All issues > Volume 67(8); 2024
Recent advances in food allergen immunotherapy
- Corresponding author: Edwin H. Kim, MD. Department of Pediatrics, University of North Carolina School of Medicine, 5004 Mary Ellen Jones Bldg, 116 Manning Dr, CB 8035, Chapel Hill, NC 27599, USA Email: edwinkim@email.unc.edu
- Received July 24, 2023 Revised September 30, 2023 Accepted October 21, 2023
- Abstract
-
Food allergies can pose significant risks and profoundly impact the quality of life of children and their families, making them a major public health concern. Allergen avoidance has been the traditional mainstay of treatment; however, recent research has focused on various approaches to food allergen immunotherapy. This review summarizes the recent advancements in oral, sublingual, and epicutaneous immunotherapies, highlighting their respective advantages and disadvantages. The ultimate goal of food allergen immunotherapy is to maximize efficacy while minimizing risks, leading to the exploration of strategies such as low-dose immunotherapy and the use of biologics. When selecting candidates for immunotherapy among patients with food allergies, factors such as allergen characteristics, the likelihood of natural resolution, age, symptom severity, and impact on quality of life require consideration, and an individualized approach should be adopted to determine the most suitable treatment method.
- Introduction
- Introduction
In recent decades, the prevalence of food allergies has increased worldwide [1-6]. Food allergies mostly begin in early childhood and can present with severe life-threatening symptoms, such as anaphylaxis, making them a significant public health concern [7,8]. Parents of children with food allergies often experience concern and anxiety about school or daycare meals and accidental food consumption as well as a fear of when severe allergic reactions may occur. These concerns can significantly restrict daily life and diminish its quality [9-11]. Despite the importance of managing food allergies, treatment remains a challenging task compared to that of other allergic conditions, and active treatments beyond avoidance have been limited [7,12].The learning early about peanut allergy (LEAP) study published in 2015 revealed that the early introduction of peanuts decreased the frequency of peanut allergies [13]. By demonstrating that food allergies can be prevented through early food introduction, the LEAP study provides valuable insight into the underlying mechanisms of food allergies. Consequently, the demand for the proactive treatment of immunoglobulin E (IgE)-mediated food allergies has remained steady [14,15].In 2020, peanut allergen powder-dnfp (Palforzia [PTAH]), an immunotherapy drug for peanut allergies, received approval from the U.S. Food and Drug Administration [16]. This marked the official recognition of oral immunotherapy (OIT) for food allergies and was a key moment in the food allergy field. In Korea, OIT using heated milk/egg was recognized as a safe and effective medical technology by the New Health Technology Assessment Committee of the Ministry of Health and Welfare in March 2022 (no. 2022-63). Based on this information, we reviewed and summarized recent studies on food allergen immunotherapy to guide the identification of applicable treatments in the clinical setting in Korea.
- Recent studies of food allergen immunotherapy
- Recent studies of food allergen immunotherapy
- 1. Oral immunotherapy
- 1. Oral immunotherapy
1) Peanut OIT
1) Peanut OIT
The PALISADE study published in 2018 was a large phase 3 trial of the peanut allergy OIT drug AR101 [16]. Based on its findings, AR101 (later officially named PTAH) was the first Food and Drug Administration–approved drug for peanut allergy in the United States (US). The study included 551 patients aged 4–55 years with peanut allergy confirmed by allergic symptoms at a dose of 100 mg or less of peanut protein (approximately one-third of a peanut kernel) during a baseline oral food challenge (OFC). The participants received either AR101 or placebo. On day 1, participants underwent an initial dose-escalation phase from 0.5 mg to 6 mg. Subsequently, they proceeded to an increasing-dose phase during which the dosage was gradually increased every 2 weeks from 3 mg to 300 mg. This was followed by a 24-week maintenance phase at a constant dosage of 300 mg. The trial lasted approximately 12 months. After 52 weeks, 67.2% of the actively treated participants (versus 4% in the placebo group) were able to consume a single dose of 600 mg or more of peanut protein without experiencing allergic symptoms. No statistically significant difference was observed between the AR101- and placebo-treated groups in the adult group (≥18 years). Adverse events (AEs) were reported in 98.7% of the AR101 group versus 95.2% of the placebo group, with severe AEs occurring in 4.3% of the AR101 group and 0.8% of the placebo group.Subsequently, a multicenter, phase 3 peanut OIT study using AR101 was conducted in children aged 4–17 in Europe. After 9 months of treatment, at the exit food challenge test with 1,000 mg of peanut protein, 58% of the AR101 group and 2% of the placebo group tolerated it [17]. Vickery et al. [18] conducted an open-label follow-up study of the PALISADE study and demonstrated that long-term daily treatment with PTAH led to improved efficacy compared to nondaily treatment. Overall, 83% of the participants experienced mild to moderate AEs, and the safety profile was superior in the daily treatment versus nondaily treatment group. Subsequently, the researchers reported a subgroup analysis of participants from the PALISADE-ARC004 study (aged 4–17 years) who received 300 mg of PTAH daily during the maintenance phase for approximately 1.5 years (group A) or 2 years (groupB). In group A, 48.1% achieved desensitization to 2,000 mg of peanut protein, whereas in group B, 80.8% achieved desensitization [19].In the recent IMPACT trial, patients aged 1–3 years were treated with a maintenance dose of 2,000 mg for 134 weeks. The study demonstrated more robust results than previous studies. At 134 weeks, the median cumulative tolerated dose was 5,005 mg in the treatment group and 5 mg in the placebo group (P<0.0001). Furthermore, “remission,” defined as passing a double-blind placebo-controlled food challenge of 5,000 mg after discontinuing treatment for 26 weeks, was achieved in 21% of the treatment group versus 2% of the placebo group. At 160 weeks, the median cumulative tolerated dose in the treatment group was 755 mg [20].2) Cow’s milk OIT
2) Cow’s milk OIT
Cow’s milk OIT has a higher rate of severe AEs than other food allergen immunotherapies. When undergoing immunotherapy with a maintenance dose of 100 mL of milk for 1 year, up to 43% of participants in the treatment group experienced AEs requiring the use of epinephrine [21]. Several recent studies aimed to improve the safety of cow’s milk OIT using low-dose milk or heated/baked milk in OIT protocols. Ogura et al. [22] conducted a study of milk, egg, and wheat OIT by dividing the participants into 100% or 25% dose groups. The study found no significant difference in efficacy between the low- and high-dose groups; however, AEs were less frequent in the low-dose group.Miura et al. [23] conducted OIT using 3 mL of milk for 1, 2, or 3 years. Short-term unresponsiveness (StU) was observed after a 2-week milk avoidance period in the treatment group as follows: 27% at 1 year, 52% at 2 years, and 61% at 3 years. The percentage of patients in the control group was 13%. In the treatment group, anaphylaxis was observed after three of 164 OIT administrations (2%) conducted in a hospital setting and after eight of 19,861 OIT administrations (0.04%) conducted at home. A study of milk OIT compared milk heated at 125°C for 30 s and unheated milk. This study involved the administration of a low dose of milk (3 mL) for 1 year. One year later, 35% and 18% of the heated-milk group and 50% and 31% of the unheated-milk group passed the 3 mL and 25 mL OFCs, respectively. The rates of moderate to severe symptoms and respiratory symptoms per administered dose were significantly lower in the heated-milk versus unheated-milk group (0.7% and 1.2% vs. 1.4% and 2.6%, respectively; P<0.001) [24].Another study used baked milk for immunotherapy. Therein, researchers added premeasured OIT powder to a cupcake or muffin batter and baked the mixture at 350°F for 30 minutes. Eleven of the 15 (73%) baked milk OIT participants reached the primary endpoint, tolerating 4,044 mg of baked milk protein after 12 months compared with 0 of 15 (0%) in the placebo group. Dose-related reactions were common; however, >95% of the reactions in both groups were mild [25]. Another aimed to reduce the risk of milk OIT by using omalizumab as an adjuvant therapy [26]. When used as monotherapy, omalizumab induced patient tolerance to ≥6,000 mg of cow’s milk protein in 34.8% of patients tested by OFC. When omalizumab was combined with milk OIT, 83.0% of patients achieved desensitization. The discontinuation of omalizumab resulted in more AEs (P=0.013) and anaphylaxis (P=0.001) compared to continued omalizumab treatment. Anaphylaxis was observed in 36.4% of patients who discontinued omalizumab, with a higher occurrence after sudden (50.0%) rather than gradual (12.5%) discontinuation.3) Egg OIT
3) Egg OIT
Low-dose and heated forms of immunotherapy have also been used to treat egg allergies. Kim et al. [27] reported that, among children allergic to unbaked eggs but tolerant of baked eggs, those treated with egg OIT were significantly more likely to achieve sustained unresponsiveness (SU) than children who ingested baked eggs after a discontinuation period of 8–10 weeks. A comparison of high-dose (6,200 mg) and low-dose (1,550 mg) egg OIT revealed no significant difference in SU rates at 1 year (20% in the high-dose group vs. 26.9% in the low-dose group) (P=0.743). However, the rate of AEs was lower in the low-dose (8.74%) versus high-dose (10.9%, P<0.05) group [22]. Palosuo et al. [28] conducted a study in which 6- to 17-year-old patients with egg allergies were treated with raw egg white powder (1 g of egg white protein) for 8 months; a desensitization rate of 44% was achieved. They reported that subjects with high egg white-specific IgE concentrations and sensitization to multiple egg allergen components at baseline benefited from prolonged treatment.Recent clinical trial results of OIT for peanut, milk, and egg allergies are presented in Table 1.
- 2. Sublingual immunotherapy
- 2. Sublingual immunotherapy
Various immunotherapy delivery modalities have been studied for immunotherapy (Table 2). Sublingual immunotherapy (SLIT) for food allergies involves the daily placement of a glycerinated food allergen under the tongue to achieve desensitization. SLIT has been attempted for treating cow’s milk allergy and pollen-food allergy syndromes, but it has mainly been studied in the context of peanut allergy [29]. A study of 40 participants aged 12–37 years compared peanut SLIT with placebo. During the 44-week treatment period at a maintenance dose of 1,386 µg, 70% of the participants in the treatment group met the responder criteria by passing a 5,000-mg OFC or increasing their tolerated peanut dose by more than 10-fold compared to baseline. The most common AE was oropharyngeal itching; excluding this symptom, 94.7% of the participants were symptom-free [30].In children aged 1–11 years with peanut allergy, daily SLIT with 2 mg of peanut protein (equivalent to 1/150th of a peanut) was administered for up to 5 years. In this study, 67% of children tolerated at least 750 mg of peanut protein (equivalent to 2.5 peanuts). The median successfully consumed dose (SCD) was 1,750 mg, and no AEs requiring epinephrine occurred. Subsequently, when the SLIT dose was increased to 4 mg, 70% of the patients tolerated 750 mg of peanut protein, and the median SCD increased to 2,350 mg, indicating improved efficacy. Similar AE levels were observed with an increased SLIT dose (4 mg) [31,32].- 3. Epicutaneous immunotherapy
- 3. Epicutaneous immunotherapy
Epicutaneous immunotherapy (EPIT)involves applying a patch to the skin with peanut protein electrostatically adhering to the inner portion of the patch. This allows for the solubilization of peanuts by sweat and the opening of skin pores, facilitating the passive transfer of peanuts to skin-based Langerhans cells with a minimal risk of systemic absorption. EPIT has been attempted for both milk and peanut allergies, with recent research predominantly focusing on the latter [33,34].A multicenter double-blind randomized placebo-controlled study was conducted in 2017, involving 74 participants aged 4–25 years. This study evaluated the efficacy of Viaskin Peanut (DBV Technologies, Montrouge, France) patches at doses of 100 µg and 250 µg as well as a placebo patch over a treatment period of 52 weeks. It aimed to assess the ability of participants to pass an OFC of 5,044 mg of peanut protein or achieve an SCD of at least 10 times the baseline value. The response rate was significantly higher in the Viaskin Peanut 100 µg (46%) and Viaskin Peanut 250 µg (48%) groups than in the placebo (12%) group. The success rate was higher in the younger age group (4–11 years) than in the older age group (>11 years). AEs were reported in 79.8% and 14.4% of the experimental and placebo groups, respectively; however, most were local patch-site reactions and generally mild [35].In the PEPITES study conducted in 2019, children aged 4–11 years were treated with a 250-µg patch for 1 year and compared to placebo. The response rate was 35.3% [36]. Recently, the EPITOPE study focused on peanut EPIT in children aged 1–3 years. The same criteria used in the PEPITES study were applied to determine efficacy, and the responder rate improved to 67%. Treatment-related anaphylaxis occurred in 1.6% of the patients in the EPIT group versus none of the patients in the placebo group [37]. The recent EPIT studies are summarized in Table 2.
- Considerations
- Considerations
- 1. Efficacy and safety
- 1. Efficacy and safety
Although OIT appears a promising treatment for pediatric food allergies, safety concerns remain a significant barrier to its widespread use. According to a meta-analysis published in 2020, among children undergoing peanut OIT, AEs requiring epinephrine occurred in 7.6% of and 6.6% discontinued therapy due to AEs [38]. Efforts are being made to improve the safety of food allergen immunotherapy. Clinical trials are underway of alternative administration methods such as SLIT and EPIT. SLIT studies of milk reported some effectiveness, although not as pronounced as that of OIT, with fewer AEs [39].Recent studies reported promising results for peanut SLIT, particularly in younger age groups and those with longer treatment durations. AEs associated with SLIT are mostly localized in the oropharynx [30-32]. EPIT is another safe and easily administered immunotherapeutic method. However, it has the lowest efficacy of all methods [33-36]. A recent study targeting children aged 1–3 years reported a high response rate, indicating promising potential [37].Patients who experience severe allergic reactions, even at very low doses, are at higher risk of developing anaphylaxis and may ultimately fail to respond to conventional-dose OIT. For such patients, the goal should be treating them in a manner that ensures that no symptoms occur during accidental exposure to allergens, thereby securing a minimal safety level and improving their quality of life. This is the rationale for the development of low-dose OIT. Yanagida et al. [40] reported low-dose OIT studies of milk and wheat. Patients with severe milk allergy were instructed to consume 3 mL of heated milk or 10 g of butter (equivalent to 2.9 mL of cow’s milk) daily for 1 year. Patients with wheat allergies were instructed to consume 2 g of boiled udon noodles daily. In an OFC using 25 mL of heated milk or 15 g of boiled udon noodles, 45% and 56% of patients, respectively, passed. When comparing the incidence of AEs between low- and conventional-dose OIT groups, the former had fewer moderate or severe symptoms than the latter. Low-dose OIT may be a favorable option for patients with severe symptoms associated with food allergies.Methods have been devised to enhance OIT safety by coadministering biological agents, such as anti-IgE, during OIT induction or maintenance [26,41-43]. Omalizumab used in conjunction with OIT showed better efficacy than omalizumab monotherapy; however, caution is required because of the higher incidence of anaphylaxis after its discontinuation. According to a recent meta-analysis, OIT with omalizumab showed significant increases in the tolerated dose of multiple foods, desensitization, improvements in quality of life, and increases in immunoglobulin G4 levels compared with conventional OIT. No major safety concerns were observed [44]. Thus, omalizumab use in OIT can be considered, especially in the initial up-dosing phase, to enhance safety. Moreover, omalizumab could become a crucial tool for reducing the risks associated with immunotherapy, especially in patients experiencing severe anaphylactic reactions at low allergen thresholds, severe allergies to food staples, multiple food allergies, or concurrent comorbidities that complicate food allergy treatment. However, when omalizumab is discontinued, clinicians should be cautious about the occurrence of AEs, and it is advisable to gradually taper rather than abruptly stop it [26].Recent research is also underway to explore OIT using ligelizumab and dupilumab in addition to omalizumab [45]. However, biological agents have not yet been approved for the treatment of food allergies and pose cost-related issues. Recent studies examined the addition of probiotic adjuvants to peanut OIT. Both probiotic-augmented and conventional OIT effectively induced SU. The addition of probiotics did not enhance OIT efficacy; however, it may offer safety benefits, especially in preschool children, compared to OIT alone [46].The efficacy and safety of various food allergen immunotherapy methods are shown in Fig. 1. Clinical trials of food allergen immunotherapy have varied in participant age, symptom severity, and maintenance immunotherapy dosage, making it challenging to quantitatively compare their effects and risks. Low-dose OIT and OIT plus omalizumab increased safety while maintaining higher efficacy rates than conventional OIT. There are no direct comparisons between SLIT and EPIT in the literature; however, 2 review articles slightly favored EPIT for safety. While anaphylactic events occurred in the EPIT studies, AEs were predominantly localized to the application site on the skin [47,48].- 2. Target foods for food allergen immunotherapy
- 2. Target foods for food allergen immunotherapy
The selection of target foods for food allergen immunotherapy is also subject to various considerations. The importance of common food allergens may vary across countries depending on dietary habits and culture and may differ in age group. According to a multicenter study in South Korea,the most common major food allergens among Korean children and adolescents (0–18 years old) were cow’s milk (28.1%), hen’s eggs (27.6%), wheat(7.9%), walnuts (7.3%), and peanuts (5.3%) [49]. Common causes of anaphylaxis in Korean children include milk (28.4%), egg whites (13.6%), walnuts (8.0%), wheat (7.2%), buckwheat (6.5%), and peanuts (6.2%) [50]. Among Korean schoolchildren, the most common food allergens were peanuts, eggs, cow’s milk, and buckwheat [4]. The importance of managing food allergies varies depending on the food’s characteristics. For example, milk is commonly found in many food products and can significantly restrict daily life, pose nutritional challenges, and cause anxiety in patients and their families, thereby significantly impairing their quality of life [51]. Another consideration is the natural course of food allergies, which can vary among food types. Milk and egg allergies tend to resolve as children mature, whereas peanut and tree nut allergies have lower natural resolution rates. However, according to recent studies, in the US, approximately 42% of children who develop a milk allergy do so by the age of 8 years, whereas in Korea, approximately 50% of children who develop a milk allergy do so at a median age of 8.7. Among Japanese children, approximately 73% who develop an egg allergy do so by the age of 6 years, whereas among Korean children, approximately 50% who develop an egg allergy do so by the age of 5.6 years [52-54]. Since recent research has indicated that milk and egg allergies may persist longer than expected, immunotherapy should be considered a proactive treatment option in such cases. However, when choosing immunotherapy for foods with the potential for natural resolution, it is necessary to carefully weigh the benefits and risks and use an individualized approach for each patient. Additionally, the establishment of biomarkers that can predict the likelihood of natural outgrowth is required.Patients with multiple food allergies present another challenging target for food allergen immunotherapy. A recent US population-based study reported that, among children with food allergies, 40% had multiple food allergies, meaning that they were allergic to at least 2 different foods [55]. Research on multifood OIT is limited, and studies to date often involved the combined use of omalizumab [41-43]. The OUtMATCH study is a phase III multicenter randomized double-blind placebo-controlled study that awaits the results of both omalizumab monotherapy and omalizumab-facilitated OIT for multifood allergy. Another recent study identified various retail food equivalents for 7 allergens, namely peanuts, milk, eggs, wheat, cashew nuts, hazelnuts, and walnuts. This study provided valuable guidance for the dietary management of patients undergoing multifood OIT [56].- 3. Age and severity
- 3. Age and severity
The decision to initiate food allergen immunotherapy and the approach chosen may vary depending on patient age and symptom severity. Recent research has suggested that food allergen immunotherapy is more effective in younger age groups. The IMPACT trial involved administering OIT to young children aged 1–3 years and demonstrated AEs that were similar to the older OIT-treated cohort efficacy but with stronger desensitization and disease remission achieved in 21% of participants. Similarly, the EPITOPE trial targeted a younger age group (1–3 years) and showed better outcomes with peanut EPIT than in the older age group [9,37]. However, children may be more likely than adults to outgrow allergies on their own, although the rate of spontaneous resolution varies depending on the specific food allergen. Therefore, determining the optimal timing for initiating food allergen immunotherapy may be challenging. For patients who experience severe symptoms, even with minimal exposure to the allergen,the goal may be to raise the threshold for the allergen that triggers allergic symptoms rather than achieving a completely unrestricted diet. For patients with such goals, it is desirable to choose immunotherapy methods that are safer than conventional OIT.In recent food allergy guidelines, based on high-certainty evidence, peanut OIT is recommended under specialist supervision with standardized evidence-based protocols using peanut products (or licensed pharmaceutical products, where appropriate) for selected children (aged 4 years and older) with clinically diagnosed severe peanut allergies to increase their peanut tolerance. Additionally, for children aged 4–11 years with peanut allergies, EPIT is recommended with moderate certainty. For patients aged 4 years and older with egg and milk allergies, OIT is recommended with moderate-certainty evidence. According to these guidelines, there is currently insufficient evidence to recommend or discourage the use of OIT for other food allergens, OIT combined with adjuvants, or alternative food allergen immunotherapy methods. The authors of the guidelines recently noted that positive immunotherapy results for peanut allergy in children under 4 years of age have been reported. However, considering the evidence of effectiveness, potential for outgrowing the allergy, and potential risks, they recommend allergen immunotherapy for the age group of 4 years and older. They also mentioned that clinicians may consider other age groups depending on the individual circumstances [57].
- Conclusion
- Conclusion
Various studies have recently aimed to develop effective and safe immunotherapy methods for food allergies. The optimal treatment approach for food allergies is likely to vary depending on factors such as the specific food allergen, natural course of the allergy, presence of accompanying allergies, age, and symptom severity. There are numerous challenges in food allergen immunotherapy research. Therefore, it is necessary to determine how to choose appropriate methods that are tailored to each patient’s treatment goals. In addition, the development of reliable biomarkers to aid in the selection of candidates for immunotherapy is essential. Specifically, for the treatment of multiple food allergies, we must explore methods such as adjuvants that can enhance therapeutic long-term safety and efficacy. While some food allergen immunotherapy methods may not yet be applicable in Korea, personalized and tailored approaches to food allergy treatment are believed to have the potential to improve patients’ quality of life and modify the natural course of allergies.
- Footnotes
-
Conflicts of interest YHJ has no conflicts of interest to declare. EHK reports advisory board membership with ALK, Kenota Health, and Ukko Inc; consultancy with AllerGenis, Belhaven Biopharma, Genentech, Nutricia, Revolo; and grants to his university from National Institutes of Health (NIH) and Food Allergy Research and Education (FARE)
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Fig. 1.
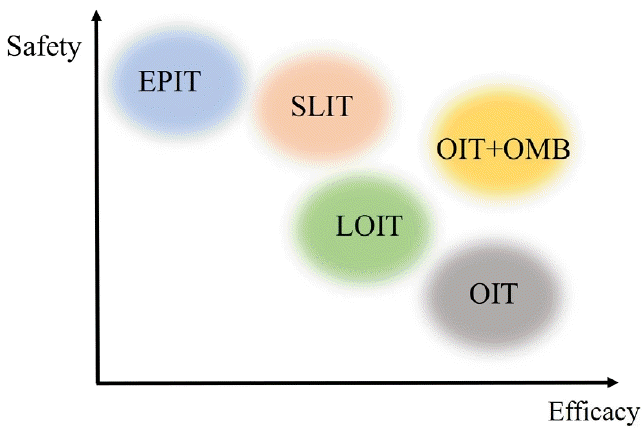
Table 1.
| Allergen/Study | Age (yr) | Numbers by group | Maintenance dose, procedure | Duration | Efficacy | Safety |
|---|---|---|---|---|---|---|
| Peanut | ||||||
| Vickery et al., 2018 [16] (PALISADE) | 4–55 | (4–17 yr) | 300 mg of peanut protein | 1 Yr | (4-17 yr) | Any AE/Severe AE |
| OIT: N=372 | Desensitization to ≥600 mg | OIT: 98.7%/4.3% | ||||
| Placebo: N=124 | OIT: 67.2% | Placebo: 95.2%/0.8% | ||||
| (18–55 yr) | Placebo: 4.0% | |||||
| OIT: N=42 | (18-55 yr) No effect | |||||
| Placebo: N=14 | ||||||
| O'B Hourihane et al., 2020 [17] (ARTEMIS) | 4–17 | OIT: N=132 | 300 mg of peanut protein | 9 Mo | Desensitized to 1,000 mg | Any AE/Severe AE |
| Placebo: N=43 | OIT: 58% | OIT: 99%/0% | ||||
| Placebo: 2% | Placebo: 98%/0% | |||||
| Vickery et al., 2021 [18] (ARC004; PALISADE follow-on study) | 4–17 | Daily dosing | 300 mg of peanut protein, Daily dosing PTAH (AR101) vs. nondaily dosing PTAH | 28–56 Wk | Desensitized to 2,000 mg | Exposure-adjusted AE/participant-year |
| PTAH Naive: N=72 | Daily dosing | Daily dosing | ||||
| Cohort 1: N=103 | PTAH Naive: 51.4% | 12.94 to 17.54 | ||||
| Cohort 3A: N=26 | Cohort 1: 48.5% | Nondaily dosing | ||||
| Nondaily dosing | Cohort 3A: 80.8% | 25.95 to 42.49 | ||||
| Cohort 2: N=38 | Nondaily dosing | |||||
| Cohort 3B: N=32 | Cohort 2: 36.8% | |||||
| Cohort 3C: N=21 | Cohort 3B: 45.4% | |||||
| Cohort 3C: 42.9% | ||||||
| Fernandez-Rivas et al., 2022 [19] (PALISADE-ARC004 post hoc longitudinal exploratory analysis) | 4–17 | Group A (28-wk maintenance): N=110 | 300 mg of peanut protein, 28-wk vs. 56-wk maintenance | 2 Yr | Desensitized to 2,000 mg | Exposure-adjusted AE (PALISADE, ARC004) |
| Group B (56-wk maintenance): N=32 | Group A: 48.1% | Group A: 20.7%, 12.8% | ||||
| Group B: 80.8% | Group B: 31.8%, 17.5% | |||||
| Jones et al., 2022 [20] (IMPACT Trial) | 1–3 | OIT: N=96 | 2,000 mg of peanut protein | 134 Wk | Desensitized to 5,000 mg | Any AE/ Severe AE |
| Placebo: N=50 | OIT group: 71% | OIT: 98%/5% | ||||
| Placebo group: 2% | Placebo: 80%/0% | |||||
| Remission: 21% vs 2% | ||||||
| Cow’s milk | ||||||
| Ogura et al., 2020 [22] | 3–15 | Low-dose OIT: N=13 | Low dose: 850 mg | 1 Yr | StU (2 wk) to 3,400 mg | Any AE per dose |
| High-dose OIT: N=13 | High-dose: 3,400 mg of CMP | Low-dose OIT: 15.4% | Low-dose OIT: 10.3% | |||
| High-dose OIT: 7.7% | High-dose OIT: 15.4$ | |||||
| Maeda et al., 2021 [21] (ORIMA) | 3–12 | OIT: N=14 | 100 mL of cow’s milk vs. avoidance | 1 Yr | Negative OFC (100 mL) | Any AE/AE requiring epinephrine |
| Control: N=14 | OIT: 50% | OIT: 86%/43% | ||||
| Control: 0% | Control: 21%/0% | |||||
| Miura et al., 2021 [23] | 5–9 | Low-dose OIT: N=36 | 3 mL of cow’s milk, 1 yr vs. 2 yr vs. 3yr | 3 Yr | 25 mL StU (2 wk) | Anaphylaxis |
| Control: N=16 | LOIT: 1 yr 27% | Hospital phase: 2% | ||||
| 2 yr 52% | Home phase: 0.04% | |||||
| 3 yr 61% | ||||||
| Control: 13% | ||||||
| Nagakura et al., 2021 [24] | 5–11 | HM-OIT: N=17 | 3 mL of cow’s milk, Heated milk vs. unheated milk | 1 Yr | Desensitized to 3 mL/ 25 mL | Mild/moderate/severe AE per home dose |
| UM-OIT: N=16 | HM-OIT: 35%/18% | HM-OIT : 7.4%/0.7%/0.02% | ||||
| UM-OIT: 50%/31% | UM-OIT: 8.1%/1.4%/0.0% | |||||
| Ibáñez-Sandín et al., 2021 [26] | 6.3–13.2 | N=58 | Omalizumab + ≥6,000 mg of CMP, omalizumab: 4 mo before OIT: omalizumab discontinued vs. continued | 1 Yr | Desensitization to ≥6,000 mg of CMP | Any AE/anaphylaxis |
| OMB discontinued: N=22 | OMB: 34.8% | OMB discontinued: 59.1%/36.4% | ||||
| OMB continued: N=16 | OMB-OIT: 83.0% | OMB continued: 25.1%/ 0% | ||||
| Dantzer et al., 2022 [25] | 3–18 | BMOIT: N=15 | 2,000 mg of BMP | 1 Yr | Desensitization to 4,044 mg of BMP | Any AE/AE requiring epinephrine |
| Placebo: N=15 | BMOIT: 73% | BMOIT: 42%/0.1% | ||||
| Placebo: 0% | Placebo: 2%/0% | |||||
| Egg | ||||||
| Kim et al., 2020 [27] | 3–16 | BE-R: N=27 | 2,000 mg of egg white protein | 2 Yr | SU (8-10 wk) to at least 4,444 mg of egg white protein | Any AE |
| OIT-R: N=23 | BE-R: 11.1% | BE-R: 2.8% | ||||
| OIT-A: N=39 | OIT-R: 43.5% | OIT-R: 3.9% | ||||
| OIT-A: 17.9% | OIT-A: 12.6% | |||||
| Ogura et al., 2020 [22] | 3–15 | Low-dose OIT: N=25 | Low dose: 1,550 mg | 1 Yr | StU (2 wk) to 6,200 mg of whole egg protein | Any AE |
| High-dose OIT: N=26 | High dose: 6,200 mg of whole egg protein | Low-dose OIT: 20% | Low-dose OIT: 8.74% | |||
| High-dose OIT: 26.9% | High-dose OIT: 10.9% | |||||
| Palosuo et al., 2021 [28] | 6–17 | OIT: N=32 | 1,000 mg of egg white protein vs avoidance, 8 mo vs. 18 mo of OIT | 8 Mo and 18 mo | Desensitization to 1,000 mg of egg white protein | Any AE in build-up phase: 82% |
| Control: N=18 | 8 mo of OIT: 44% | Severe AE: 0% | ||||
| 18 mo of OIT: 72% | ||||||
| Control: 4.8% | ||||||
| Multifood | ||||||
| Sindher, 2022 [43] | 2–25 | Low-dose mOIT: N=30 | Low dose: 300 mg | 18 Wk | IgG4/IgE ratio increase from baseline ≥25% for at least 2 allergens | Any AE/treated AE |
| High-dose mOIT: N=30 | High dose: 1,200 mg of total protein (2–5 allergens), pre-treated with 3 doses Omalizumab | Low-dose mOIT: 70% | Low-dose mOIT: 19.4%/3.6% | |||
| High-dose mOIT: 70% | High-dose mOIT: 16.8%/3.9% |
OIT, oral immunotherapy; AE, adverse event; PTAH, peanut allergen powder; StU, short-term unresponsiveness; OFC, oral food challenge; LOIT, low-dose OIT; HM-OIT, heated milk oral immunotherapy; UM-OIT, unheated milk oral immunotherapy; OMB, omalizumab; CMP, cow’s milk protein; BMOIT, baked milk oral immunotherapy; BMP, baked milk protein; SU, sustained unresponsiveness; OMB, omalizumab; BE-R, baked egg-randomized; OIT-R, egg OIT-randomized; OIT-A, egg OIT-assigned; mOIT, multifood oral immunotherapy.
Table 2.
| Study | Age (yr) | Numbers by group | Maintenance dose, procedure | Duration | Efficacy | Safety |
|---|---|---|---|---|---|---|
| SLIT | ||||||
| Fleischer et al., 2013 [30] | 12–37 | N=40 | 1,386 µg of peanut protein SLIT vs. placebo SLIT | 44 Wk and 68 wk | Passing a 5,000 mg OFC or at least ≥10 fold peanut powder than the baseline | AE beyond the oropharynx of all doses: 4.8% |
| Peanut SLIT: 70% | ||||||
| Placebo SLIT: 15% | ||||||
| Median SCD: 496 mg (44 wk) : 996 mg (68 wk) | ||||||
| Kim et al., 2019 [31] | 1–11 | N=48 | 2 mg of peanut protein SLIT | 3–5 Yr | Passing a 750 mg DBPCFC: 67% | Oropharyngeal pruritis (%) of all doses taken: 3.6% |
| Passing a 5,000 mg DBPCFC: 25% | Doses requiring epinephrine: 0% | |||||
| Median SCD:1,750 mg | ||||||
| Kim et al., 2023 [32] | 1–11 | N=54 | 4 mg of peanut protein SLIT | 4 Yr | Clinically significant desensitization (SCD > 800 mg): 70% | Oropharyngeal itching and lip swelling (%) of all doses taken: 3.7% |
| Full desensitization (SCD = 5,000 mg): 36% | Skin symptoms: 0.1% | |||||
| Abdominal symptoms: 0.1% | ||||||
| Median SCD: 2,723 mg | Doses requiring epinephrine: 0% | |||||
| EPIT | ||||||
| Fleischer et al., 2019 [36] (PEPITES) | 4–11 | Peanut patch: N=238 | 250 µg of peanut protein patch | 1 Yr | Responder criterion | Any AE/anaphylaxis |
| (1) Baseline eliciting dose | Peanut patch: 95.4%/3.4% | |||||
| Placebo patch: N=118 | ≤10 mg; posttreatment eliciting dose ≥300 mg | Placebo patch: 89%/0.4% | ||||
| (2) Baseline eliciting dose | ||||||
| >10–300 mg; ≥1,000 mg | ||||||
| Responder rate | ||||||
| Peanut patch: 35.3% | ||||||
| Placebo patch: 13.6% | ||||||
| Greenhawt et al., 2023 [37] (EPITOPE) | 1–3 | Peanut patch: N=244 | 100 µg/250 µg of peanut protein patch | 1 Yr | Responder criterion | Any AE/treatment-related anaphylaxis |
| Placebo patch: N=118 | (1) Baseline eliciting dose | Peanut patch: 100%/1.6% | ||||
| ≤10 mg; posttreatment eliciting dose ≥300 mg | Placebo patch: 99.2%/0% | |||||
| (2) Baseline eliciting dose | ||||||
| >10 mg; ≥1,000 mg | ||||||
| Responder rate | ||||||
| Peanut patch: 67% | ||||||
| Placebo patch: 33.5% |
- References
- 1. Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol 2011;127:594–602.
[Article] [PubMed]2. Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep 2020;20:6.
[Article] [PubMed] [PMC]3. Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in seoul. Allergy Asthma Immunol Res 2014;6:131–6.
[Article] [PubMed] [PMC]4. Kim M, Lee JY, Jeon HY, Yang HK, Lee KJ, Han Y, et al. Prevalence of immediate-type food allergy in Korean schoolchildren in 2015: a nationwide, population-based study. Allergy Asthma Immunol Res 2017;9:410–6.
[Article] [PubMed] [PMC]5. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011;128:e9–17.
[Article] [PubMed]6. Barni S, Liccioli G, Sarti L, Giovannini M, Novembre E, Mori F. Immunoglobulin E (IgE)-mediated food allergy in children: epidemiology, pathogenesis, diagnosis, prevention, and management. Medicina (Kaunas) 2020;56:111.
[Article] [PubMed] [PMC]7. Lee S. IgE-mediated food allergies in children: prevalence, triggers, and management. Korean J Pediatr 2017;60:99–105.
[Article] [PubMed] [PMC]8. Lee S. Food allergy and food-induced anaphylaxis in children: an increasing critical public health issue. Korean J Pediatr 2019;62:431–2.
[Article] [PubMed] [PMC]9. Dilley MA, Rettiganti M, Christie L, O'Brien E, Patterson M, Weeks C, et al. Impact of food allergy on food insecurity and health literacy in a tertiary care pediatric allergy population. Pediatr Allergy Immunol 2019;30:363–9.
[Article] [PubMed]10. Jeong K, Kim J, Chang HY, Song TW, Kim JH, Shin M, et al. Maternal posttraumatic stress symptoms and psychological burden in mothers of Korean children with anaphylaxis. Allergy Asthma Immunol Res 2022;14:742–51.
[Article] [PubMed] [PMC]11. Kim S, Kim M, Kim J, Park B, Min N, Jung M, et al. Quality of life in food allergy: validation of the Korean version of the food allergy quality of life questionnaire parent form (K-FAQLQPF) and risk factor analysis. Allergy Asthma Immunol Res 2023;15:43–54.
[Article] [PubMed] [PMC]12. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018;141:41–58.
[Article] [PubMed]13. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–13.
[Article] [PubMed] [PMC]14. Kim EH, Patel C, Burks AW. Immunotherapy approaches for peanut allergy. Expert Rev Clin Immunol 2020;16:167–74.
[Article] [PubMed]15. Costa C, Coimbra A, Vitor A, Aguiar R, Ferreira AL, Todo-Bom A. Food allergy-from food avoidance to active treatment. Scand J Immunol 2020;91:e12824.
[Article] [PubMed]16. PALISADE Group of Clinical Investigators; Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 Oral immunotherapy for peanut allergy. N Engl J Med 2018;379:1991–2001.
[Article] [PubMed]17. O'B Hourihane J, Beyer K, Abbas A, Fernandez-Rivas M, Turner PJ, Blumchen K, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health 2020;4:728–39.
[Article] [PubMed]18. Vickery BP, Vereda A, Nilsson C, du Toit G, Shreffler WG, Burks AW, et al. Continuous and daily oral immunotherapy for peanut allergy: results from a 2-year open-label follow-on study. J Allergy Clin Immunol Pract 2021;9:1879–89.e13.
[PubMed]19. Fernandez-Rivas M, Vereda A, Vickery BP, Sharma V, Nilsson C, Muraro A, et al. Open-label follow-on study evaluating the efficacy, safety, and quality of life with extended daily oral immunotherapy in children with peanut allergy. Allergy 2022;77:991–1003.
[Article] [PubMed] [PMC]20. Jones SM, Kim EH, Nadeau KC, Nowak-Wegrzyn A, Wood RA, Sampson HA, et al. Efficacy and safety of oral immunotherapy in children aged 1-3 years with peanut allergy (the Immune Tolerance Network IMPACT trial): a randomised placebo-controlled study. Lancet 2022;399:359–71.
[Article] [PubMed] [PMC]21. Maeda M, Imai T, Ishikawa R, Nakamura T, Kamiya T, Kimura A, et al. Effect of oral immunotherapy in children with milk allergy: the ORIMA study. Allergol Int 2021;70:223–8.
[Article] [PubMed]22. Ogura K, Yanagida N, Sato S, Imai T, Ito K, Kando N, et al. Evaluation of oral immunotherapy efficacy and safety by maintenance dose dependency: a multicenter randomized study. World Allergy Organ J 2020;13:100463.
[Article] [PubMed] [PMC]23. Miura Y, Nagakura KI, Nishino M, Takei M, Takahashi K, Asaumi T, et al. Long-term follow-up of fixed low-dose oral immunotherapy for children with severe cow's milk allergy. Pediatr Allergy Immunol 2021;32:734–41.
[PubMed]24. Nagakura KI, Sato S, Miura Y, Nishino M, Takahashi K, Asaumi T, et al. A randomized trial of oral immunotherapy for pediatric cow's milk-induced anaphylaxis: Heated vs unheated milk. Pediatr Allergy Immunol 2021;32:161–9.
[PubMed]25. Dantzer J, Dunlop J, Psoter KJ, Keet C, Wood R. Efficacy and safety of baked milk oral immunotherapy in children with severe milk allergy: a randomized, double-blind, placebo-controlled phase 2 trial. J Allergy Clin Immunol 2022;149:1383–91.e17.
[Article] [PubMed]26. Ibáñez-Sandín MD, Escudero C, Candon Morillo R, Lasa EM, Marchan-Martin E, Sanchez-Garcia S, et al. Oral immunotherapy in severe cow's milk allergic patients treated with omalizumab: real life survey from a Spanish registry. Pediatr Allergy Immunol 2021;32:1287–95.
[Article] [PubMed]27. Kim EH, Perry TT, Wood RA, Leung DYM, Berin MC, Burks AW, et al. Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. J Allergy Clin Immunol 2020;146:851–62.e10.
[Article] [PubMed] [PMC]28. Palosuo K, Karisola P, Savinko T, Fyhrquist N, Alenius H, Makela MJ. A randomized, open-label trial of Hen's egg oral immunotherapy: efficacy and humoral immune responses in 50 children. J Allergy Clin Immunol Pract 2021;9:1892–901.e1.
[Article] [PubMed]29. Schworer SA, Kim EH. Sublingual immunotherapy for food allergy and its future directions. Immunotherapy 2020;12:921–31.
[Article] [PubMed] [PMC]30. Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013;131:119–27.e1-7.
[PubMed] [PMC]31. Kim EH, Yang L, Ye P, Guo R, Li Q, Kulis MD, et al. Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2019;144:1320–6.e1.
[Article] [PubMed] [PMC]32. Kim EH, Keet CA, Virkud YV, Chin S, Ye P, Penumarti A, et al. Open-label study of the efficacy, safety, and durability of peanut sublingual immunotherapy in peanut-allergic children. J Allergy Clin Immunol 2023;151:1558–65.e6.
[Article] [PubMed] [PMC]33. Kim EH, Burks AW. Food allergy immunotherapy: oral immunotherapy and epicutaneous immunotherapy. Allergy 2020;75:1337–46.
[Article] [PubMed]34. Lanser BJ, Leung DYM. The current state of epicutaneous immunotherapy for food allergy: a comprehensive review. Clin Rev Allergy Immunol 2018;55:153–61.
[Article] [PubMed]35. Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol 2017;139:1242–52.e9.
[Article] [PubMed] [PMC]36. Fleischer DM, Greenhawt M, Sussman G, Begin P, NowakWegrzyn A, Petroni D, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA 2019;321:946–55.
[PubMed] [PMC]37. Greenhawt M, Sindher SB, Wang J, O'Sullivan M, du Toit G, Kim EH, et al. Phase 3 trial of epicutaneous immunotherapy in toddlers with peanut allergy. N Engl J Med 2023;388:1755–66.
[PubMed]38. Grzeskowiak LE, Tao B, Knight E, Cohen-Woods S, Chataway T. Adverse events associated with peanut oral immunotherapy in children - a systematic review and meta-analysis. Sci Rep 2020;10:659.
[Article] [PubMed] [PMC]39. Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol 2012;129:448. –55. 455.e1-5.
[Article] [PubMed] [PMC]40. Yanagida N, Okada Y, Sato S, Ebisawa M. New approach for food allergy management using low-dose oral food challenges and low-dose oral immunotherapies. Allergol Int 2016;65:135–40.
[Article] [PubMed]41. Andorf S, Manohar M, Dominguez T, Block W, Tupa D, Kshirsagar RA, et al. Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol 2017;13:51.
[Article] [PubMed] [PMC]42. Andorf S, Purington N, Kumar D, Long A, O'Laughlin KL, Sicherer S, et al. A phase 2 randomized controlled multisite study using omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. EClinicalMedicine 2019;7:27–38.
[Article] [PubMed] [PMC]43. Sindher SB, Kumar D, Cao S, Purington N, Long A, Sampath V, et al. Phase 2, randomized multi oral immunotherapy with omalizumab 'real life' study. Allergy 2022;77:1873–84.
[Article] [PubMed]44. Zuberbier T, Wood RA, Bindslev-Jensen C, Fiocchi A, Chinthrajah RS, Worm M, et al. Omalizumab in IgE-mediated food allergy: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2023;11:1134–46.
[Article] [PubMed]45. Sindher SB, Hillier C, Anderson B, Long A, Chinthrajah RS. Treatment of food allergy: oral immunotherapy, biologics, and beyond. Ann Allergy Asthma Immunol 2023;131:29–36.
[PubMed] [PMC]46. Loke P, Orsini F, Lozinsky AC, Gold M, O'Sullivan MD, Quinn P, et al. Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): a multicentre, randomised, phase 2b trial. Lancet Child Adolesc Health 2022;6:171–84.
[Article] [PubMed]47. Alghamdi R, Alshaier R, Alotaibi A, Almutairi A, Alotaibi G, Faqeeh A, et al. Immunotherapy effectiveness in treating peanut hypersensitivity: a systemic review. Cureus 2022;14:e21832.
[Article] [PubMed] [PMC]48. Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: evidence and outlook. Allergol Select 2022;6:285–92.
[Article] [PubMed] [PMC]49. Jeong K, Kim J, Ahn K, Lee SY, Min TK, Pyun BY, et al. Agebased causes and clinical characteristics of immediate-type food allergy in Korean children. Allergy Asthma Immunol Res 2017;9:423–30.
[Article] [PubMed] [PMC]50. Lee SY, Ahn K, Kim J, Jang GC, Min TK, Yang HJ, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res 2016;8:535–40.
[Article] [PubMed] [PMC]51. Abrams EM, Kim H, Gerdts J, Protudjer JLP. Milk allergy most burdensome in multi-food allergic children. Pediatr Allergy Immunol 2020;31:827–34.
[Article] [PubMed]52. Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow's milk allergy. J Allergy Clin Immunol 2007;120:1172–7.
[Article] [PubMed]53. Kim M, Lee JY, Yang HK, Won HJ, Kim K, Kim J, et al. The natural course of immediate-type cow's milk and egg allergies in children. Int Arch Allergy Immunol 2020;181:103–10.
[Article] [PubMed]54. Ohtani K, Sato S, Syukuya A, Asaumi T, Ogura K, Koike Y, et al. Natural history of immediate-type hen's egg allergy in Japanese children. Allergol Int 2016;65:153–7.
[Article] [PubMed]55. Warren CM, Aktas ON, Manalo LJ, Bartell TR, Gupta RS. The epidemiology of multifood allergy in the United States: A population-based study. Ann Allergy Asthma Immunol 2023;130:637–48.e5.
[PubMed]56. Groetch M, Mudd K, Woch M, Schaible A, Gray BE, Babineau DC, et al. Retail food equivalents for post-oral immunotherapy dosing in the omalizumab as monotherapy and as adjunct therapy to multi-allergen oral immunotherapy in food-allergic children and adults (OUtMATCH) clinical trial. J Allergy Clin Immunol Pract 2023;11:572–80.e2.
[Article] [PubMed] [PMC]

 About
About Browse articles
Browse articles For contributors
For contributors

