All issues > Volume 67(5); 2024
Children’s health affected by parent’s behavioral characteristics: a review
- Corresponding author: Jongin Lee MD, PhD. Department of Occupational and Environmental Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul 06591, Korea Email: leejongin.md@gmail.com
- Received January 18, 2023 Revised April 19, 2023 Accepted July 19, 2023
- Abstract
-
Exposure of an individual to occupational and environmental risk factors for a certain disease affects them and their family. Children are highly vulnerable in this setting because they are family-dependent. This review discusses diseases that occur in children according to the occupational and behavioral characteristics of their parents. Toxic agents in the home environment can affect children’s health. Maternal exposure to substances during pregnancy may directly affect fetal outcomes. The Industrial Accident Compensation Insurance Act in Korea was amended in 2023 to compensate for children’s adverse health effects due to their parents’ occupational risks. The long working hours and smoking behaviors of parents and toxic materials in the home environment are highlighted. To control for the diverse factors affecting children’s health in medical research, this review introduces directed acyclic graphs. Pediatric, occupational, and environmental medicine must collaborate to prevent childhood diseases related to environmental factors.
- Introduction
- Introduction
Many diseases have distinct pathological mechanisms. Those that are closely related to occupations and environments are called occupational diseases. For example, pneumoconiosis is a typical disease prevalent in people working in mines, which is characterized by large amounts of dust. Inhaled dust is deposited in the terminal bronchioles and alveoli, resulting in the formation of nodules that contain dust-laden macrophages [1]. Heavy metal poisoning, such as lead poisoning, is another typical case that many people suffered from in the past, although it has become rare in recent years [2].Many countries, including South Korea, study occupational diseases in the fields of occupational and environmental medicine. In the United States, occupational and environmental medicine is subdivided into different specialties, which include injury care, clinical specialties, and management [3]. In South Korea, the field of occupational and environmental medicine mainly covers special medical examinations and workplace healthcare regarding risk factors. The problem of work-related diseases is perceived as an individual problem of workers themselves, and research into the impact of the work environment on nearby members are limited.Occasionally, exposure to risk factors for a certain disease affects both the person involved and his/her family. Children are particularly vulnerable in this setting because they are family-dependent. Second-hand smoking (SHS) is among the most well-known examples. Long-term exposure to parental smoking is a strong risk factor for lung cancer in offspring [4]. Similarly, the aforementioned occupational risk factors also affect members of the corresponding workers’ families. For example, evidence of asbestosis was observed on imaging studies of spouses and children of shipyard workers exposed to asbestos [5]. Finally, studies have discussed lead poisoning in the family members of workers exposed to lead [6].While diseases of adults are considered contributed to by their surrounding environment, the most important factor in diseases of children is the environment provided by their parents. The present review discusses diseases that occur in children according to their parents’ occupational and behavioral characteristics.
- Paraoccupational exposure to parents’ occupational hazards
- Paraoccupational exposure to parents’ occupational hazards
Carcinogenic agents, including chemical substances to which workers are exposed, may also affect their family members, albeit at lower concentrations. There are 2 main mechanisms by which the toxicity of occupational exposure in parents manifests in their children. The first mechanism is the transmission of contaminants by clothing or body hair that workers bring into the household, defined as “paraoccupational” or “take home” exposure. Exposure to asbestos, as mentioned earlier in this review, is a typical case. Several studies have examined paraoccupational asbestos exposure, and the majority have suggested a correlation with workers such as miners, shipyard workers, and insulators [7]. Asbestos handling was banned in South Korea in 2009 [8], and there has been little concern regarding asbestos exposure in children.Meanwhile, a variety of other hazardous substances are consistently used in workplace, and concerns for take home exposure of these substances persist. A recent pilot study conducted in Boston reported the risk of exposure to arsenic, chromium, copper, lead, manganese, nickel and tin in the families of construction laborers [9]. Exposure to multiple trace metals can affect children’s neurodevelopment. A recent study reported a decrease in full-scale/verbal intelligence quotient and attention-deficit/hyperactivity disorder– like behaviors in children after combined exposure to arsenic, cadmium, manganese, and lead [10].Previous studies mainly investigated preclinical symptoms, such as cognitive impairment, caused by paraoccupational exposure; however, considering the disease burden, preventing the occurrence of malignant tumors may be more important. In 1987, a study by the American Institute for Cancer Research investigated the association between the risk of childhood leukemia and parents’ occupational and home exposure [11]. In this case-control study, occupational exposure to chlorinated solvents, spray paints, dyes, or pigments showed significant odds ratios for childhood leukemia risk. A similar case-control study in Greece investigated the risk of childhood leukemia according to the level of exposure to occupational risk factors defined by the standard occupational classification of the parents’ jobs. This study reported an increased odds ratio of childhood leukemia according to exposure level, although it was not as statistically significant as in previous studies [12]. A recent case-control study conducted by the Childhood Leukemia International Consortium (CLIC) reported that acute lymphoblastic leukemia in children may be associated with the father’s exposure to crystalline silica, chromium, and diesel engine exhausts [13]. Regarding pesticide exposure, an association between lymphoma or solid tumors and maternal exposure has been suggested; however, the difference was not statistically significant [14].Most studies investigating malignant tumors focused on occupational risks without specifying the exposure substances. Because certain workers can be concurrently exposed to multiple chemicals at the workplace, it is difficult to assess disease risk using individual substances, and controlling for only one factor may be ineffective. Therefore, the International Agency for Research on Cancer classified certain occupations as carcinogenic in recent years, and occupational exposure as a painter has been classified as group 1, that is, definite carcinogen [15].
- Toxicity from maternal exposure
- Toxicity from maternal exposure
Maternal exposure to risk factors during pregnancy may affect fetal outcomes. Certain drugs are clearly defined as teratogenic, and occupational risk factors other than drugs can also directly influence fetal status. For example, increased lead levels in the maternal bone are independently associated with neurodevelopmental disorders in 24-month-old children [16]. In addition, exposure to polybrominated diphenyl [17], pesticides and phthalate [18] is reportedly related to delayed fetal growth. The occupations exposed to these substances include veterinarians, horticultural workers, hairdressers, and printing workers. Disinfectant use recently became frequent owing to the coronavirus disease 2019 (COVID-19) pandemic, and exposure to disinfectants in pregnant women has been suggested as a risk factor for allergic diseases such as asthma and eczema in their children [19]. The possible paraoccupational exposures discussed above are presented in Table 1.Epigenetics is another mechanism that can affect the fetus. External factors, such as DNA methylation, histone modification, and microRNAs, can alter genetic expression, thereby causing various diseases [20]. For example, maternal exposure to malnutrition, hypoxia, excess hormones, and endocrine disruptors can cause obesity in mothers and children through epigenetic mechanisms [21]. Although these mechanisms are currently well explained in animal models [22], the epidemiological evidence in humans seems insufficient. A cohort study of the effects of parental pesticide exposure on the epigenome of their children was recently conducted in Morocco [23].In addition to the aforementioned toxic materials, there have been concerns regarding the risk of adverse health outcomes from maternal toxic exposure. In many cases, workers are exposed to toxic materials. If a worker develops a disease from a toxin, he or she can generally be compensated according to laws regarding industrial accident compensation. Until now, industrial accident compensation insurance in South Korea covered only the workers themselves. Starting in 2023, through the revision of related laws, the compensation of the insurance was expanded to include the health deterioration of children whose mothers had been exposed to risk factors. The risk factors specified by the law are listed in Table 2. Although the revised compensation system is in effect, evidence of its implementation is lacking, and there are many differences in the detailed compensation plan. As research on the effects of parental exposure to occupational risk factors on children’s health accumulates, we expect more children to benefit from this system.
- Working hours
- Working hours
Among the studies on occupational and environmental medicine, research on working hours greatly influenced our society. Long working hours are associated with the risk of coronary heart disease and stroke [24], and this medical evidence plays a decisive role in limiting weekly working hours.Dual-income families have recently become predominant in South Korea, and parents’ working hours can affect their children’s behaviors and experiences [25]. While many studies have investigated children’s behavioral and psychosocial changes, some focused on changes in medically defined conditions, especially overweight and obesity. Many studies have identified an association between maternal working hours and overweight/obesity. Notably, the clinical correlation is more evident in studies conducted in South Korea [26,27]. A recent meta-analysis showed that the risk of obesity in children increases when their mothers engage in long-term labor [28]. An Australian study that followed 434 children reported a significant relationship between paternal nonstandard working hours and obesity prevalence in children [29], while both maternal and paternal working hours mattered in a German study [30]. This discrepancy can be explained by differences in primary caregivers across countries. Additionally, dyslipidemia was related to the parents’ long working hours [31]. In a corresponding study conducted in South Korea, the influence of maternal working hours was more pronounced.Prolonged parental working hours leads to insufficient time to care for their children, which may cause problems with their eating habits. Maternal employment is linked with reduced caregiving hours used to shop for food materials, cook, eat, and play with children [32]. Many previous studies indicated obesity as an outcome because it is relatively easy to measure; however, changes in children’s health behaviors in association with parental labor are expected to be more complex. Notably, the risk of smoking among adolescents increased as maternal working hours increased [33]. Income level may be an associated variable for children’s behavioral changes, which can affect biological changes [34]. From this perspective, there are concerns that health inequalities caused by long working hours may be transmitted across generations.Furthermore, parental working hours can affect children’s psychological health. In 2013, a study in Australia evaluated the effect of parental working hours on children’s psychological health using the Child Behavior Checklist (CBCL) [35]. Children whose parents spent more time working had a high externalizing behavioral index. In addition to the changes in behavioral disorders measured by the CBCL, other factors related to psychological health are expected to be affected by parental working hours. However, studies investigating these psychological factors are lacking, especially compared to those investigating the relationship with obesity. Further studies of this issue are needed in the near future. The main results presented in this section are summarized in Table 3.
- Smoking
- Smoking
Parental smoking is a typical behavioral factor that can significantly influence children. SHS is closely related to an increase in all-cause mortality in children under 5 years of age [36]. In addition to the toxicity of SHS, exposed children tend to start smoking earlier in their own lives [37].Childhood obesity or overweight is also related to parental smoking. The large cohort Nurses’ Health Study II (NHS II) showed that maternal smoking during pregnancy was associated in a dose-response manner with overweight and obesity in the daughter across adolescence and adulthood [38].Smoking is a common carcinogen, and there has been a marked increase in cancer of the lungs, mouth, head and neck, esophagus, stomach, and parts of the body physically affected by cigarette smoking [39,40]. The CLIC has published several comprehensive studies on the impact of parental smoking on leukemia in children. Additional analyses of smoking were also conducted in some studies that showed a relationship between parental exposure to occupational hazards and childhood acute lymphoblastic leukemia; however, the results were inconsistent. A meta-analysis conducted in 2016 showed a significant odds ratio for acute myeloid leukemia (AML) among Hispanic children whose mothers had a history of smoking during pregnancy, but the difference from other ethnic groups was insignificant. Maternal smoking before and after pregnancy was not associated with childhood AML in all ethnicities [41].Many studies have attempted to identify a link between parental smoking and cancers other than leukemia. In 2003, Pang et al. [42] conducted a large case-control study and reported an association between childhood hepatoblastoma risk and parental smoking, although the risk was not generally significant. In contrast, a Jordanian case-control study reported a statistically significant relationship between parental smoking and childhood cancer [43]. Despite the inconsistencies in these studies, encouraging parents to quit smoking is important because the harmful effects of SHS on children are clearly explained.
- Home exposure to toxic agents
- Home exposure to toxic agents
Most products used at home are purchased by parents, and children are directly exposed to toxic products. The humidifier disinfectant case in South Korea is a typical example. Before this humidifier disinfectant issue emerged, pediatricians collected data on the prevalence of unexplained interstitial lung disease in winter and spring [44]. In 2011, the cause of this interstitial lung disease was humidifier disinfectants, and the disease was newly termed humidifier disinfectant-associated lung injury (HDLI). The clinical presentation of HDLI differs from that of other interstitial lung diseases. Many children die of the disease, and survivors live with serious sequelae [45]. In addition to HDLI, humidifier disinfectant exposure has a clear epidemiological correlation with asthma and other forms of interstitial lung disease, and its correlation with systems other than the respiratory system has been suggested in animal models [46].In addition to humidifier disinfectants, many other chemical products are used at home, some of which cause adverse health effects in children. The use of disinfectants has recently increased, primarily due to the COVID-19 pandemic, and the authors have already introduced a study of childhood allergic diseases related to maternal exposure. In addition, children’s direct exposure to COVID-19 disinfectants may increase the prevalence and acute exacerbation of asthma, which requires special attention [47].
- Methodology for observational studies
- Methodology for observational studies
As discussed in this review, parental factors, such as exposure to occupational risk factors, working hours, and smoking, are related to children’s health, and these factors have an intermediate mediating effect. For example, people with long working hours may have a high smoking rate [48], and smoking habits vary considerably depending on their occupation [49]. Long working hours affect not only smoking habits but also a wide range of health behaviors such as drinking alcohol, exercise, and sleep [50].The previously cited NHS II cohort study reported that obesity and overweight in adolescents were associated with both maternal and paternal smoking. Based on these results, the first problem that must be considered is the association between paternal and maternal smoking. This relationship is illustrated in Fig. 1A. The results should be adjusted for maternal smoking (confounder) to determine the relationship between paternal smoking and obesity in children. The results of the corresponding study were reported after the adjustment for various confounders, including maternal smoking.The mechanism by which maternal smoking affects fetal health can be explained by placental transmission. Hundreds of toxic substances in cigarettes are transmitted to the fetus through the placenta and can cause metabolic disorders. Conversely, paternal smoking may be related to working hours, which may contribute to obesity in children (Fig. 1B). It is intuitive to present these relationships in pictures rather than words, and many epidemiological studies have drawn directed acyclic graphs (DAG)to estimate causal relationships in observational studies of various factors.DAG is also recommended in the field of pediatric research, and some review studies provided sufficient guidance in this regard. Williamson et al. [51] published a review that provided a detailed analysis of a causal diagram for the research question, “Does personal smoking affect the risk of subsequent asthma?” While smoking and asthma are considered directly related, personal factors such as childhood asthma, underlying atopic dermatitis, chronic bronchitis, and decreased pulmonary function as well as other factors such as parental smoking, sex, and socioeconomic status can affect the results. Parental asthma may affect their smoking habits. Should the results be adjusted for all of these variables? Adjusting all variables can cause overcorrection, which may distort the results. When variables with which both explanatory variables and outcome variables have a causal relationship are adjusted, “colliders” may occur and distortion of the results may be exacerbated.When considering the impact of parental factors on children, a more complex relationship is involved because there are generally 2 parents. A review by Williams et al. [52] applying DAG in pediatric research provided insight utilizing DAG in various research topics including “the association between screen time and obesity in children.” Although DAG is based on researchers’ thesis and is limited by its lack of considering bias degree [53], it provides an adequate understanding of studies that require the consideration of multiple confounders.Because of the features of DAG, researchers are often confused when distinguishing structural equation modeling (SEM) from path analysis. SEM is a multivariate analysis technique used to ascertain causality among variables [54]. SEM models are composed of latent variables that are conceptually derived from observed variables. The models were examined using a multiple regression analysis, a latent variable and its observed variables, observation errors, other latent variables, and its latent errors. The strength of the associations and statistical significance were considered in the SEM models; however, they were not confirmed in the DAG model. Because it involves a qualitative assessment by experts, consensus cannot be reached. Performing validating multiple models and sensitivity analyses may overcome this limitation.Returning to Fig. 1, the confounders assumed in (B) and (C) are the same. However, depending on how the relationship between confounders was established, the inclusion of confounders in the statistical analysis model was determined. Although the detailed methodology was not explained in this review, it was favorable to include all confounders in case (B) and only paternal long work hours in case (C). In summary, the use of DAG to control for appropriate confounders and prevent under- or over-adjustment in pediatric observation studies is recommended.
- Conclusion
- Conclusion
Here we reviewed the effects of occupational and behavioral characteristics of parents on their children regarding exposure to occupational risk factors, fetal outcomes due to maternal exposure, long working hours, smoking, and the use of toxic substances at home. Occupational risk factors and long working hours are related to occupational diseases and can have negative impacts on the health of workers and their families, especially their children.We also introduced the DAG as a research methodology and emphasized that various factors related to children’s health should be properly investigated and controlled. In the studies discussed here, the effect of exposure to occupational risk factors was transmissible to children and preclinical symptoms were possible. However, the risk of diseases such as leukemia was mainly managed according to occupation type, and the detailed mechanism of carcinogen transmission remains obscure. Several studies have investigated the association between parents’ long working hours and childhood obesity, but evidence regarding the impact of parents’ long working hours on psychological or other health issues in children is still lacking.As observed in the case of humidifier disinfectants, despite the widespread use of HDLI in the clinical field, systematic investigations began 17 years after the first humidifier disinfectant was released. There is criticism that the investigation was late; however, the number of victims affected by external environmental factors would have increased even more if this investigation had not been conducted. There may be childhood diseases related to environmental factors, and preventive measures (e.g., mass recall of humidifier disinfectants) after a systemic investigation of these diseases can prevent many casualties. To successfully accomplish this goal, pediatrics and occupational and environmental medicine must collaborate.
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Fig. 1.
Sample-directed acyclic graphs used to estimate causal relationship between paternal smoking and obesity. (A) A single confounder: maternal smoking should be adjusted. (B) Multiple confounders: all the variables including maternal long work, maternal smoking, paternal long work should be adjusted. (C) Multiple confounders with mediating effects: only paternal long work should be adjusted.
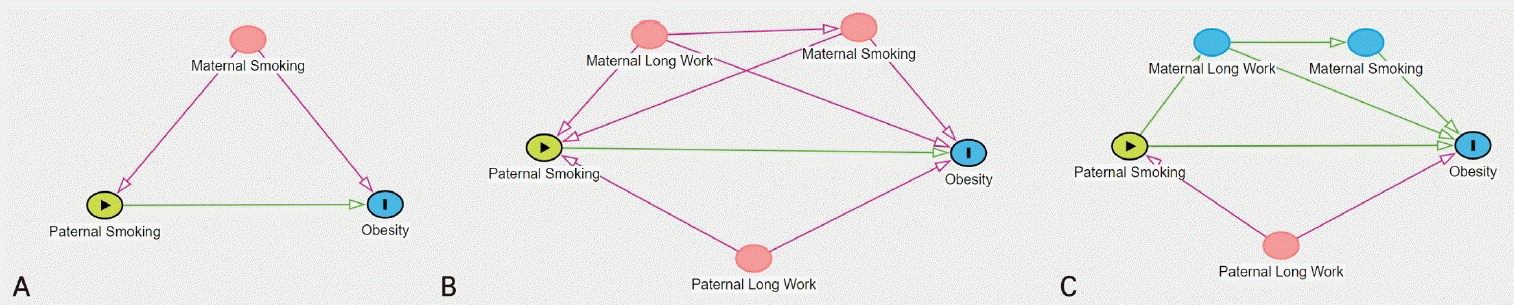
Table 1.
Possible paraoccupational exposure agents and the children’s possible medical conditions
| Hazards | Source of related jobs | Possible conditions of children | Reference |
|---|---|---|---|
| Asbestos | Miners, shipyard workers, insulators | Asbestosis | Kilburn et al. [5] |
| Lung cancer | Donovan et al. [7] | ||
| Heavy metals | Construction workers | Metal poisoning | Ceballos et al. [9] |
| Neurodevelopment problems | Stein et al. [10] | ||
| Solvents, paint, dyes, pigments | Hairdressers, painters | Leukemia | Lowengart et al. [11] |
| Kyriakopoulou et al. [12] | |||
| Crystalline silica, chromium, diesel engine exhausts | Tunnel workers, Miners | Leukemia | Onyije et al. [13] |
| Pesticides | Agricultural workers | Lymphoma, solid tumors | Rossides et al. [14] |
| Lead, polybrominated diphenyl ether, phthalates | Environmental exposure to mother, veterinarians, horticultural workers, hairdressers, printing workers | Mental retardation | Gomaa et al. [16] |
| Fetal growth restriction | Jin et al. [17] | ||
| Shirangi et al. [18] |
Table 2.
Diseases in workers’ children from occupational hazards compensated for by the new Industrial Accident Compensation Insurance Act
Table 3.
Children’s health affected by parental working hours
| The most related condition | Main results | Vulnerable groups | Reference |
|---|---|---|---|
| Mothers' long working hours | Overweight | Boys aged 13–18 with mothers working under 40 hr per week | Lee et al. [26] |
| Obesity | Girls aged 6–12 with mothers working 49–60 hr per week | ||
| Girls aged 13–18 with mothers working more than 60 hr per week | |||
| Mothers' long working hours | Body mass index | Children with mothers working 69 hr per week or more | Kim et al. [27] |
| Mothers' nutrient condition | Waist circumference | Mothers with overweight or obesity | |
| Mothers with frequently consuming energy-dense, nutrient-poor foods | |||
| Mothers' long working hours | Overweight | Meta-analysis from 22 observational studies | Lou et al. [28] |
| Obesity | Mothers working more than 35–40 hr per week | ||
| Fathers' nonstandard work schedule | Overweight | Children with paternal nonstandard work schedules | Champion et al. [29] |
| Obesity | |||
| Mothers' long working hours | Overweight | Preschool children with mothers working more than 35 hr per week | Li et al. [30] |
| Fathers' long working hours with mothers' part-time job | Obesity | Fathers working more than 55 hr with maternal part-time working | |
| Mothers' long working hours | Dyslipidemia | Children with mothers working 52 hr per week or more | Ahn et al. [31] |
| The association was greater in elementary school children | |||
| Mothers' long working hours | Smoking | Boy adolescents with mothers working more than 52 hr per week | Park et al. [33] |
| Fathers' long working hours | Child Behavior Checklist | Children with fathers working more than 55 hr per week | Johnson et al. [35] |
| Externalizing behavior |
- References
- 1. Fujimura N. Pathology and pathophysiology of pneumoconiosis. Curr Opin Pulm Med 2000;6:140–4.
[Article] [PubMed]2. Riva MA, Lafranconi A, D'Orso MI, Cesana G. Lead poisoning: historical aspects of a paradigmatic "occupational and environmental disease". Saf Health Work 2012;3:11–6.
[Article] [PubMed] [PMC]3. Harber P, Rose S, Bontemps J, Saechao K, Liu Y, Elashoff D, et al. Occupational medicine practice: one specialty or three? J Occup Environ Med 2010;52:672–9.
[Article] [PubMed]4. Sandler DP, Everson RB, Wilcox AJ, Browder JP. Cancer risk in adulthood from early life exposure to parents' smoking. Am J Public Health 1985;75:487–92.
[Article] [PubMed] [PMC]5. Kilburn KH, Lilis R, Anderson HA, Boylen CT, Einstein HE, Johnson SJ, et al. Asbestos disease in family contacts of shipyard workers. Am J Public Health 1985;75:615–7.
[Article] [PubMed] [PMC]6. Hipkins KL, Materna BL, Payne SF, Kirsch LC. Family lead poisoning associated with occupational exposure. Clin Pediatr (Phila) 2004;43:845–9.
[Article] [PubMed]7. Donovan EP, Donovan BL, McKinley MA, Cowan DM, Paustenbach DJ. Evaluation of take home (para-occupational) exposure to asbestos and disease: a review of the literature. Crit Rev Toxicol 2012;42:703–31.
[Article] [PubMed]8. Lee ES, Kim YK. Asbestos exposure level and the carcinogenic risk due to corrugated asbestos-cement slate roofs in Korea. Int J Environ Res Public Health 2021;18:6925.
[Article] [PubMed] [PMC]9. Ceballos DM, Dong Z, Peters JL, Herrick RF, Gupta P, Spengler JD. Metals dust in workers' homes and potential for take home in the Greater Boston area: pilot study. Environ Res 2022;209:112893.
[Article] [PubMed]10. Stein CR, Wu H, Bellinger DC, Smith DR, Wolff MS, Savitz DA. Exposure to metal mixtures and neuropsychological functioning in middle childhood. Neurotoxicology 2022;93:84–91.
[Article] [PubMed] [PMC]11. Lowengart RA, Peters JM, Cicioni C, Buckley J, Bernstein L, Preston-Martin S, et al. Childhood leukemia and parents' occupational and home exposures. J Natl Cancer Inst 1987;79:39–46.
[PubMed]12. Kyriakopoulou A, Meimeti E, Moisoglou I, Psarrou A, Provatopoulou X, Dounias G. Parental occupational exposures and risk of childhood acute leukemia. Mater Sociomed 2018;30:209–14.
[Article] [PubMed] [PMC]13. Onyije FM, Olsson A, Erdmann F, Magnani C, Petridou E, Clavel J, et al. Parental occupational exposure to combustion products, metals, silica and asbestos and risk of childhood leukaemia: Findings from the Childhood Cancer and Leukaemia International Consortium (CLIC). Environ Int 2022;167:107409.
[Article] [PubMed] [PMC]14. Rossides M, Kampitsi CE, Talbäck M, Mogensen H, Wiebert P, Tettamanti G, et al. Occupational exposure to pesticides in mothers and fathers and risk of cancer in the offspring: a register-based case-control study from Sweden (1960-2015). Environ Res 2022;214(Pt 1): 113820.
[Article] [PubMed]15. Myong JP, Cho Y, Choi M, Kim HR. Overview of occupational cancer in painters in Korea. Ann Occup Environ Med 2018;30:10.
[Article] [PubMed] [PMC]16. Gomaa A, Hu H, Bellinger D, Schwartz J, Tsaih SW, GonzalezCossio T, et al. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics 2002;110(1 Pt 1): 110–8.
[Article] [PubMed]17. Jin Y, Li J, Deng X, Xia B, Song Q, Zhao Y, et al. Association between fetal growth restriction and maternal exposure to polybrominated diphenyl ethers. Ecotoxicol Environ Saf 2020;198:110623.
[Article] [PubMed]18. Shirangi A, Wright J, Blair EM, McEachan RR, Nieuwenhuijsen MJ. Occupational chemical exposures in pregnancy and fetal growth: evidence from the Born in Bradford Study. Scand J Work Environ Health 2020;46:417–28.
[Article] [PubMed] [PMC]19. Kojima R, Shinohara R, Kushima M, Horiuchi S, Otawa S, Yokomichi H, et al. Prenatal occupational disinfectant exposure and childhood allergies: the Japan Environment and Children's study. Occup Environ Med 2022;79:521–6.
[Article] [PubMed]20. Zhang L, Lu Q, Chang C. Epigenetics in health and disease. Adv Exp Med Biol 2020;1253:3–55.
[Article] [PubMed]21. Panera N, Mandato C, Crudele A, Bertrando S, Vajro P, Alisi A. Genetics, epigenetics and transgenerational transmission of obesity in children. Front Endocrinol (Lausanne) 2022;13:1006008.
[Article] [PubMed] [PMC]22. Terrazas-Salgado L, García-Gasca A, Betancourt-Lozano M, Llera-Herrera R, Alvarado-Cruz I, Yáñez-Rivera B. Epigenetic transgenerational modifications induced by xenobiotic exposure in zebrafish. Front Cell Dev Biol 2022;10:832982.
[Article] [PubMed] [PMC]23. Menouni A, Duca RC, Berni I, Khouchoua M, Ghosh M, El Ghazi B, et al. The parental pesticide and offspring's epigenome study: towards an integrated use of human biomonitoring of exposure and effect biomarkers. Toxics 2021;9:332.
[Article] [PubMed] [PMC]24. Kivimäki M, Jokela M, Nyberg ST, Singh-Manoux A, Fransson EI, Alfredsson L, et al. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet 2015;386:1739–46.
[Article] [PubMed]25. Lewis J, Noden P, Sarre S. Parents’ working hours: adolescent children's views and experiences. Child Soc 2008;22:429–39.
[Article]26. Lee G, Kim HR. Mothers' working hours and children's obesity: data from the Korean National Health and Nutrition Examination Survey, 2008-2010. Ann Occup Environ Med 2013;25:28.
[Article] [PubMed] [PMC]27. Kim J, Park EC, Choi Y, Park S. Are mothers' working hours associated with general and abdominal obesity in children and adolescents? The Korean National Health and Nutrition Examination Survey (2008-2012). Matern Child Health J 2018;22:474–84.
[Article] [PubMed]28. Lou Y, Zhu Y, You Q, Jiang Q, Meng X, Di H, et al. Maternal long working hours and offspring's weight-related outcomes: A systematic review and meta-analysis. Obes Rev 2022;23:e13439.
[Article] [PubMed]29. Champion SL, Rumbold AR, Steele EJ, Giles LC, Davies MJ, Moore VM. Parental work schedules and child overweight and obesity. Int J Obes (Lond) 2012;36:573–80.
[Article] [PubMed]30. Li J, Kaiser T, Pollmann-Schult M, Strazdins L. Long work hours of mothers and fathers are linked to increased risk for overweight and obesity among preschool children: longitudinal evidence from Germany. J Epidemiol Community Health 2019;73:723–9.
[Article] [PubMed]31. Ahn J, Lee DW, Kang MY, Myong JP, Chung MH, Kim HR, et al. The association between long working hours of parents and dyslipidemia in their children. Front Public Health 2022;10:894609.
[Article] [PubMed] [PMC]32. Cawley J, Liu F. Maternal employment and childhood obesity: a search for mechanisms in time use data. Econ Hum Biol 2012;10:352–64.
[Article] [PubMed]33. Park TH, Ahn YD, Rhie JB. Maternal working hours and smoking and drinking in adolescent children: based on the Korean National Health and Nutrition Examination Survey VI and VII. Ann Occup Environ Med 2021;33:e25.
[Article] [PubMed] [PMC]34. Heymann SJ, Earle A. The impact of parental working conditions on school-age children: the case of evening work. Community Work Fam 2001;4:305–25.
[Article]35. Johnson S, Li J, Kendall G, Strazdins L, Jacoby P. Mothers' and fathers' work hours, child gender, and behavior in middle childhood. J Marriage Fam 2013;75:56–74.
[Article]36. Wu H, Sun J, Xi B. Parental tobacco and indoor secondhand smoking exposure and the risk of offspring under-five mortality in low- and middle-income countries. Indoor Air 2021;31:2188–99.
[Article] [PubMed]37. Wang MP, Ho SY, Lam TH. Parental smoking, exposure to secondhand smoke at home, and smoking initiation among young children. Nicotine Tob Res 2011;13:827–32.
[Article] [PubMed]38. Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes (Lond) 2013;37:1356–63.
[Article] [PubMed] [PMC]39. Jethwa AR, Khariwala SS. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev 2017;36:411–23.
[Article] [PubMed] [PMC]40. Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol 2010;15:135–44.
[Article] [PubMed]41. Metayer C, Petridou E, Aranguré JM, Roman E, Schüz J, Magnani C, et al. Parental tobacco smoking and acute myeloid leukemia: the Childhood Leukemia International Consortium. Am J Epidemiol 2016;184:261–73.
[Article] [PubMed] [PMC]42. Pang D, McNally R, Birch JM. Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer 2003;88:373–81.
[Article] [PubMed] [PMC]43. Alyahya MS, Al-Sheyab NA, Amro B. Parental smoking behavior and childhood cancer: a case-control study. Am J Health Behav 2020;44:572–90.
[Article] [PubMed]44. Cheon CK, Jin HS, Kang EK, Kim HB, Kim BJ, Yu J, et al. Epidemic acute interstitial pneumonia in children occurred during the early 2006s. Clin Exp Pediatr 2008;51:383–90.
[Article]45. Kim HJ, Lee MS, Hong SB, Huh JW, Do KH, Jang SJ, et al. A cluster of lung injury cases associated with home humidifier use: an epidemiological investigation. Thorax 2014;69:703–8.
[PubMed]46. Song JH, Ahn J, Park MY, Park J, Lee YM, Myong JP, et al. Health effects associated with humidifier disinfectant use: a systematic review for exploration. J Korean Med Sci 2022;37:e257.
[Article] [PubMed] [PMC]47. Hicks A, Hicks P. Disinfection in the time of COVID: Safe solutions are critical for schools. Paediatr Child Health 2022;27:324–6.
[Article] [PubMed] [PMC]48. Cho YS, Kim HR, Myong JP, Kim HW. Association between work conditions and smoking in South Korea. Saf Health Work 2013;4:197–200.
[Article] [PubMed] [PMC]49. Kim S, Kim J. The associations between smoking and occupational categories: the Korea National Health and Nutrition Examination Survey from 2008 to 2010. Asia Pac J Public Health 2015;27:NP1752–64.
[Article] [PubMed]50. Lee DW, Jang TW, Kim HR, Kang MY. The relationship between working hours and lifestyle behaviors: evidence from a population-based panel study in Korea. J Occup Health 2021;63:e12280.
[Article] [PubMed] [PMC]51. Williamson EJ, Aitken Z, Lawrie J, Dharmage SC, Burgess JA, Forbes AB. Introduction to causal diagrams for confounder selection. Respirology 2014;19:303–11.
[Article] [PubMed]52. Williams TC, Bach CC, Matthiesen NB, Henriksen TB, Gagliardi L. Directed acyclic graphs: a tool for causal studies in paediatrics. Pediatr Res 2018;84:487–93.
[Article] [PubMed] [PMC]

 About
About Browse articles
Browse articles For contributors
For contributors