All issues > Volume 67(8); 2024
Effects of diethylene glycol contamination of pharmaceutical products on unexplained acute kidney injury in children: a systematic review
- Corresponding author: Sani Rachman Soleman, MD, MSc. Department of Public Health, Faculty of Medicine, Universitas Islam Indonesia, Jalan Kaliurang km 14.5, Ngaglik, Sleman, Yogyakarta 55584, Indonesia Email: sani.rachman@uii.ac.id
- Received August 2, 2023 Revised October 10, 2023 Accepted October 21, 2023
- Abstract
-
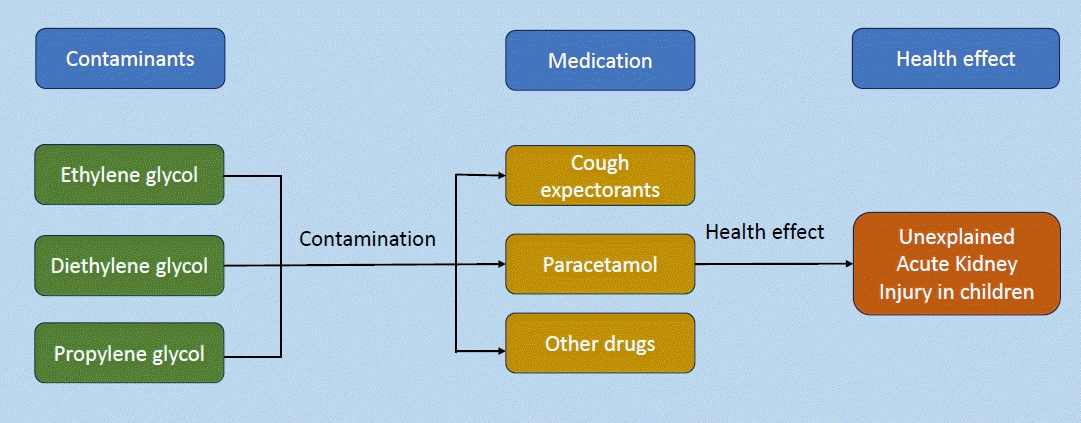
Unexplained acute kidney injury (AKI) in children owing to diethylene glycol (DEG) contamination during drug production has gained attention in recent years. This qualitative study investigated the effects of DEG exposure on the incidence of unknown AKI in children. A systematic review following the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines was proposed to search for studies using predefined search terms in the PubMed, EBSCO, and Web of Science data-bases without publication date restrictions. The inclusion criteria are observational study, case study, case report, and case series design; and having provided accurate data for DEG poisoning and AKI diagnosis in children. All authors performed the study screening, data extraction, and data synthesis processes. Consensus was reached by mutual agreement. The data synthesis was conducted according to the DEG and unexplained AKI in children by examining the statistical data using Microsoft Excel 2017 and storing the data using the cloud service of Universitas Islam Indonesia. Of the 115 included studies, 21 met the inclusion criteria, including 2 case-control studies, 1 cross-sectional study, 4 case studies, and 14 case reports. DEG-contaminated paracetamol caused unexplained AKI in children. Other drugs including cough expectorants, antihistamines, and sedatives were administered. Chemicals other than DEG, such as propylene glycol and ethylene glycol, also induce AKI owing to overprescription and unintentional exposure. A recent epidemic of unexplained AKI showed contaminated paracetamol as the poisoning agent regardless of formula.
Graphical abstract.
- Introduction
- Introduction
Acute kidney injury (AKI) in children has attracted considerable attention. One quarter of hospitalized children in low- and middle-income countries suffer from AKI, which is associated with a significant risk of death [1]. Global estimates have revealed a 26% pooled AKI incidence, with 14% of cases being moderate to severe. Moreover, the overall pooled hospital mortality rate of AKI is reportedly 11%, dominated by moderate to severe cases (15%) [1]. Several countries also faced challenges in its incidence and mortality, such as Nigeria, which suffered from a 43.9% mortality rate [2], and the United States of America, which also dealt with a mortality rate of 14.4%, although the incidence was slightly higher at 39.8% [3]. A chemical agent as a primary cause of morbidity and mortality related to unexplained AKI in children, namely diethylene glycol (DEG) and its parent compound, ethylene glycol (EG), which contaminates pharmaceuticals during the manufacturing processes, remains debatable.DEG, a clear, colorless, odorless, and viscous liquid with a sweet taste, is widely used in industrial products, including antifreeze formulations, brake fluids, cosmetics, and lubricants [4]. DEG is used as a solvent for water-insoluble chemicals and drugs because of its tremendous physical properties [5]. Drug diluents, including glycerin and propylene glycol (PG), are inert components in pharmaceutical formulations as vehicles that deliver drug products to the body’s cells. However, they can poison pharmaceutials due to errors in diluent manufacturing. Both glycerin and PG were developed as substitutes for DEG-containing products [6]. Upon ingestion, DEG is promptly absorbed and distributed to well-perfused organs and metabolized to a toxic metabolite in the liver [4]. Further effects include tubular necrosis within the kidneys due to the scattered deposition of calcium oxalate crystals [7].Contemporary reports have indicated that DEG-contaminated pharmaceuticals are a source of poisoning. Moreover, paracetamol, which is mainly used to treat fever in children, is likely to be contaminated with DEG during processing. Several outbreaks of mass paracetamol poisoning in children with unexplained AKI have been reported in Haiti [8], Bangladesh [9], and Nigeria [10]. These outbreaks had a >70% mortality rate in children. Additionally, the combination of paracetamol with other drugs, such as diphenhydramine hydrochloride [10] and promethazine [11], as antihistamine agents was confirmed as a source of contamination. However, neither of the additional antihistamine drugs had a detectable DEG concentration in their formulation. Paracetamol, or a mixture thereof, was not the only source of contamination causing a poisoning. Other drugs, such as cough expectorants [12] and sedative agents [13], reportedly caused DEG contamination in India. Notably, the DEG concentration in the bottle formula has not been holistically evaluated because of fewer diagnostic tools, loss of information, and a lack of resources. The number of unexplained AKI cases was also significantly reduced after the discontinuation of the drug’s manufacture and banning of its distribution.Studies are limited of unexplained AKI induced by DEG-contaminated pharmaceuticals. This systematic review observes the circulating debate regarding the primary source of unexplained drug-induced AKI in children. Other drugs also exist, as paracetamol is not the only contaminant. Therefore, this study aimed to determine whether paracetamol plays a vital role in pediatric drug-induced AKI. This study will help prevent further cases of poisoning related to pharmaceutical products.
- Methods
- Methods
- 1. Study identification and selection
- 1. Study identification and selection
We performed a qualitative analysis to observe the effect of DEG contamination on the incidence of unexplained AKI in children guided by the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) [14]. Eligible studies published in English before December 2022 were retrieved from the PubMed, EBSCO, and Web of Science (WoS) databases. We manually searched the reference list of each included study to identify other relevant studies.Table 1 lists the search terms used in the databases. After removing duplicates, the authors (MLA, HAS, SBM, AAT, HAF, and AAK) screened the titles and abstracts and then evaluated the full text, if applicable, according to the inclusion criteria. Two authors (MDP and EAC) discussed the retrieved papers and agreed upon which should be included. If necessary, another author (SRS) was consulted to reach consensus. The corresponding authors of the retrieved studies were contacted for clarification as needed.We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines to perform a certainty analysis [15].- 2. Inclusion and exclusion criteria
- 2. Inclusion and exclusion criteria
The inclusion criteria focused on the relationship between DEG contamination and the incidence of AKI in children. Clinical signs or laboratory results were approved as DEG contamination; AKI parameters were confirmed by clinical and laboratory results; every observational study design was applicable; review papers, case studies, and case reports that provided actual data were pertinent; proceedings that offered actual data were eligible; and languages other than English were excluded. A case report includes a single case of poisoning, while a case study includes a few cases. Furthermore, editorials, opinions, and perspectives that did not contain actual information or for which full texts were unavailable were excluded. Children were defined as those aged <18 years. From each included study, we obtained data for children and excluded those for adults.- 3. Data coding and quality assessment
- 3. Data coding and quality assessment
Coded and extracted data included author information, incidence year, country, study design, population, contamination sources, outcomes, and summary findings. The authors (MLA, HAS, SBM, AAT, HAF, and AAK) independently conducted the data extraction, discussed the findings, and reached consensus. Another author (SRS) was consulted in cases of doubt and redundancy. The synthesis data provided unique statistical findings, including odds ratios (ORs), relative risks, and confidence intervals (CIs). The corresponding authors were contacted if statistical data were missing from the retrieved papers and the effect size was calculated independently. All extracted and tabulated data were analyzed using Microsoft Excel 2017 and Mendeley and stored in cloud services provided by Universitas Islam Indonesia. The quality assessment was performed using the The ROBINS-E tool (Risk Of Bias In Non-randomized Studies - of Exposures), which encompassed 7 risk of bias domains [16]. Three authors (MLA, MDP, and EAC) independently assessed the risk of bias. All authors reviewed and agreed upon the results. The protocol number for PROSPERO is CRD42023416240.
- Results
- Results
- 1. Studies of effect of DEG in unexplained AKI
- 1. Studies of effect of DEG in unexplained AKI
Two case-control studies reported an outbreak of AKI in children in Haiti [8] and Bangladesh [9]. Both elucidated the history of paracetamol administration and identified DEG in children with unexplained AKI. However, only O’Brien et al. [8] revealed a median 14% (1.2%–19.6%) concentration of DEG from 36 acetaminophen syrup analyses. Other antipyretics [9] and vitamins [8] were contaminated as well, although both studies reported that paracetamol elixir, and syrup were contaminated with DEG. A cross-sectional study by Akuse et al. [10] confirmed an unknown AKI outbreak in Nigeria. Their study elaborated on the history of an ingested mixture of paracetamol (120 mg/5 mL) with diphenhydramine hydrochloride and reported that brand name my pikin (6.25 mg/5 mL) had been contaminated with DEG. Of the 95 children with AKI who consumed several drugs prior to being hospitalized, 60 ingested my pikin syrup, 56 consumed other brands of paracetamol, 23 used herbal medicines, and 6 consumed other brands of teething tonics [10].Five case studies reported DEG in an epidemic of AKI of unknown etiology in Nigeria [17,18], India [12], and South Africa [13] in which total of 47 children were admitted to the hospital [17]. Obebi Cliff-Eribo et al. [18] in Nigeria reported that 7 cases of AKI were correlated with the use of contaminated paracetamol/diphenhydramine chloride and herbal medicines for teething problems. This study revealed that 4 of 7 patients ingested paracetamol and the remaining patients consumed herbal medicine. Singh et al. [12] reported that 17.5% (v/v) of unknown AKI outbreaks in India were caused by DEG-contaminated cough syrups (4.25-mg bromhexine hydrochloride and 27.4-mg pseudoephedrine hydrochloride per 5 mL). Similar to the report by Singh et al. [12] in India, Hari et al. [19] reported that 11 children suffered from unexplained AKI due to the use of DEG-contaminated paracetamol, with a 2.3%–23% w/w concentration. Additionally, 7 children in South Africa were diagnosed with unexplained AKI caused by DEG-contaminated sedative use [13].Most studies have been case reports of a single patient with DEG-induced unexplained AKI [7,20-29]. Some case reports described unintentional EG contamination or DEG-induced oliguria or anuria [21,24,27,29] after the consumption of a DEG-contaminated acetaminophen elixir [26], PG-containing intravenous phenobarbital and pentobarbital for uncontrolled seizures [28], DEG ingestion suicide attempts [7], ergocalciferol-containing high-dose vitamin D dissolved in PG for diagnosing nutritional rickets [22], intentional EG poisoning in baby formula [25], DEG-contaminated fever and cough medication [23], and EG contamination of laxatives [20].High-quality studies of AKI induced by DEG and other glycol-induced AKI are limited. Our study summarized that most studies revealed that the outbreak of unexplained AKI originated from DEG-contaminated pharmaceuticals, including paracetamol, a mixture of paracetamol and diphenhydramine hydrochloride, a mixture of bromhexine HCL and pseudoephedrine HCL, a sedative mixture, adverse drug reactions from the teething tincture, unspecific cough syrups, and unintentional poisoning. Few studies have reported intentional or unintentional EG poisoning and PG-containing high-dose drugs.- 2. DEG-contaminated paracetamol-induced unexplained AKI
- 2. DEG-contaminated paracetamol-induced unexplained AKI
DEG-contaminated paracetamol has primarily been reported. Any DEG-contaminated paracetamol (OR, 18.2; 95% CI, 4.4–75.2; P<0.001) [9] and syrup (OR, 52.7; 95% CI, 15.2–197.2) [8,17] was strongly correlated with unexplained AKI in children. Among 339 children with unknown AKI in the study of Hanif et al. [9], paracetamol elixir ingestion was 13.3 times more likely to cause unexplained AKI compared to 90 cases of identified AKI, any fever medication among 308 children was 22 (12.2–39.3; P<0.001) times more likely to cause unexplained renal failure paralleled to 28 cases of known AKI; the ingestion of DEG-containing branded paracetamol was confirmed among only children with unexplained AKI [9]. That study also revealed that 19 of 69 paracetamol elixirs from different brand products contained DEG as a diluent, while the remaining 50 paracetamol elixirs contained PG as a diluent. However, this study could not obtain elixir samples for further analysis from patients that may have been strongly contaminated with DEG. Therefore, the unexplained AKI outbreak in Bangladesh caused 185 deaths due to AKI and 157 deaths (85%) due to the consumption of an unknown elixir for fever [9].A similar study by Hari et al. [19] reported that 11 children suffered unexplained AKI, 6 of whom had a history of ingesting elixir paracetamol containing 2.3%–22.3% w/w (median: 15.4 w/w). Scalzo [26] corroborated former studies reporting that contaminated elixir paracetamol caused unexplained AKI. However, this case study revealed no DEG or parent compounds in the patient specimen analysis. DEG-contaminated paracetamol was confirmed based on the history of ingestion in outbreak cases in Haiti.O’Brien et al. [8] investigated 109 cases of unexplained renal failure caused by contaminated paracetamol syrup. Paracetamol syrup consumption revealed an OR of 52.7 (95% CI, 15.2–197.2) compared to other drugs, and the remaining drug evaluation confirmed no statistical significance on unexplained AKI, although a laboratory examination revealed that these drugs were also contaminated with DEG. Further statistical analyses were performed when DEG-contaminated drugs demonstrated an OR of 44.2 (95% CI, 13.4–156.6) [8]. The median DEG concentration of 36 bottles was 14.4% (95% CI, 1.2%–19.6%); this was the equivalent of 1.34 mL/kg in 32 children (95% CI, 0.22–4.22 mL/kg) and 1.0 mL/kg in 12 children. The primary source of DEG contamination in this study was a formulation with 50% glycerin, which was comparable to the 12% DEG concentration in the final products [8].Okuonghae et al. [17] reported that 47 pediatric patients died after ingesting paracetamol syrup. However, this study revealed that paracetamol syrups were not the only drugs consumed by the patients; other drugs such as chloroquine, promethazine, and cough syrups were also administered to them. Laboratory examinations revealed no information about the concentrations of these drugs. Thus, predicting paracetamol as a primary source of contamination is impossible because a few drugs were ingested.Akuse et al. [10] revealed that the ingestion of my pikin teething syrups composed of paracetamol (120 mg/5 mL) and diphenhydramine hydrochloride (6.25/5 mL) caused unexplained acute renal failure in 60 of 119 children. Of the 119 children with unknown AKI, 95 consumed drugs before developing the disease, including my pikin by 60, other paracetamol brands by 56, native herbs by 23, and other brands by 6. However, only my pikin ingestion showed statistically significant differences compared to the other drugs. Furthermore, laboratory evaluations of 6 of the 7 residue bottles confirmed 17%–21% DEG contamination by weight in the sample [10]. This study could not reflect the actual exposure to DEG in my pikin because the children also ingested other drugs and the DEG concentration was not examined. The examination of 6 residue bottles was unable to conclude that my pikin was the primary source of the poisoning, as other brands of paracetamol existed.Obebi Cliff-Eribo et al. [18] reported a similar series of 8 patients with unexplained drug-induced AKI. The authors concluded that 4 cases were caused by DEG in my pikin teething mixture, 3 by the herbal teething mixture, and one by dihydroartemisinin. Additionally, diphenhydramine is not the only antihistamine that causes AKI; promethazine has also contributed. All 56 cases of unknown AKI in children in Gambia revealed that a mixture of paracetamol and promethazine syrup was the cause [11].- 3. Contribution of other contaminated drugs to unexplained AKI
- 3. Contribution of other contaminated drugs to unexplained AKI
DEG-contaminated paracetamol is not the only cause of vehicle poisoning. Other drugs such as cough expectorants [12,23], sulfanilamides [30], and sedatives can contribute [13]. Apart from the history of paracetamol contamination, Singh et al. [1] revealed interesting findings that a cough reliever was a primary source of unknown AKI in 36 children. However, their investigation revealed that only one cough syrup, comprising 4.25 mg of bromhexine hydrochloride and 27.4 mg of pseudoephedrine hydrochloride, was contaminated with DEG. Furthermore, one nonspecific cough syrup sample was contaminated with EG, one paracetamol syrup sample was negative for any contamination, and 4 drugs, including paracetamol and cough syrup from different manufacturers, were negative for DEG and EG [12]. One sample in the cough syrup investigation was insufficient to conclude that the drug was vehicle-contaminated. Additionally, the paracetamol drugs that were reportedly contaminated tested negative for harmful substances. Therefore, this study does not address the relationship between exposure and outcomes. Jain et al. [23] confirmed that cough syrup use was associated with unexplained AKI in a boy with fever and cough. This study did not reveal the drug’s ingredients; however, we assumed that the cough syrup contained paracetamol and expectorants.One study recognized sedative agents as the cause of death of 7 children [13]. They investigated that 2 brand name sedatives (Pronap and Plaxim) substituted PG for DEG during the manufacturing process. All sedative drugs used in their study were obtained from parent interviews. However, the data were limited due to a lacking further examination of DEG concentrations and statistical analysis, making this study prone to bias.PG overconcentration and EG contamination are common medications, such as antiseizure agents [28], vitamin D treatments [22], and laxative agents [20]. One study reported the adverse drug effects of high-dose intravenous phenobarbital and pentobarbital for uncontrolled seizures after the failure to prescribe standard medicine for seizures. During the 8-day treatment, 6.53 g/day of pentobarbital and 6.70 g/day of phenobarbital were infused, comparable to the ingestion of 90.3 g/day of PG.Another study revealed that oral vitamin D contains 100,000 international units (IU) of ergocalciferol every 2 hours for a total of 600,000 IU over 12 hours, which induces unwanted AKI [22]. Ergocalciferol contains 103.6 g/100 mL of PG, and 600,000 IU is equivalent to 75 mL of the solution or 77.7 g of PG. Another study by Aly et al. [20] revealed that an EG-contaminated laxative was the primary cause of AKIin a boy with a complicated congenital disease. Based on the renal biopsy evaluation, the drugs were suspected to be contaminated with EG. Several studies of PG and EG have been performed on a single patient without a standardized methodology. The patients had nutritional and congenital comorbidities that probably affected their homeostatic functions during treatment. Thus, knowledge of the adverse effects of PG during treatment is limited due to its common use as a vehicle to enhance drug solubility.- 4. Other factors related to unexplained AKI
- 4. Other factors related to unexplained AKI
Several cases of unintentional DEG toxicity and parent compound-induced unwanted AKI have been confirmed, and some were intentionally poisoned. Vale et al. [29] reported the case of a child who accidentally ingested 100 mL of EG, comparable to 26.9 g of EG and 73 mg of oxalate, on dialysis. Similarly, Walker et al. [27] fortuitously ingested nonspecific drugs suspected of containing EG. They postulated that the poisoning caused kidney enlargement, and a biopsy revealed crystal oxalate. Another study by Harry et al. [21] confirmed unwanted ingested antifreeze contained 41% of EG. Unwanted AKI was diagnosed based on laboratory examinations, such as metabolic acidosis with an anion gap of 29 mmol/L, an osmolar gap of 50 mOsm/L, and a chromatography result of 3.1 g/L. The last unintentional case was reported by Brophy et al. [24], who revealed accidental poisoning combined with DEG using tetraethylene glycol and penta-ethylene glycol. They reported no metabolic acidosis or anion and osmolar gaps; otherwise, chromatography revealed 1.7-mg/dL DEG. Most cases of unintentional poisoning have been reported in single patients without robust scientific analyses and statistical methodologies. Moreover, one study did not report the EG concentration in the specimens, resulting in bias of the reporting results.Two other intentional cases of DEG poisoning were suicide [7] and assassination [25]. Gupta et al. [7] revealed that DEG was used in suicide attempts by the ingestion of brake fluid containing DEG as the primary constituent. A laboratory evaluation revealed an osmolar gap, and chromatography revealed a DEG concentration <1 mg/dL. Greenky et al. [25] described a case of the intention to kill an 8-day-old neonate by the addition of DEG to milk formula. The laboratory findings indicated signs of poisoning, including metabolic acidosis, anion and osmolar gaps, and the presence of oxalate. However, the DEG concentration was not determined. Two described cases of unintentional and intentional poisoning included limited data. Thus, we could not reflect on the exposure and outcome assessments in this case. Furthermore, this finding indicates that DEG and EG are commonly neglected owing to their accessibility and affordability in the market.
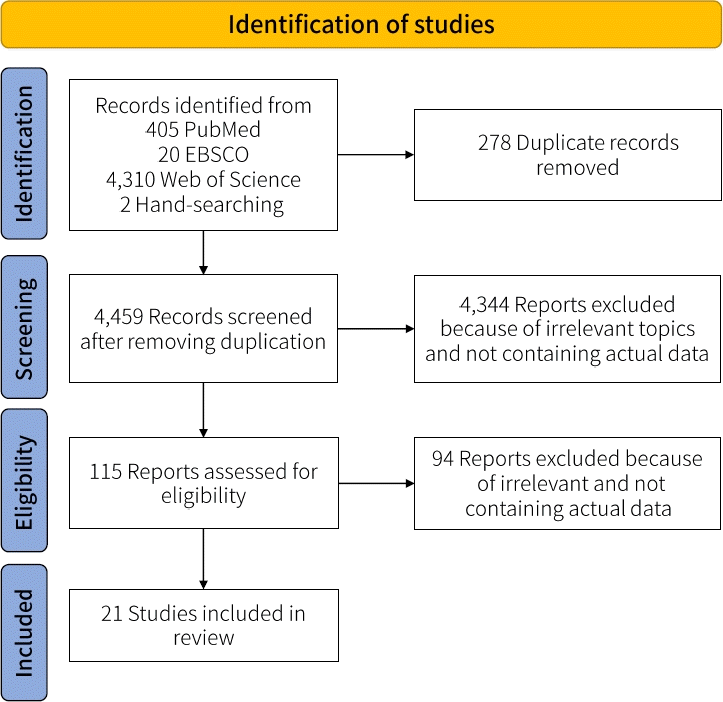
Fig. 1 illustrates the study search and identification process. We used the search terms in Table 1 to search the PubMed, EBSCO, and WoS databases. The electronic and manual searches retrieved a total of 4,737 papers. The title and abstract screening process and removal of duplicates yielded 4,459 papers. Of them, 4,344 were excluded due to irrelevance, leaving 115 eligible papers. Another 94 papers were eliminated for not including actual data. Thus, the analysis included 21 papers: 2 case-control studies, 1 cross-sectional study, 4 case studies, and 14 case reports (Supplementary Table 1).
- Discussion
- Discussion
This qualitative review summarizes that paracetamol and other drugs become contaminated with DEG during the manufacturing process. In addition to DEG, EG as the parent compound andPG have also been reported. However, EG is mainly used intentionally or unintentionally, and PG poisoning originates from high-dose drugs. Despite the limited number of studies with low evidence, unexplained AKI in children is primarily caused by DEG-contaminated paracetamol.Contaminated paracetamol, regardless of formulation as an elixir, syrup, or mixture with antihistamines, caused unexplained AKI in children. Our study revealed that paracetamol, PG, and glycerin are adulterants during the pharmaceutical process that are accidentally replaced by inexpensive DEG. The reason for this proposed by Alkahtani et al. [31] is that DEG has been neglected in the drug industry. They hypothesized that the financial profits of pharmaceuticals are enormous;hence, small manufacturers maximize their profits by using cheaper agents during production. Additionally, counterfeit drugs sometimes contain no or minimal concentrations of active ingredients compared with diluents and active compounds [31]. However, the evidence of foreign manufacturers, distributors, or importer-substituted diluents with DEG remains unclear. 8-11,19) Moreover, the affordability of EG and DEG in the local Nigerian market was a source of unexplained AKI epidemics in children [9].Apart from paracetamol, other drugs contain DEG as a contaminant. A recent epidemic in Indonesia confirmed that almost all 103 DEG- and EG-contaminated drug syrups were paracetamol. In addition to paracetamol and other antipyretics, cough syrups under different brand names were also contaminated with DEG [32]. Cough syrups are the most often contaminated, affecting hundreds of children in similar cases in Gambia [33]. No further information was available on why the cough syrup was neglected during manufacturing. This question is similar to the hypothesis proposed by Alkahtani et al. [31] Identical cases involving different antifungal and sedative agents have also been reported. These cases appeared a few decades ago when the monitoring of surveillance drugs on the market was limited; thus, the remaining DEG were accessible and affordable. Our study revealed that an overdose of drugs, including PG, causes unknown AKI in children. PG is a less toxic agent than DEG, but the overuse of drugs that contain PG also contributes to unknown AKI [22,28]. Thus, discontinuation of the drug prescription improved the patient’s condition.DEG and its derivative EG have been intentionally or unintentionally misused in addition to being neglected during pharmaceutical processing. DEG is commonly used as an antifreeze chemical; thus, a broken container presents a poisoning risk when grabbed by an infant. DEG is not commonly used for suicide attempts, such as the hydrogen sulfide trend in automobiles in 2006–2010 [34-36], but this chemical should be considered in cases of intentional suicide attempts [36].This study had several limitations. Studies of the effects of DEG poisoning on the incidence of unexplained AKI in children are limited. The few studies that have used case-control and cross-sectional designs involved suspected sampling bias, while the remaining case studies and case reports may have had inaccurate methodologies. Meta-analysis–related issues have been caused by the limited observational studies in the pediatric population. Several cases of DEG poisoning in adults [37-41] were excluded from this study.Individual studies conducted in similar countries have reported similar results. A few studies also lacked AKI and DEG poisoning diagnostic data; thus, they were excluded from the further analysis. Our study revealed that low-quality papers were included in the GRADE summary (Table 2) due to failure to control for confounding factors, self-reported poisoning, statistical data limitations, and bias risk, which confirmed the weakness of the included studies (Table 3).In conclusion, DEG-contaminated paracetamol causes AKI in children. Unfortunately, this antipyretic is not the only involved drug; cough expectorants, antihistamines, and sedative agents are also involved. In addition to DEG, the corresponding chemical, EG, also reportedly causes unintentional poisoning in children.In comparison, PG contributes to unexplained AKI due to overdose prescriptions for the treatment of underlying diseases. The negligible use of DEG during the manufacturing process poses concerns about toxicity and the need to monitor drug production. In addition to these limitations, more studies using robust methods are essential to provide accurate data and observe children’s outcomes after DEG exposure.
Supplementary materials
Supplementary materials
Supplementary Table 1 can be found via https://doi.org/10.3345/cep.2023.01375.Supplementary Table 1. Included studies reporting the contribution of DEG and parent compound contamination of pharmaceuticals on unexplained AKI in childrencep-2023-01039-Supplementary-Table-1.pdf
- Footnotes
-
Conflicts of interest No potential conflict of interest re levant to this article was reported.
Funding This study was supported by Faculty of Medicine, Universitas Islam Indonesia through Systematic Review Grant 2023.
-
Table 1.
Study search strategy and terms
Table 2.
GRADE summary for certainty
Table 3.
Risk of bias of the included observational studies
| Study | Study design | Sample size | Confounding | Measurement of exposure | Selection of participants | Postexposure intervention | Missing data | Measurement of the outcome | Selection reported of result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Hanif et al. [9] | Case control | 339 Children | High | High | Some concern | High | Some concern | High | Some concern | High |
| O’brien et al. [8] | Case control | 109 Children | High | High | Some concern | High | Some concern | High | Some concern | High |
| Akuse et al. [10] | Cross-sectional | 119 Children | High | High | Some concern | High | Some concern | High | Some concern | High |
- References
- 1. Meena J, Mathew G, Kumar J, Chanchlani R. Incidence of acute kidney injury in hospitalized children: a meta-analysis. Pediatrics 2023;151:e2022058823.
[Article] [PubMed]2. Olowu WA, Adelusola KA. Pediatric acute renal failure in southwestern Nigeria. Kidney Int 2004;66:1541–8.
[Article] [PubMed]3. Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 2014;9:2036–43.
[Article] [PubMed] [PMC]4. Schep LJ, Slaughter RJ, Temple WA, Beasley DMG. Diethylene glycol poisoning. Clin Toxicol 2009;47:525–35.
[Article]5. Schier JG, Barr DB, Li Z, Wolkin AF, Baker SE, Lewis LS, et al. Diethylene glycol in health products sold over-the-counter and imported from Asian countries. J Med Toxicol 2011;7:33–8.
[Article] [PubMed] [PMC]6. Schier JG, Rubin CS, Miller D, Barr D, McGeehin MA. Medication-associated diethylene glycol mass poisoning: A review and discussion on the origin of contamination. J Public Health Policy 2009;30:127–43.
[Article] [PubMed]7. Gupta A, Diaz FJ, Lal A, Sung L, Aaron CK. Basal ganglion hemorrhage as delayed complication of diethylene glycol ingestion. Am J Forensic Med Pathol 2017;38:39–42.
[Article] [PubMed]8. O’Brien KL, Selanikio JD, Hecdivert C, Placide MF, Louis M, Barr DB, et al. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. JAMA 1998;279:1175–80.
[Article] [PubMed]9. Hanif M, Mobarak MR, Ronan A, Rahman D, Donovan john J, Bennish ML. Fatal renal failure caused by diethylene glycol in paracetamol elixir: the Bangladesh epidemic. BMJ 1995;311:88.
[Article] [PubMed] [PMC]10. Akuse RM, Eke FU, Ademola AD, Fajolu IB, Gbelee HO, Ihe jiahi U, et al. Diagnosing renal failure due to diethylene glycol in children in a resource-constrained setting. Pediatr Nephrol 2012;27:1021–8.
[Article] [PubMed]11. Bastani P, Jammeh A, Lamar F, Malenfant JH, Adewuyi P, Cavanaugh AM, et al. Acute kidney injury among children likely associated with diethylene glycol–contaminated medications — The Gambia, June–September 2022. MMWR Morb Mortal Wkly Rep 2023;72:217–22.
[Article] [PubMed] [PMC]12. Singh J, Dutta AK, Khare S, Dubey NK, Harit AK, Jain NK, et al. Diethylene glycol poisoning in Gurgaon,India, 1998. Bull World Health Organ 2001;79:88–95.
[PubMed] [PMC]14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
[Article] [PubMed] [PMC]15. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94.
[Article] [PubMed]16. Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R, et al. Risk of bias in non-randomized studies-of exposure (ROBINS-E). Launch version, 1 June 2022 ROBINS-E Development Group [Internet]. riskofbias.info 2022;[cited 2023 May 20]. Available from: https://www.riskofbias.info/welcome/robins-e-tool.17. Okuonghae HO, Ighogboja IS, Lawson JO, Nwana EJC. Diethylene glycol poisoning in Nigerian children. Ann Trop Paediatr 1992;12:235–8.
[Article] [PubMed]18. Obebi Cliff-Eribo K, Sammons H, Star K, Ralph Edwards I, Osakwe A, Choonara I. Adverse drug reactions in Nigerian children: a retrospective review of reports submitted to the Nigerian Pharmacovigilance Centre from 2005 to 2012. Paediatr Int Child Health 2016;9047(January 2017): 1–5.19. Hari P, Jain Y, Kabra SK. Fatal encephalopathy and renal failure caused by diethylene glycol poisoning. J Trop Pediatr 2006;52:442–4.
[Article] [PubMed]20. Aly R, Acharya R, Zeng X, Upadhyay K. Polyethylene glycol 3350 crystal nephropathy in association with glomerular mesangial immunoglobin a deposition. J Med Cases 2022;13:475–81.
[Article] [PubMed] [PMC]21. Harry P, Jobard E, Briand M, Caubet A, Turcant A. Ethylene glycol poisoning in a child treated with 4-methylpyrazole. Pediatrics 1998;102:3–5.
[Article]22. Jelley DH, Ali DZM, Condren M. Acute renal failure secondary to inadvertent propylene glycol overdose with single-day high-dose vitamin d (stosstherapy). Case Rep Endocrinol 2019;2019:1482727.
[Article] [PubMed] [PMC]23. Jain R, Randev S, Kumar P, Guglani V. Acute kidney injury and encephalopathy in a child: diethylene glycol poisoning. Indian J Pediatr 2021;88:194–5.
[Article] [PubMed]24. Brophy PD, Tenenbein M, Gardner J, Bunchman TE, Smoyer WE. Childhood diethylene glycol poisoning treated with alcohol dehydrogenase inhibitor fomepizole and hemodialysis. Am J Kidney Dis 2000;35:958–62.
[Article] [PubMed]25. Greenky D, Ball TT, Murray B. A neonate with metabolic acidosis: intentional ethylene glycol poisoning. Am J Emerg Med 2021;44:478.e5–478.e6.
[Article] [PubMed]26. Scalzo AJ. Diethylene glycol toxicity revisited: the 1996 Haitian epidemic. J Toxicol Clin Toxicol 1996;34:513–6.
[Article] [PubMed]27. Walker JT, Keller MS, Katz SM. Computed tomographic and sonographic findings in acute ethylene glycol poisoning. J Ultrasound Med 1983;2:429–31.
[Article] [PubMed]28. Yorgin PD, Theodorou AA, Al-Uzri A, Davenport K, BoyerHassen LV, Johnson MI. Propylene glycol-induced proximal renal tubular cell injury. Am J Kidney Dis 1997;30:134–9.
[Article] [PubMed]29. Vale J, Bluett NH, Widdop B. Ethylene glycol poisoning. Postgrad Med J 1976;52:598–602.
[Article] [PubMed] [PMC]30. Geiling EMK, Cannon PR. Pathologic effects of elixir of sulfanilamide (diethylene glycol) poisoning. A clinical and experimental correlation: final report. JAMA 1938;111:919–26.
[Article]31. Alkahtani S, Sammons H, Choonara I. Epidemics of acute renal failure in children (diethylene glycol toxicity). Arch Dis Child 2010;95:1062–4.
[Article] [PubMed]32. Fikri E, Firmansyah YW. A case report of contamination and toxicity of ethylene glycol and diethylene glycol on drugs in Indonesia. Environ Ecol Res 2023;11:378–84.
[Article]33. Chopra H, Attia MS, Badshah SF, Dhama K, Emran TB. Cough syrups: silent killer of Gambian children. Int J Surg 2023;109:150–2.
[Article] [PubMed] [PMC]34. Morii D, Miyagatani Y, Nakamae N, Murao M, Taniyama K. Japanese experience of hydrogen sulfide: the suicide craze in 2008. J Occup Med Toxicol 2010;5:28.
[Article] [PubMed] [PMC]35. Ruder J, Ward J, Taylor S, Higgins T, Haan J. Hydrogen sulfide suicide: a new trend and threat to healthcare providers. J Burn Care Res 2015;36:e23–5.
[PubMed]36. Centers for Disease Control and Prevention (CDC). Chemical suicide in automobiles-six states, 2006-2010. MMWR Morb Mortal Wkly Rep 2011;60:1213–5.
[PubMed]37. Ballentine C. Sulfanilamide disaster. U.S. FDA consumer magazine [Internet]. Silver Spring (MD), U.S. Food and Drug Administration. 1981;[cited 2023 June 15]. Available from: https://www.fda.gov/media/110479/download?attachment.38. Cantarel M, Fort J, Camps J, Sans M, Piera L. Acute intoxication due to topical application of diethylene glycol. Ann Intern Med 1987;106:478–9.
[Article]39. Ferrari LA, Giannuzzi L. Clinical parameters, postmortem analysis and estimation of lethal dose in victims of a massive intoxication with diethylene glycol. Forensic SciInt 2005;153:45–51.
[Article]

 About
About Browse articles
Browse articles For contributors
For contributors