vitamin D levels in the asthmatics. The total number of exacerbations during the previous year was much lower in the vitamin D-sufficient group than in the vitamin D-insufficient group. The number of children with controlled asthma was also higher in the sufficient group, suggesting a positive correlation between serum vitamin D level and asthma control [30].
- Effects of vitamin D supplementation on clinical outcomes in pediatric asthma
- Effects of vitamin D supplementation on clinical outcomes in pediatric asthma
Interestingly, few clinical trials have examined the effects of vitamin D supplementation on clinical outcomes in children with asthma. A double-blind randomized placebo-controlled trial examined whether a 100,000 IU dose of supplemental vitamin D could rapidly increase serum 25(OH)D levels in preschoolers with asthma. At 3 months, all children in the intervention group versus only approximately 50% of the control group had serum 25(OH)D levels >30 ng/mL [31].In a study of Japanese children with asthma, an assessment of the frequency and severity of asthmatic episodes was improved by the administration of vitamin D3 supplements (800 IU/day) for 2 months [32]. In a trial of Caucasian children who received 2,000 IU/day of vitamin D for 15 weeks, supplementation significantly decreased the number of missed school days. However, there were no other positive changes in asthma parameters compared to those in the placebo group [33].In a double-blind randomized placebo-controlled trial [34], children with mild asthma, a provocation concentration of methacholine required to induce a 20% decrease in FEV1<16 mg/mL, and a vitamin D level<30 ng/mL, the oral vitamin D group received oral vitamin D 14,000 once weekly. Despite significant increases in blood vitamin D levels, no difference was found in the effects of vitamin D and placebo.In another trial [35], children with moderate-to-severe asthma and a vitamin D level < 25 ng/mL were randomized to receive vitamin D as a loading dose or for 12 months. Alansari et al. [35] examined the effects of 300,000 or 600,000 IU of vitamin D2 injected as a loading dose followed by 400 IU of oral vitamin D3 daily versus oral therapy only in 231 children (aged 2–14 years) [35]. The loading dose versus maintenance vitamin D supplementation in children with the lowest levels resulted in short- but not long-term reductions in asthma exacerbations [35].Additionally, the World Allergy Organization reported in 2016 that, based on currently available evidence, it did not support the hypothesis that vitamin D supplementation reduces the risk of developing allergic diseases, including asthma, in children. The panel suggested not administering vitamin D to pregnant women, breastfeeding mothers, or healthy infants in an effort to prevent the development of allergic diseases [36].Two recent double-blind randomized placebo-controlled trials support this recommendation [37,38]. Among children with persistent asthma and low vitamin D levels, vitamin D3 supplementation versus placebo did not significantly improve the time to severe asthma exacerbation, and vitamin D supplementation as an adjunct to standard treatment did not improve asthma control in children [37,38]. However, a recent interventional study of 68 asthmatic children found that asthma severity, FEV1, FVC, and FEV1/FVC indicators were significantly increased after vitamin D supplementation [39].In summary, evidence from the highlighted studies demonstrated an association between vitamin D supplementation and clinical outcomes of pediatric asthma; however, reports from recent clinical trials are inconclusive. More prospective research is needed to clarify whether vitamin D supplementation relieves symptoms associated with asthma.
- Vitamin D and obesity-related asthma in children
- Vitamin D and obesity-related asthma in children
The prevalence of obesity in children has increased significantly since the coronavirus disease 2019 pandemic due to losses of daily routines.40) Adipose tissue may act as an endocrine organ releasing soluble factors, and excess adipose tissue predisposes an individual to an enhanced inflammatory state and may contribute to the pathogenesis and aggrevation of asthma [41]. Two recent retrospective cohort studiesanalyzed big data for 150,000 subjects [42,43]. One study was divided into 3 groups (aged 2–6 years, 7–11 years, and 12–17 years); before 12 years of age, females had a higher risk for obesity-related asthma; however, after 12 years of age, obese males had a higher asthma risk [42]. Overall, obesity was a major preventable risk factor for pediatric asthma in 2 studies. Therefore, obesity may substantially contribute to childhood asthma morbidity and healthcare costs.Recent exciting reports stated that obesity and vitamin D deficiency are associated with increased asthma symptoms [25] and that vitamin D supplementation could decrease asthma aggravation in children [44]. There are several well-established studies on this topic (Table 3). Epidemiological studies have reported low serum 25(OH)D levels in children with difficultto-treat asthma irrespective of body weight. According to a nationwide study using National Health and Nutrition Examination Survey data, children with asthma (n=1,192) were likelier to have a higher BMI z score and a lower serum vitamin D level [25]. Low serum vitamin D (25(OH)D) levels were reported in children with asthma or who were obese, making children who have both asthma and obesity particularly at risk for a low vitamin D level [28,45]. A population-based cohort study found that a 25(OH)D level > 10 nmol/L was associated with 0.46% predicted higher FEV, a 0.46% predicted higher FVC, and a 0.24 ppb lower FeNO level in obese adults with a BMI ≥ 30 kg/m2 [27]. Thus, higher 25(OH)D levels were associated with better lung function and lower airway inflammation in an obese subject [27]. Turer et al. [45] observed that 79% of children who were overweight (BMI 85th–95th percentile for age and sex) and 86% of children with obesity (BMI ≥95th percentile) met the criteria for vitamin D insufficiency (serum vitamin D measured as 25(OH)D <30 ng/mL).In a study examining vitamin D levels in 235 children with asthma, only 76 were considered obese, and the mean serum 25(OH)D level was 20.6 ng/mL (interquartile range, 13.5–26.0 ng/mL) [46]. Children with asthma and a low serum 25(OH)D level are predisposed to worse asthma outcomes. Lautenbacher et al. [47] reported that vitamin D deficiency was associated with pulmonary function decline among obese children of Hispanic and African-American descent but not their healthy-weight controls. In another study, Bose et al. [48] showed a relationship between indoor air quality, asthma, and vitamin D levels in obese children in an urban environment. Three-way interaction models demonstrated significantly greater fine particular matter-associated effects on daytime asthma symptoms only among obese children with low 25-OH D levels. They observed that higher serum 25(OH)D levels mitigated the effects of increased indoor air pollution [48].It has been hypothesized that vitamin D supplementation may benefit this patient population. A Cochrane review noted that the dose of vitamin D supplementation for asthma (and, therefore, obesity-related asthma) remains uncertain [49]. A study comparing vitamin D bioavailability in normal-weight versus obese adults attributed decreased vitamin D levels to the deposition of vitamin D in body fat compartments [50]. However, data on vitamin D pharmacokinetics in children with obesity are lacking. Thus, addressing this critical gap in our understanding of vitamin D pharmacokinetics is an essential first step in investigating the role of vitamin D as a treatment for pediatric obesity-related asthma.In summary, there is ample evidence that both asthma and obesity are inflammatory conditions associated with decreased serum 25(OH)D levels and that vitamin D supplementation may decrease the inflammatory properties of both diseases.
- Conclusion
- Conclusion
The incidence and socioeconomic burden of asthma has been increasing among children and adolescents in Korea and other countries. The role of vitamin D in asthma pathogenesis has been a topic of debate for the past 2 decades. This systematic review evaluated the relationship between asthma and vitamin D levels in children. Unfortunately, protocols (duration and dosage) varied among the birth cohorts and double-blind randomized placebo-controlled trials. These clinical trials are not without limitations. Thus, future research must identify the optimal dose and duration of vitamin D supplementation for distinct groups based on sex, ethnicity, age, BMI, and asthmatic phenotype, since all these factors affect vitamin D absorption and bioavailability. Additionally, it is important to determine whether these observations reflect long-term effects on immune regulation.
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Table 1.
Summary of effects of vitamin D supplementation on asthma onset in children by study included in the systematic review
| Study | Design | Subjects | Description | |
|---|---|---|---|---|
| Hollams et al. [12] 2011 | Large unselected cohort study | 989 (6-yr-olds)/1,380 (14-yr-olds) children: 689 subjects were assessed at both ages | Vitamin D levels at age 6 years were significant predictors of subsequent atopy/ asthma-associated phenotypes at age 14 years. | |
| Clinical and immunological phenotyping at ages 6 and 14 years | ||||
| Gazibara et al. [13] 2015 | Population-based prospective cohort study during 6 years | 3,130 Mothers/ their children | Low 25(OH)D levels at birth were associated with a higher airway resistance in childhood. | |
| Serum 25(OH)D levels in midgestation and at birth | ||||
| Chiu et al. [14] 2015 | Birth cohort of children aged from 0 to 4 years | A total of 164 mother-child pairs | Low maternal 25(OH)D levels appear not only to be associated with an increase in the prevalence of allergic sensitization but also the risk of asthma in early childhood. | |
| Serum 25(OH)D levels in maternal blood before delivery, cord blood, and at ages 1.5, 3, and 4 | ||||
| Mensink-Bout et al. [15] 2019 | A population-based prospective cohort | 4,951 Mothers/their children | The associations of 25(OH)D concentrations in midgestation with FEV1/FVC and FEF75% at age 10 years | |
| Maternal (midgestation)/umbilical cord (birth) blood | ||||
| At age 10 years, lung function, question- naire, and inhalant allergic sensitization by skin prick tests | ||||
| Hypponen et al. [17] 2004 | The Northern Finland Birth Cohort | Those who attended clinical examination (n=6,007) were compared with those who were not invited or were invited but did not attend (n=5,630). | Association between vitamin D supplemen- tation in infancy and an increased risk of atopy and allergic rhinitis later in life, but not asthma | |
| Vitamin D supplementation during the first year of life | ||||
| Women during pregnancy and their offspring are followed up at ages 1, 14, and 31 years. | ||||
| Bäck et al. [16] 2009 | A prospective birth cohort study up to the age of 6 years. | 123 (6-yr-olds) children | Prevention through modified vitamin D3 supplementation in infancy could reduce allergic diseases. | |
| The relationship between lower or higher vitamin D3 intake and atopic illness later in childhood was assessed. | ||||
| Goldring et al. [18] 2013 | A randomized controlled trial | 180 Pregnant women at 27 weeks gestation | Prenatal vitamin D supplementation in late pregnancy was not associated with de- creased wheezing in offspring at age 3 years. | |
| Assessed offspring at 3 years | - No vitamin D (n=60) | |||
| - Daily 800 IU ergocalciferol (n=60) | ||||
| - Single 200,000 IU bolus of cholecalciferol (n=60) | ||||
| Grant et al. [20] 2016 | A randomized, double-blind, placebo-con- trolled parallel-group trial | A total of 260 mother-child pairs | Vitamin D supplementation during pregnancy and infancy reduces the proportion of children sensitized to mites at age 18 months. | |
| Pregnant women (from 27-week gestation to birth) and their infants (from birth to 6 months) | - Placebo (n=87) | |||
| - Lower dose oral vitamin D (n=87) | There were study group differences in the proportion of children with primary care visits described by the doctor as being for asthma. | |||
| Woman/infant pairs: placebo/placebo, 1,000/400 IU, or 2,000/800 IU | - Higher dose oral vitamin D (n=86) | |||
| Litonjua et al. [19] 2016 | A randomized, double-blind, placebo-con- trolled trial: (1) parental report of physician-diagnosed asthma or recurrent wheezing through 3 years of age; (2) third trimester maternal 25(OH)D level | 881 Pregnant women (from 10 to 18 weeks' gestation) at high risk of having children with asthma | The incidence of asthma and recurrent wheezing in their children at age 3 years was lower by 6.1%, but this did not meet statistical significance. | |
| - Daily 4,000 IU vitamin D+prenatal vita- min containing 400 IU vitamin D (n=440) | ||||
| - Placebo+prenatal vitamin containing 400 IU vitamin D (n=436) | ||||
Table 2.
Summary of the effects of vitamin D on asthma severity in pediatric patients by study included in the systematic review
| Study | Design | Subjects | Description | |
|---|---|---|---|---|
| Brehm et al. [7] 2009 | Cross-sectional study | 616 Children with asthma (aged: 6–14 years) | Lower vitamin D levels are associated with increased markers of allergy and asthma severity. | |
| Hospitalization/use of anti-inflammatory medications in the previous year | ||||
| Alyasin et al. [21] 2011 | Case-control study and cross-sectional study | 50 Childhood asthmatics & 50 healthy controls (aged: 6–18 years) | 25(OH)D was significantly lower in asthmatic patients than controls and positively correlated with FEV1 and FEV1/FVC. | |
| Serum vitamin D level, pulmonary function test, and eosinophil counts were examined | No correlation with eosinophil counts, asthma duration, number of hospitalizations, or unscheduled | |||
| Chinellato et al. [23] 2011 | Case-control study and cross-sectional study | 45 Asthmatic & 59 healthy children (aged: 9–11 years) | No significant difference in the 25(OH)D level between the 2 groups. 25(OH)D positively correlated with FVC and FEV1 but negatively with exercise induced bronchoconstriction. | |
| Serum vitamin D level, pulmonary function test, and exercise challenge test were examined. | ||||
| Maalmi et al. [22] 2012 | Case-control and cross-sectional study | 39 Children with controlled asthma/30 controls (aged: 6–16 years): age- and sex- matched | Vitamin D deficiency was higher in asthma compared to control; vitamin D sufficiency was lower in asthma than control. | |
| Vitamin D, Th1, Th2, Th17, Treg, and pulmonary function test | Th1/Th2 ratio and CD25(þ) Foxp3(þ) Treg cells were positively related to 25(OH) D level while IL-17 was negatively correlated. | |||
| Ehlayel et al. [24] 2011 | Case-control study and cross-sectional study | 483 Asthma & 483 controls (aged: <15 years): age, gen- der & ethnicity matched | 25(OH)D levels were significantly lower, and IgE significantly higher in cases than in controls, with a negative correlation evident. | |
| Serum vitamin D level and IgE | Vitamin D deficiency was the strongest predictor of asthma. | |||
| Dabbah et al. [26] 2015 | Cross-sectional study | 71 Nonobese children with asthma (aged: 6–18 years) | No correlation was found between vitamin D level and response to the methacholine challenge test, FeNO, IgE levels, eosinophil counts. | |
| Methacholine challenge test/FeNO) | ||||
| Serum vitamin D, total IgE, blood eosinophil counts | ||||
| Han et al. [25] 2017 | Using data from the National Health and Nutrition Examination Survey from 2001 to 2010 | Current asthma or wheeze in 10,860 children (6–17 years)/24,115 adults (18–79 years) | Positive correlation between vitamin D insufficiency (<30 ng/ mL) and current asthma or current wheeze in children and adults | |
| Lung function in a subset of participants | Children with asthma (n=1,192) were likelier to have a higher BMI z score and a lower serum vitamin D level. | |||
| Gupta et al. [28] 2011 | Case-control and cross-sectional study | 36 Children with steroid-resis- tant asthma, 26 with mode- rate asthma, and 24 healthy controls (aged: 6–16 years) | 25(OH)D levels were significantly lower in steroid-resistant asthma than either mild asthmatics or controls and inversely correlated with airway smooth muscle mass, bronchodilator response and IgE but positively correlated with asthma control, FEV1 and FVC. | |
| 25(OH)D, asthma control test, spirometry, corticosteroid use, and exacerbations were assessed. | ||||
| Fiberoptic bronchoscopy, bronchoalveolar lavage, and endobronchial biopsy (severe, therapy-resistant asthma) | Low 25(OH)D was correlated with asthma exacerbation and medication usage | |||
| Brehm et al. [29] 2012 | Case-control and cross-sectional study | 287 Asthmatic/ 273 healthy children (aged: 6–14 years). | No significant difference in 25(OH)D between cases and controls | |
| Serum vitamin D level, pulmonary function test, and specific IgE | Lower 25(OH)D correlated with severe asthma exacerbation, atopy, and a lower FEV1/FVC in cases | |||
| Turkeli et al. [30] 2016 | Case-control and cross-sectional study | 102 Preschool children with asthma/102 healthy controls in winter (aged: 1–4 years) | The frequency of vitamin D deficiency and insufficiency was higher in children with asthma, compared to the controls. | |
| In the vitamin D-sufficient group, total number of exacerbations during the previous year was much lower compared to the vitamin D-insufficient group. | ||||
| Yoseph et al. [34] 2015 | Double-blind, randomized, placebo- controlled trial | Children with mild asthma, PC20-FEV1 | No difference could be demonstrated between the effect of vitamin D and placebo | |
| Methacholine challenge test, skin prick tests, FeNO, and exhaled breath con- densate collection | <16 mg/mL, and vitamin D <30 ng/mL for 6 weeks of treatment | |||
| - Oral vitamin D 14,000 in 2-mL units once weekly | ||||
| - Placebo (2 mL of olive oil) | ||||
| Jensen et al. [31] 2016 | Double-blind, randomized, placebo- controlled trial | 22 preschool-aged children with asthma | Following 100,000 IU vitamin D3, all children reached serum 25OHD ≥75 nmol/L (>30 ng/mL), compared with half who received placebo. | |
| Serum 25(OH)D: baseline, 10 days, 3/6 months | - 100,000 IU vitamin D3 (intervention)/placebo (control) | |||
| - Followed by 400 IU vitamin D3 daily for 6 months | ||||
| Tachimoto et al. [32] 2016 | Double-blind, randomized, placebo- controlled trial | Japanese schoolchildren with asthma | Low-dose, short-term vitamin D supplemen- tation in addition to standard treatment may improve levels of asthma control in schoolchildren. | |
| Childhood asthma control test scores at 2, 6 months. | - Vitamin D3 supplements (800 IU/day) with placebo for 2 months | |||
| Kerley et al. [33] 2016 | Double-blind, randomized, placebo- controlled trial | Caucasian 51 children from 44 urban (aged: 6–16 years) | Vitamin D3 supplementation led to a signifi- cant decreased school days missed. | |
| Assessments were completed at base- line and after 15 weeks of supplemen- tation. | - Vitamin D supplementation (2,000 IU/day) | There were nonsignificant, advantageous changes in the placebo group compared with the vitamin D3 group in subjective asthma control and lung function, particularly percentage of predicted FEV1. | ||
| Alansari et al. [35] 2017 | Randomized, controlled trial | Children with moderate-to-severe asthma and vitamin D levels<25 ng/mL for 12 months (aged: 2–14 years) | Rapid compared to maintenance vitamin D supplementation for children with the lowest levels resulted in short- but not long-term reduction in asthma exacer- bations. | |
| - IM+ Oral group (n=116): 400 IU/d + 300,000 IU (IM, <5 years) or 600,000 IU (IM+ oral, >5 years) | ||||
| - Oral-only group (n=115) : 400 IU/day | ||||
| Kalmarzi et al. [39] 2020 | Interventional study | 68 Asthmatic children | Therapeutic prescription of vitamin D is very effective in improving the clinical status of asthmatic children. | |
| Serum levels of 25(OH)D, asthma se- verity and pulmonary function tests before and after therapeutic prescrip- tion of vitamin D | - Vitamin D levels <10 ng/mL: 800-IU capsules (#4) a day for 12 weeks or 50,000-IU tablets (#1) a day for up to 6 days at secondary care level: cholecalciferol 300,000-unit ampoules once a month for 3 months | |||
| - Vitamin D levels: 10–30 ng/mL: Vitamin D was administered based on the patient's calcium level | ||||
| Forno et al. [37] 2020 | Double-blind, randomized, placebo-con- trolled trial | 400 with low-dose inhaled corticosteroids and serum 25(OH) D levels <0 ng/mL (aged: 6–16 years) | Among children with persistent asthma and low vitamin D levels, vitamin D3 supple- mentation, compared with placebo, did not significantly improve the time to a severe asthma exacerbation. | |
| Pulmonary function test | - Vitamin D3, 4,000 IU/d (n=96) | |||
| Asthma Control Test (≥12 years)/Child- hood ACT (<12 years) | - Placebo (n=96) for 48 weeks | The findings do not support the use of vitamin D3 supplementation to prevent severe asthma exacerbations in this group of patients. | ||
| Maintained with fluticasone propionate | ||||
| Thakur et al. [38] 2021 | Double-blind, randomized, placebo- controlled trial | 60 Children with moderate persistent asthma and placebo (n=30) (aged: 6–11 years) | Vitamin‐D supplementation as an adjunct to standard treatment does not improve asthma control in children. | |
| Childhood asthma control test scores at 12 weeks | ||||
| FEV1, FeNO, asthma exacerbations, use of systemic steroids, number of emer- gency visits, postintervention vitamin D levels, and adverse outcomes | - 2,000 IU per day of vitamin D | |||
25(OH)D, 25-hydroxyvitamin D; FEF75%, forced expiratory flow at 75%; FeNO, fraction of exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IgE, immunoglobulin E; IU, international unit; Treg, regulatory T cells; PC20, provocation concentration of methacholine required to induce a 20% decrease in FEV1.
Table 3.
Summary of vitamin D and obesity-related asthma in children by study included in the systematic review
| Study | Design | Subjects | Description | |
|---|---|---|---|---|
| Turer et al. [45] 2013 | Cross-sectional study (the 2003–2006 National Health and Nutrition Examination Survey) | 12,292 Children (aged: 6–18 years) | Compared with healthy-weight children, overweight, obese, and severely obese children had significantly greater adjusted odds of vitamin D deficiency. | |
| Age- and gender-specific BMI- percentile cut point | - 79% of children with overweight (BMI 85th–95th percentile for age and gender) | |||
| - 86% of children with obesity (BMI≥ 95th percentile) | Vitamin D deficiency is highly prevalent in overweight and obese children. | |||
| Lautenbacher et al. [47] 2016 | Cross-sectional study | 72 Obese and 71 normal-weight Hispanic and African-American children with asthma recruited at an urban children's hospital (aged: 7–11 years) | Vitamin D deficiency was associated with pulmonary function deficits among obese children, but not among normal- weight children with asthma, an association that was independent of Th1 and Th2 serum inflammatory measures. | |
| Pulmonary function test, serum vitamin D and cytokines | ||||
| Reinehr et al. [46] 2018 | NIKI cohort | 235 Children (60% boys, age 9.3±1.7 years) with obesity, ADHD, BA, and AD | Vitamin D concentrations were not lower in children with obesity, ADHD, BA, and AD compared to healthy children. | |
| Multicenter study between 2013 and 2016 | 3,352 children from a healthy population | Vitamin D levels were not linked to the severity of asthma measured as FEV1. | ||
| Bose et al. [48] 2019 | Longitudinal cohort study | 120 Children with physician-diagnosed asthma (aged: 5–12 years) | Among obese urban children with asthma, low vitamin 25(OH) D enhanced adverse respiratory effects associated with indoor PM2.5. | |
| Indoor PM2.5, serum 25(OH)D levels and asthma symptoms | 25-OH D was protective against asthma symptoms in high PM2.5 environments. | |||
- References
- 1. Lee-Sarwar KA, Bacharier LB, Litonjua AA. Strategies to alter the natural history of childhood asthma. Curr Opin Allergy Clin Immunol 2017;17:139–45.
[Article] [PubMed] [PMC]2. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Res Pract 2017;3:1.
[Article] [PubMed] [PMC]3. Jang Y, Shin A. Sex-based differences in asthma among preschool and school-aged children in Korea. PLoS One 2015;10:e0140057.
[Article] [PubMed] [PMC]4. Sol IS, Kim YH, Kim SY, Choi SH, Kim JD, Kim BO BO, et al. Prescription patterns and burden of pediatric asthma in Korea. Allergy Asthma Immunol Res 2019;11:280–90.
[Article] [PubMed] [PMC]5. Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007;120:1031–5.
[Article] [PubMed]6. Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, et al. High prevalence of vitamin D deficiency among innercity African American youth with asthma in Washington, DC. J Pediatr 2010;156:948–52.
[Article] [PubMed] [PMC]7. Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179:765–71.
[Article] [PubMed] [PMC]8. Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol 2010;125:995–1000.
[Article] [PubMed] [PMC]9. Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 2007;85:853–9.
[Article] [PubMed]10. Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 2010;121:297–300.
[Article] [PubMed]11. Andersen R, Mølgaard C, Skovgaard LT, Brot C, Cashman KD, Chabros E, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur J Clin Nutr 2005;59:533–41.
[Article] [PubMed]12. Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J 2011;38:1320–7.
[Article] [PubMed]13. Gazibara T, den Dekker HT, de Jongste JC, McGrath JJ, Eyles DW, Burne TH, et al. Associations of maternal and fetal 25-hydroxyvitamin D levels with childhood lung function and asthma: the Generation R Study. Clin Exp Allergy 2016;46:337–46.
[Article] [PubMed]14. Chiu CY, Huang SY, Peng YC, Tsai MH, Hua MC, Yao TC, et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol 2015;26:337–43.
[Article] [PubMed]15. Mensink-Bout SM, van Meel ER, de Jongste JC, Voortman T, Reiss IK, De Jong NW, et al. Maternal and neonatal 25-hydroxyvitamin D concentrations and school-age lung function, asthma and allergy. The Generation R Study. Clin Exp Allergy 2019;49:900–10.
[Article] [PubMed] [PMC]16. Bäck O, Blomquist HK, Hernell O, Stenberg B. Does vitamin D intake during infancy promote the development of atopic allergy? Acta Derm Venereol 2009;89:28–32.
[Article] [PubMed]17. Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci 2004;1037:84–95.
[Article] [PubMed]18. Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS One 2013;8:e66627.
[Article] [PubMed] [PMC]19. Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART clinical trial. JAMA 2016;315:362–70.
[Article] [PubMed] [PMC]20. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy 2016;71:1325–34.
[Article] [PubMed]21. Alyasin S, Momen T, Kashef S, Alipour A, Amin R. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res 2011;3:251–5.
[Article] [PubMed] [PMC]22. Maalmi H, Berraies A, Tangour E, Ammar J, Abid H, Hamzaoui K, et al. The impact of vitamin D deficiency on immune T cells in asthmatic children: a case-control study. J Asthma Allergy 2012;5:11–9.
[PubMed] [PMC]23. Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, et al. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J 2011;37:1366–70.
[Article] [PubMed]24. Ehlayel MS, Bener A, Sabbah A. Is high prevalence of vitamin D deficiency evidence for asthma and allergy risks? Eur Ann Allergy Clin Immunol 2011;43:81–8.
[PubMed]25. Han YY, Forno E, Celedón JC. Vitamin D insufficiency and asthma in a US study. J Allergy Clin Immunol Pract 2017;5:790–6.
[PubMed]26. Dabbah H, Yoseph RB, Livnat G, Hakim F, Bentur L. Bronchial reactivity, inflammatory and allergic parameters, and vitamin D levels in children with asthma. Respir Care 2015;60:1157–63.
[Article] [PubMed]27. Rafiq R, Thijs W, Prein R, de Jongh RT, Taube C, Hiemstra PS, et al. Associations of serum 25(OH)D concentrations with lung function, airway inflammation and common cold in the general population. Nutrients 2018;10:35.
[Article] [PubMed] [PMC]28. Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med 2011;184:1342–9.
[Article] [PubMed]29. Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012;186:140–6.
[Article] [PubMed] [PMC]30. Turkeli A, Ayaz O, Uncu A, Bas VN, Tufan AK, Yilmaz O, et al. Effects of vitamin D levels on asthma control and severity in preschool children. Eur Rev Med Pharmacol Sci 2016;20:26–36.
[PubMed]31. Jensen ME, Mailhot G, Alos N, Rousseau E, White JH, Khamessan A, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials 2016;17:353.
[Article] [PubMed] [PMC]32. Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double blind, placebo-controlled trial. Allergy 2016;71:1001–9.
[Article] [PubMed]33. Kerley CP, Hutchinson K, Cormican L, Faul J, Greally P, Coghlan D, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol 2016;27:404–12.
[Article] [PubMed]34. Yoseph RB, Livnat G, Schnapp Z, Hakim F, Dabbah H, Goldbart A, et al. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: maintenance vitamin D supplementation in deficient children with asthma to prevent exacerbations. Chest 2017;152:527–36.
[Article] [PubMed]35. Alansari K, Davidson BL, Yousef KI, Mohamed ANH, Alattar I. Rapid vs maintenance vitamin D supplementation in deficient children with asthma to prevent exacerbations. Chest 2017;152:527–36.
[Article] [PubMed]36. Yepes-Nunez JJ, Fiocchi A, Pawankar R, Cuello-Garcia CA, Zhang Y, Morgano GP, et al. World Allergy Organization-McMaster University guidelines for allergic disease prevention (GLAD-P): vitamin D. World Allergy Organ J 2016;9:17.
[Article] [PubMed] [PMC]37. Forno E, Bacharier LB, Phipatanakul W, Guilbert TW, Cabana MD, Ross K, et al. Effect of supplementation on severe asthma exacerbations in children with asthma and low veletereecetea randomized controlled trial (ViDASTA trial). Pediatr Pulmonol 2021;56:1427–33.
[PubMed]38. Thakur C, Kumar J, Kumar P, Goyal JP, Singh K, Gupta A. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: a randomized controlled trial (ViDASTA Trial). Pediatr Pulmonol 2021;56:1427–33.
[Article] [PubMed]39. Kalmarzi RN, Ahmadi S, Rahehagh R, Fathallahpour A, Khalafi B, Kashefi H, et al. The vitamin D supplementation on clinical outcomes of asthmatic children with vitamin D insufficiency. Endocr Metab Immune Disord Drug Target 2020;20:149–55.40. Hong YH. Pediatric obesity: life cycle approach of pediatrician and society. Clin Exp Pediatr 2022;65:29–30.
[Article] [PubMed]41. O’Sullivan BP, James L, Majure JM, Bickel S, Phan LT, Gonzalez MS, et al. Obesity-related asthma in children: a role for vitamin D. Pediatr Pulmonol 2021;56:354–61.
[Article] [PubMed]42. Lang JE, Bunnell HT, Lima JJ, Hossain MJ, Wysocki T, Bacharier L, et al. Effects of age, sex, race/ethnicity, and allergy status in obesity-related pediatric asthma. Pediatr Pulmonol 2019;54:1684–93.
[Article] [PubMed]43. Lucas JA, Marino M, Fankhauser K, Bailey SR, Ezekiel-Herrera D, Kaufmann J, et al. Oral corticosteroid use, obesity, and ethnicity in children with asthma. J Asthma 2020;57:1288–97.
[Article] [PubMed]44. Bates J. Physiological mechanisms of airway hyperresponsiveness in obese asthma. Am J Respir Cell Mol Biol 2016;24:618–23.
[Article]45. Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics 2013;131:152–61.
[Article] [PubMed]46. Reinehr T, Langrock C, Hamelmann E, Lücke T, Koerner-Rettberg C, Holtmann M, et al. 25-Hydroxvitamin D concentrations are not lower in children with bronchial asthma, atopic dermatitis, obesity, or attentiondeficient/hyperactivity disorder than in healthy children. Nutr Res 2018;52:39–47.
[Article] [PubMed]47. Lautenbacher LA, Jariwala SP, Markowitz ME, Rastogi D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr Pulmonol 2016;51:1276–83.
[Article] [PubMed] [PMC]48. Bose S, Diette GB, Woo H, Koehler Ki, Romero K, Rule AM, et al. Vitamin D status modifies the response to indoor particulate matter in obese urban children with asthma. J Allergy Clin Immunol Pract 2019;7:1815–22.
[Article] [PubMed] [PMC]

 About
About Browse articles
Browse articles For contributors
For contributors