All issues > Volume 67(11); 2024
Efficacies of different treatment strategies for infants hospitalized with acute bronchiolitis
- Corresponding author: Man Yong Han, MD. Department of Pediatrics, CHA Bundang Medical Center, CHA University School of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam 13496, Korea Email: drmesh@gmail.comCo-corresponding author Eun Kyo Ha, MD. Department of Pediatrics, Hallym University Kangnam Sacred Hospital, 12 Beodeunaru-ro 7-gil, Yeongdeungpo-gu, Seoul 07247, Korea Email: dmsry1@gmail.com
- Received December 6, 2023 Revised May 20, 2024 Accepted May 31, 2024
- Abstract
-
- Background
- Background
- Acute bronchiolitis is a common cause of hospitalization during infancy that carries significant morbidity and mortality rates.
- Purpose
- Purpose
- This study compared the efficacy of different treatment modalities for infants with bronchiolitis in terms of hospital stay and clinical severity scores.
- Methods
- Methods
- The PubMed database was searched for relevant studies. Eligibility criteria included double-blind randomized controlled trial design, assessment of the effect of treatment on bronchiolitis in infants under 2 years of age, and publication in English from inception through July 31, 2020. The primary efficacy outcome was the length of hospital stay, while the secondary outcome was the clinical severity score. The standardized treatment effect and standard error of the effect size were calculated.
- Results
- Results
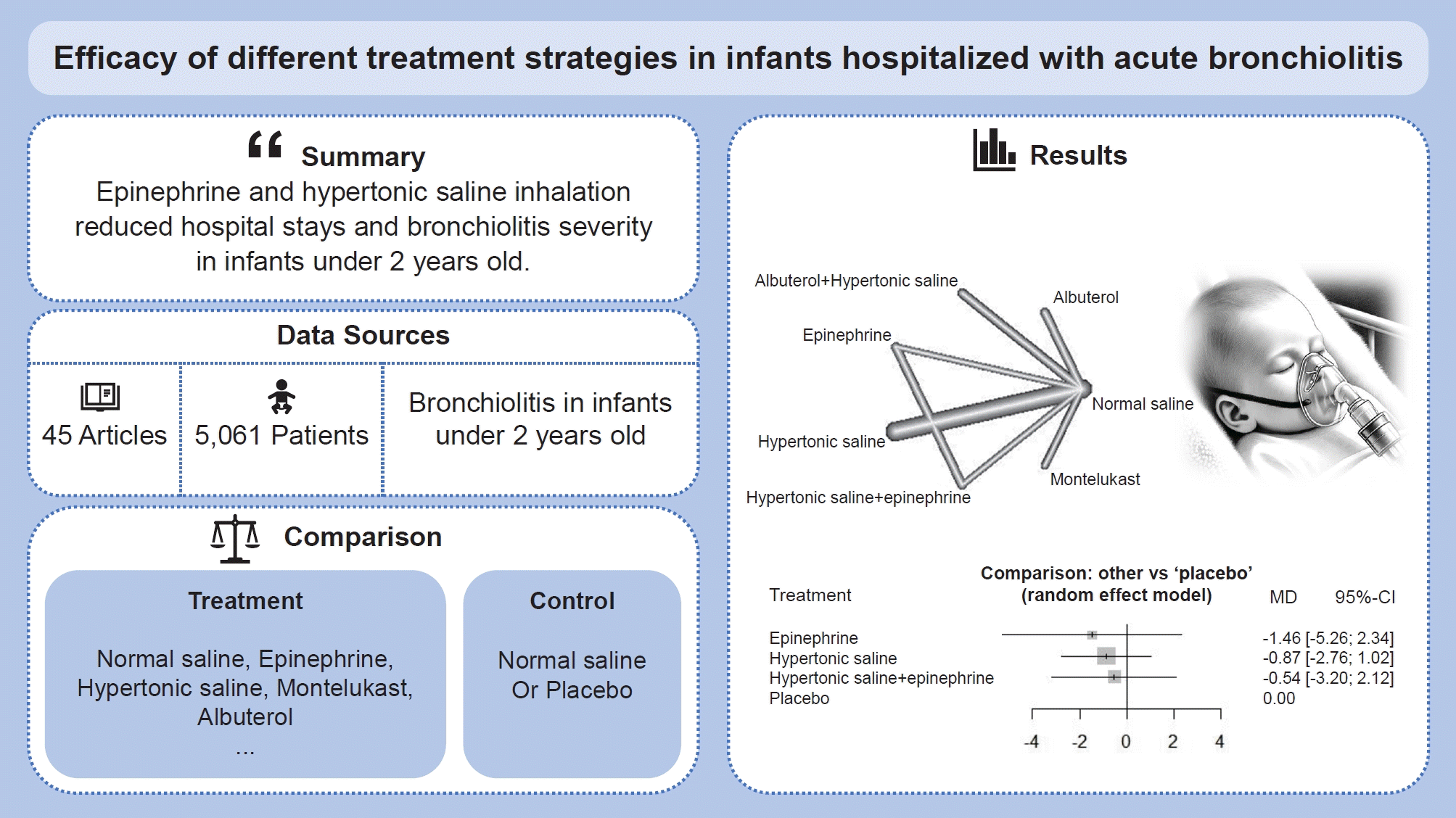
- We identified 45 randomized controlled trials of 24 pairwise comparisons. These 45 trials included 5,061 participants and investigated 13 types of interventions (12 active, 1 placebo). Inhalation therapy with epinephrine (standard mean difference [SMD], -0.41; 95% confidence interval [CI], -0.8 to -0.03) and hypertonic saline (SMD, -0.29; 95% CI, -0.55 to -0.03) reduced the length of hospital stay compared with normal saline. Hypertonic saline was the most effective at improving the clinical severity score (SMD, -0.52; 95% CI, -0.95 to -0.10).
- Conclusion
- Conclusion
- Inhalation therapy with epinephrine and hypertonic saline reduced the length of hospital stay and the clinical severity of bronchiolitis among infants under 2 years of age.
Graphical abstract. MD, mean difference; CI, confidence interval.
- Introduction
- Introduction
Acute bronchiolitis is the leading cause of morbidity among young children and infants under 2 years old world wide [1-4]. Respiratory syncytial virus (RSV) is the major etiologic agent for bronchiolitis [1,2]. Despite bronchiolitis having a significant impact on public health, its clinical management strategies vary considerably [3]. Supportive care including fluid intake, ensuring oxygen exchange, and feeding, has been the standard treatment for acute bronchiolitis [4]. Nevertheless, several studies have sought to find the most appropriate treatment for this condition [5].Randomized controlled trials (RCTs) conducted to examine the efficacy of corticosteroids, bronchodilators, and other treatments, such as epinephrine, hypertonic saline, ribavirin, and rhDNase [6-10]. None of these, however, was found to significantly affect the rate of clinical improvement. A meta-analysis of studies assessing bronchiolitis treatment modalities might enable more practical and methodological approaches. To date, more than 20 meta-analyses have been conducted to evaluate the efficacy of epinephrine, bronchodilators, and hypertonic saline in the treatment of bronchiolitis [11,12]. These conventional meta-analyses involved only pairwise comparisons, making it hard to compare multiple treatment modalities or to assess the interactions of multiple therapies [13]. However, meta-analyses based on both direct and indirect comparisons for the same outcome may enable assessment of the relative efficacy of several treatments [13,14].The present study was designed to integrate evidence of interventions currently used to manage bronchiolitis in infants, by comparing the effectiveness of these modalities on length of hospital stay and Wang score representing clinical severity [15]. The PubMed database was searched to identify RCTs comparing therapeutic regimens in infants with bronchiolitis, followed by a network meta-analysis to determine effective treatments for this condition.
- Methods
- Methods
- 1. Information sources and search strategy
- 1. Information sources and search strategy
PubMed was searched for relevant articles in English published through 31 July 2020. The main search terms were “bronchiolitis,” “wheezing,” “respiratory syncytial virus,” and “RSV.”Included studies were restricted to “clinical trials” and “randomized controlled trials.” The titles and abstracts of studies identified by the initial search were reviewed to determine their relevance. The bibliographies of selected studies were also reviewed.- 2. Eligibility criteria
- 2. Eligibility criteria
Studies were included if their populations consisted of inpatients aged <24 months who were hospitalized with bronchiolitis, wheezing, and/or RSV infection; if these studies compared 2 or more types of treatment; and if they reported Wang scores and/or length of hospital stay. Retrospective and long/short term follow-up studies were excluded, as were studies in populations with other underlying diseases, such as cystic fibrosis, chronic pulmonary diseases, congenital heart disease, and immunodeficiency. Also excluded were trials that examined other outcomes, including gastrointestinal symptoms, pulmonary function, oxygen saturation, hospitalization rate, hypothalamic-pituitary-adrenal axis function, serum cytokine and chemokine concentrations, and heart rate. In addition, trials that examined the effects of herbs and Chinese traditional medicines were excluded.- 3. Study selection
- 3. Study selection
Two investigators (MYH and HJ)independently screened articles by title and abstract according to the inclusion criteria. These investigators subsequently read the full text of selected articles. Studies were excluded if they (1) did not report data on length of hospital stay or average Wang score after hospitalization, (2) did not include statistical comparisons, or (3) were performed to assess chest physiotherapy. In addition, studies were excluded if they were the only studies to assess certain treatments. The mean and standard deviation for the 2 primary outcomes; length of hospital stay and Wang scores, were determined. Data unavailable from the original publications were calculated using presented results. Trial details (e.g., study ID, first author, journal, publication year, countries, patient characteristics, treatments, and outcomes) were recorded on a spreadsheet.- 4. Quality assessment and risk of bias
- 4. Quality assessment and risk of bias
Overall risk of bias was evaluated on each included study using the revised Cochrane risk of bias tool for randomized clinical trials [16]. This tool consists of 5 domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. Results were scored as low, high, or some concerns.- 5. Data synthesis and statistical analysis
- 5. Data synthesis and statistical analysis
The network meta-analysis was performed using a random effects model with a frequentist approach.All statistical analyses were conducted using the netmeta package (version 1.2-1) R version 4.0.2. For data synthesis in the analysis, the standardized treatment effect (TE) and standard error of effect size were calculated using EffectSizeCalculator (CEM, Ushaw college, Durham, England). Normal saline was distinguished from placebo because normal saline may have active effects on bronchiolitis [9]. Normal saline was defined as the common comparator in this study. Heterogeneity was assessed using the τ2 measure. I2 statistics are commonly used to measure heterogeneity in meta-analyses, with I2 ranging from 0% to 100%. These statistics are easy to interpret and do not vary with the number of studies [17]. However, the I2 value can increase with the number of patients included in the meta-analysis. Because I2 statistics for heterogeneity can increase with the number of patients included in the meta-analysis, global statistical heterogeneity was evaluated across all comparisons with the τ2 measure using the netmeta statistical package. Each treatment was ranked by P score, which is analogous to the surface under the cumulative ranking curve in Bayesian network meta-analysis with a higher score indicating a greater probability of being beneficial. Forest plots were calculated to visualize the directionality and confidence intervals (CIs) of each TE. Network diagrams were plotted to show the overall structure of the treatment network for each outcome.
- Results
- Results
- 1. Study selection
- 1. Study selection
Fig. 1 shows the flow of study selection. The initial database search identified 51,279 articles. Of the 929 studies eligible for title and abstract screening, 698 did not meet our inclusion criteria and were excluded, as were 25 articles not available in full text version. Overall, 45 articles with a total of 5,061 patients were included in the network meta-analysis.- 2. Study characteristics
- 2. Study characteristics
Table 1 shows the number of included studies by treatment comparison, and Table 2 shows the detailed characteristics of the included studies. The included studies reported 24 treatment comparisons. Most involved hypertonic saline as an intervention, with 10 studies comparing hypertonic saline with normal saline. These 45 trials were performed in numerous countries and evaluated the effects of 12 active treatments, including normal saline, epinephrine, magnesium sulfate, hypertonic saline, montelukast, ribavirin, systemic steroids (dexamethasone and prednisolone), furosemide, antibiotics, ipratropium bromide, albuterol, and rhDNase. All included studies were conducted in the inpatient setting. Ten studies reported Wang scores.- 3. Risk of bias
- 3. Risk of bias
Eighteen studies were categorized as possibly having risk of overall bias based on outcome measurements (Table 3). These 18 studies did not clearly describe the criteria for measuring outcomes. Five studies were categorized as having a high risk for overall bias, notably for the randomization process and outcome measurements. Blinding of participants and staff was not feasible owing to the nature of the intervention, thus limiting protection against performance and detection bias. The funnel plots are shown in Supplementary Fig. 1.- 4. Network meta-analysis
- 4. Network meta-analysis
Figs. 2A and 3A display the efficacy estimated using network meta-analysis of indirect comparisons of each treatment with that of normal saline, as measured by duration of hospitalization and averageWang score, respectively. Epinephrine (standardized mean difference [SMD], -0.41; 95% CI, -0.8 to -0.03) and hypertonic saline (SMD, -0.29; 95% CI -0.55 to -0.03) were the most effective treatments for decreasing duration of hospital stay. Compared with normal saline, epinephrine and hypertonic saline reduce 0.41 days and 0.29 days of hospitalization, respectively.The estimated heterogeneity (τ2) was 0.147 for duration of hospital stay and 0.233 for Wang score, which represented a moderate degree of heterogeneity. Inconsistencies of comparisons are presented in Supplementary Fig. 2.For clinical scores, 10 studies had useable data. Hypertonic saline is considered to have an effect on improving Wang score (SMD, -0.52; 95% CI-0.95 to -0.1), which means reducing clinical severity. Forest plots presenting all comparisons based on both direct and indirect evidence for duration of hospitalization and Wang scores are shown in Supplementary Fig. 3A and B, respectively. The area of each box surrounding the estimate was proportional to the weighting in the meta-analysis.A network graph (Figs. 2B and 3B) showed that the network is a sparsely connected intersection of 2 ‘star’ networks, the principal comparators being placebo and normal saline for length of hospital stay, and normal saline for Wang score. The thickness of each edge corresponds to the number of trials and represents the precision of the estimate. Montelukast had the highest probability of being the best treatment for bronchiolitis (P score=0.833), followed by azithromycin (P score=0.792), systemic steroids (P score=0.682), and epinephrine (P score=0.675) (Table 4).
- Discussion
- Discussion
This systematic review and meta-analysis comparing different therapy strategies in infants hospitalized for bronchiolitis included 45 RCTs and 5,061 patients. Based on both direct and indirect evidence, this study compared the therapeutic effects of 12 types of intervention on length of hospital stay and Wang score. Of these 12 treatments, epinephrine and hypertonic saline were found to be most beneficial in reducing length of hospital stay, and hypertonic saline was found to help improve Wang score. Twenty RCTs compared epinephrine, hypertonic saline, normal saline and placebo, with 8 of these studies yielding results similar to ours [18-25].Among the infants with bronchiolitis, approximately 1%–2% of whom require hospitalization [26]. In the United States, it is reported at 18% of all hospitalizations in children under 2 years old in 2016. The proportion of infants with bronchiolitis requiring intensive care unit admission has previously been accounted for 6% to 22% [27]. Based on our results, epinephrine and hypertonic saline can be considered as effective treatment options.Nebulized epinephrine is generally used to treat significant respiratory distress in children. Epinephrine possesses both alpha-adrenergic and beta-adrenergic properties. Its alpha-adrenergic properties reduce airway edema and vasoconstriction [28,29]. Thus, epinephrine can be theoretically possible to be a benefit of managing bronchiolitis. A Cochrane review [28] demonstrated that epinephrine significantly decrease the risk of hospitalization In addition,the combination of epinephrine and hypertonic saline has been reported to reduce the length of hospital stay [10]. Although several studies found that epinephrine improved clinical outcomes in outpatients [7,30,31]. other studies have yielded conflicting results [32]. Hypertonic saline also can improve mucociliary clearance and reduce edema of the airway through absorbing water from the mucosa and submucosa [4]. Two meta-analysis studies [23,33] derived that nebulized hypertonic saline has the potential to reduce the risk of hospitalization in infants with bronchiolitis. These properties would be helpful to infants with bronchiolitis. We also calculated the P score to identify the relative therapeutic superiority of interventions. Although montelukast had the highest P score, it should be noted that CIs must also be taken into account to determine the best treatment [34,35]. There are only 2 trials comparing the effect of montelukast and placebo for the length of hospital stay, and each trial suggested contradictory results. In this context, we considered both the P score and forest plot. Consequently, epinephrine and hypertonic saline are regarded to have an effect to reduce the length of hospital stay. The strength of our study includes comprehensive integration of individual RCT on effective treatment for bronchiolitis in infants. Contrary to standard meta-analysis which can only statistically combine 2 interventions, the methodology used in this study enabled indirect comparisons of all pairs of treatments for the same outcomes. Furthermore, we assessed relative superiority between treatments that have not been directly compared in RCT. This result could provide research insights to choose the best treatments in managing bronchiolitis.There are several limitations in the present study. First, we selected 2 primary outcomes, the length of hospital stays and the Wang score, due to the lack of sufficient other outcome data. However, this approach is less precise because of high heterogeneity. Since the measurement times for the Wang score varied by trial, we decided to use the Wang score from the second day after enrollment. In this process, it was difficult to obtain sufficient data for our analysis, which may be the reason for the high heterogeneity. There are many different outcomes reflecting clinical improvement in bronchiolitis, such as the respiratory distress assessment instrument score, oxygen saturation, and respiratory rate. Thus, different results may be derived depending on the outcomes selected. Second, several included trials did not adequately describe the randomization process, outcome measurement sequence, or deviations from intended interventions. These limitations in the individual included trials could lead to potential bias in this study. Third, we did not account for variations within the same treatment—specifically, we were unable to thoroughly compare differences in concentrations of hypertonic saline or the effects of epinephrine versus racemic epinephrine. Such variations could result in outcome deviations across different doses.In summary, epinephrine and hypertonic saline can be beneficial to reduce length of hospital stay and epinephrine is considered to improve Wang score in bronchiolitis in infants under 2 years old.Further studies need for validating the effectiveness of treatment listed in this study.
Supplementary materials
Supplementary materials
Supplementary Figs. 1-3 can be found via https://doi.org/10.3345/cep.2023.01676.Supplementary Fig. 1. (A) Funnel plot: length of hospital stays. (B) Funnel plot: Wang score.cep-2023-01676-Supplementary-Fig-1.pdfSupplementary Fig. 2. Heat plot based on random-effects model.cep-2023-01676-Supplementary-Fig-2.pdfSupplementary Fig. 3. (A) Forest plot displaying all comparisons for which there is both direct and indirect evidence: length of hospital stays. (B) Forest plot displaying all comparisons for which there is both direct and indirect evidence: Wang Score. CI, confidence interval.cep-2023-01676-Supplementary-Fig-3.pdf
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article was reported.
Funding This study was supported by grant HR22C1605-C1 from the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea.
Author contribution Conceptualization: MYH; Data curation: MYH; Formal analysis: HJ, HH; Funding acquisition: MYH; Methodology: MYH; Project administration: HJ, DP; Visualization: HJ; Writing- original draft: HJ; Writingreview & editing: HJ, EKH, JHK, JS, HB, YHS, HMJ
-
Fig. 1.
Flow diagram of study selection process. RSV, respiratory syncytial virus; LOS, length of stay.
Fig. 2.
(A) Forest plot of lengths of hospital stay. (B) Network graph of lengths of hospital stay. The nodes indicate treatments, while the interconnecting lines (edges) indicate direct comparisons. The thickness of each edge is proportional to the precision of the compared estimate. MD, mean difference; CI, confidence interval.
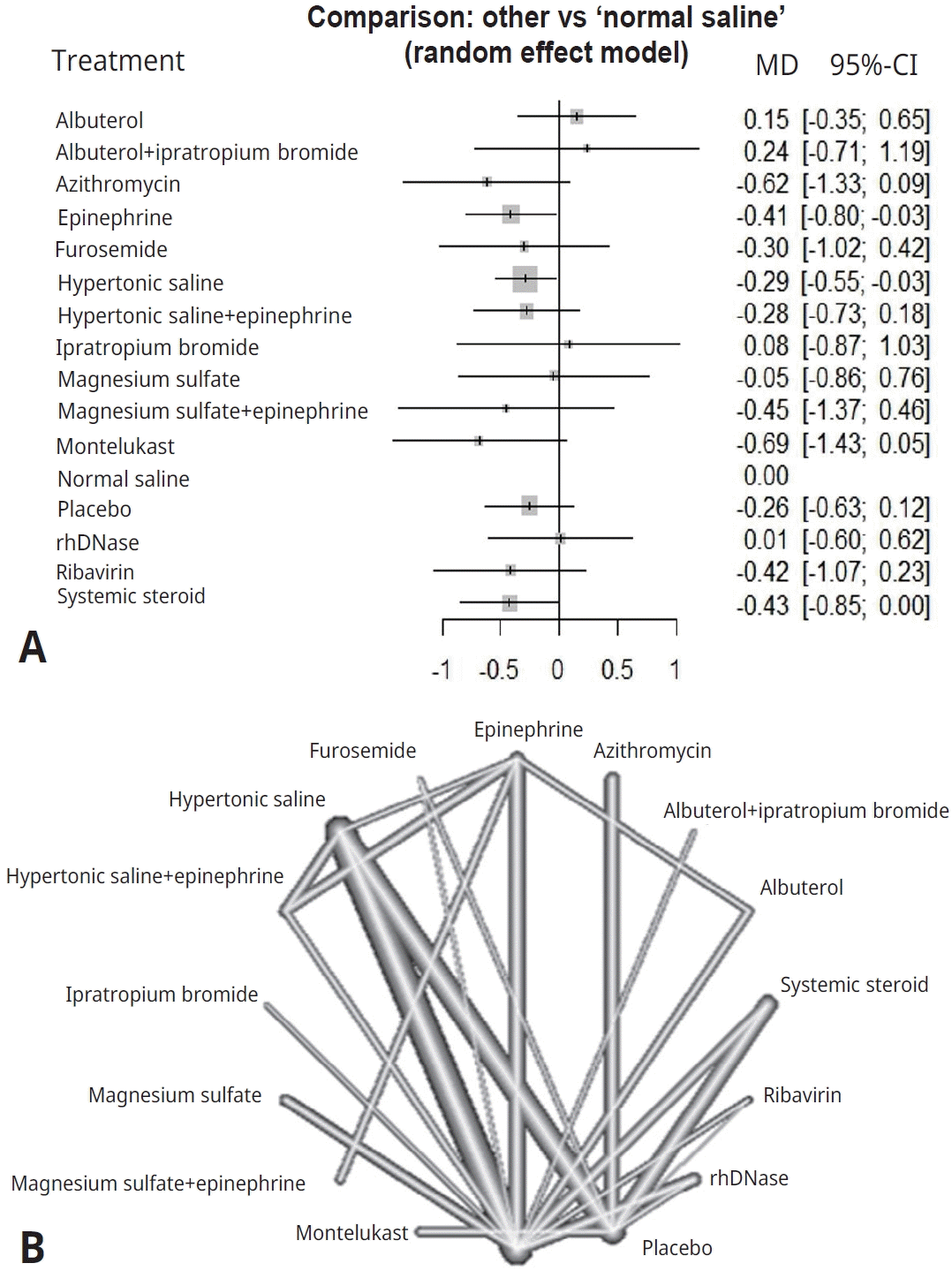
Fig. 3.
(A) Forest plot of Wang scores representing clinical severity. (B) Network graph of Wang scores representing clinical severity. MD, mean difference; CI, confidence interval.
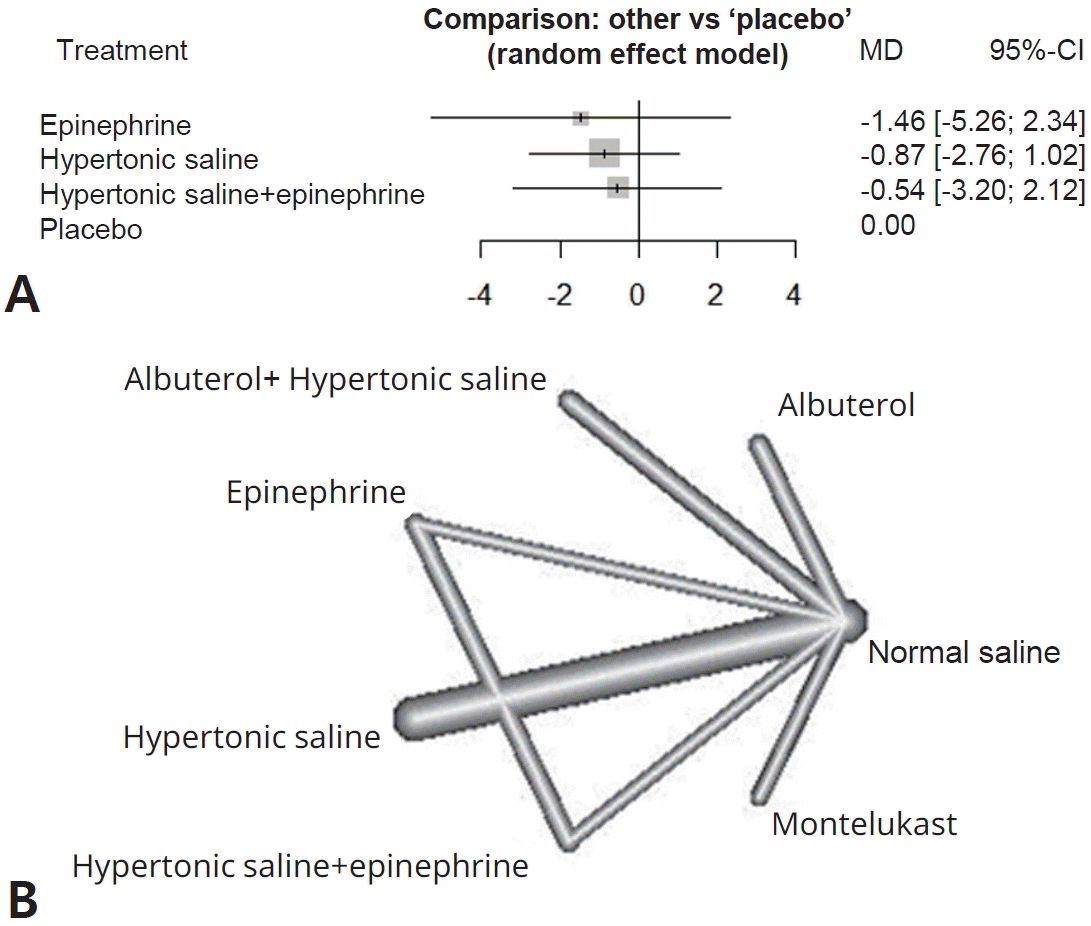
Table 1.
Number of included studies by intervention and control group
Table 2.
Characteristics of included studies
| Study | Country | No. | Treatment | Age, mean±SD | Outcome | Age, mean±SD |
|---|---|---|---|---|---|---|
| Jaquet-pilloud et al. (2020) [36] | Switzerland | 120 | Hypertonic salinea) | 7.7 mo | Length of hospital days | 47±33.9 hr |
| Placebo | 7.5 mo | 50.4±40.9 hr | ||||
| Morikawa et al. (2018) [37] | Japan | 128 | Hypertonic salinea) | 4.4±3.1 mo | Length of hospital days | 4.81±2.14 days |
| Normal saline | 4.2±3.0 mo | 4.61±2.18 days | ||||
| Williamson et al. (2018) [38] | USA | 46 | Furosemide | 7.7±5.5 mo | Length of hospital days | 2.8±2.35 days |
| Placebo | 8.1±6.8 mo | 3.0±2.45 days | ||||
| Alansari et al. (2017) [39] | Qatar | 162 | Magnesium sulfate | 4±3 mo | Length of hospital days | 24.1±20.76 hr |
| Normal saline | 5±4 mo | 25.3±25.87 hr | ||||
| Flores et al. (2016) [40] | Portugal | 68 | Hypertonic salinea) | 3.3±2.4 mo | Length of hospital days | 5.6±2.3 days |
| Normal saline | 3.8±2.5 mo | 5.4±2.1 days | ||||
| Flores-González et al. (2016) [18] | Spain | 185 | Hypertonic salinea)+epinephrine | 2.10±2.37 mo | Length of hospital days | 3.94±1.88 days |
| Hypertonic salinea) | 2.12±2.08 mo | 4.82±2.3 days | ||||
| Everard et al. (2015) [41] | UK | 291 | Hypertonic salinea) | 3.3±2.6 mo | Length of hospital days | 90.4±73.2 hr |
| Placebo | 3.4±2.8 mo | 88.9±67.9 hr | ||||
| Modaresi et al. (2015) [42] | Iran | 120 | MgSO4+epinephrine | 5.2±2.1 mo | Length of hospital days | 84.3±9.7 hr |
| Epinephrine | 4.8±3.2 mo | 84. 7±10.1 hr | ||||
| Khanal et al. (2015) [43] | Nepal | Hypertonic saline+epinephrineb) | 9.82±5.06 mo | Wang score | 2.73±1.37 | |
| Normal saline+epinephrineb) | 9.51±4.28 mo | 3.6±0.97 | ||||
| Ojha et al. (2014) [44] | Nepla | 59 | Hypertonic saline | 8.61±5.74 mo | Length of hospital days, Wang score | 44.82±23.15 hr, 4.00±1.56 hr |
| Normal saline | 8.51±4.24 mo | 43.60±28.25 hr, 3.83±1.90 hr | ||||
| Everard et al. (2014) [45] | UK | 291 | Hypertonic salinea) | 3.3±2.6 mo | Length of hospital days | 100.6±76.9 hr |
| Placebo | 3.4±2.8 mo | 101.3±84.4 hr | ||||
| Jacobs et al. (2013) [46] | USA | 101 | Hypertonic salinec)+epinephrined) | 6.0±3.9 mo | Length of hospital days | 4.1±0.9 days |
| Normal saline+epinephrined) | 5.6±3.3 mo | 3.9±4.0 days | ||||
| Alansari et al. (2013) [47] | Qatar | 200 | Dexamethasone | 3.4±2.2 mo | Length of hospital days | 18.60±20.92 hr |
| Placebo | 3.9±2.0 mo | 27.10±29.04hr | ||||
| Sharma et al. (2012) [48] | India | 248 | Hypertonic salinea | 4.93±4.31 mo | Length of hospital days | 63.93±22.43 hr |
| Normal saline | 4.18±4.24 mo | 63.51±21.27 hr | ||||
| Pinto et al. (2012) [49] | Brazil | 184 | Azithromycin | 3.08±2.23 mo | Length of hospital days | 5±2 days |
| placebo | 3.12±2.29 mo | 5±2 days | ||||
| Miraglia Del Giudice et al. (2012) [19] | Italy | 106 | Hypertonic salinea)+epinephrine | 4.8±2.3 mo | Length of hospital days, Wang score | 4.9±1.3 days, 7.4±1.6 days |
| Normal saline | 4.2±1.6 mo | 5.6±1.6 days, 8.3±1.7 days | ||||
| Ipek et al. (2011) [50] | Turkey | 120 | Albuterol | 8.13±4.75 mo | Wang score | 2.47±2.16 |
| Albuterol+hypertonic salinea) | 7.90±3.57 mo | 2.47±1.93 | ||||
| Hypertonic salinea) | 8.40±4.19 mo | 2.27±2.07 | ||||
| Normal saline | 7.40±3.08 mo | 3.10±2.43 | ||||
| Anil et al. (2010) [51] | Turkey | 148 | Epinephrine | 10.4±5.7 mo | Wang score | 2.33±1.07 |
| Epinephrine+hypertonic salinea) | 9.4±5.0 mo | 2.47±1.33 | ||||
| Albuterol | 9.0±6.2 mo | 2.1±2.1 | ||||
| Albuterol+hypertonic salinea) | 9.7±6.2 mo | 2.63±0.97 | ||||
| Normal saline | 9.1±4.4 mo | 2.2±1.2 | ||||
| Gupta et al. (2010) [52] | India | 64 | Epinephrine | 7.10±6.58 mo | Length of hospital days | 96.03±111.40 hr |
| Hypertonic salinea) | 5.27±3.82 mo | 82.91±65.94 hr | ||||
| Al-Ansari et al. (2010) [53] | Qatar | 113 | Hypertonic salinee) | 4.02±2.56 mo | Length of hospital days | 1.56±1.38 days |
| Normal saline | 3.30±2.43 mo | 1.88±1.76 days | ||||
| Luo et al. (2010) [25] | China | 112 | Hypertonic salinea) | 5.9±4.1 mo | Length of hospital days, Wang score | 4.8±1.2 days, 3.5±1.1 days |
| Normal saline | 5.8±4.3 mo | 6.4±1.4 days, 5.9±1.5 days | ||||
| Zedan et al. (2010) [54] | Egypt | 85 | Montelukast | 3.50±2.37 mo | Length of hospital days | 3.34±1.38 days |
| Placebo | 3.30±2.36 mo | 5.42±3.47 days | ||||
| Luo et al. (2009) [23] | China | 93 | Hypertonic salinea) | 6±4.3 mo | Length of hospital days | 6.0±1.2 days |
| Normal saline | 5.6±4.5 mo | 7.4±1.5 days | ||||
| Amirav et al. (2008) [55] | Israel | 53 | Montelukast | 3.2±2.8 mo | Length of hospital days | 4.65±1.97 days |
| Bar et al. (2008) [56] | Israel | 32 | Furosemide | 75±37 days | Length of hospital days | 76.3±35.4 hr |
| Normal saline | 69±50 days | 87.0±45.9 hr | ||||
| Kneyber et al. (2008) [57] | Netherland | 71 | Azithromycin | 3.0±0.6 mo | Length of hospital days | 132.0±10.8 hr |
| Placebo | 3.6±0.5 mo | 139.6±7.7 hr | ||||
| Kuzik et al. (2007) [24] | UAE | 96 | Hypertonic salinea) | 4.4±3.7 mo | Length of hospital days | 2.6±1.9 days |
| Normal saline | 4.6±4.7 mo | 3.5±2.9 days | ||||
| Teeratakulpisarn et al. (2007) [58] | Thailand | 174 | Dexamethasone | 10.2±5.5 mo | Length of hospital days | 54.2±29.9 hr |
| Normal saline | 11.2±5.9 mo | 67.6±41.8 hr | ||||
| Boogaard et al. (2007) [59] | Netherland | 222 | rhDNase | 2.1 mo | Length of hospital days | 4.40±2.69 days |
| Normal saline | 2.3 mo | 3.80±2.42 days | ||||
| Tal et al. (2006) [20] | Israel | 41 | Hypertonic salinea)+epinephrine | 2.8±1.2 mo | Length of hospital days, Wang score | 3.5±1.7 days, 5.35±1.35 days |
| Normal saline+epinephrine | 2.3±0.7 mo | 2.6±1.4 days, 6.45±1.00 days | ||||
| Bentur et al. (2005) [60] | Israel | 61 | Dexamethasone | 3.3±2.5 mo | Length of hospital days | 6.5±1.7 days |
| Normal saline | 3.8±2 mo | 9.1±1.9 days | ||||
| Langley et al. (2005) [61] | Canada | 62 | Epinephrinec | 5.49 mo | Length of hospital days | 2.60±1.62 days |
| Salbutamol | 3.32 mo | 3.40±2.16 days | ||||
| Zhang et al. (2003) [62] | Brazil | 52 | Prednisolone | 4±2.5 mo | Length of hospital days | 6.00±3.85 days |
| Placebo | 3.4±1.8 mo | 5.0±3.2 days | ||||
| Wainwright et al. (2003) [63] | Australia | 194 | Epinephrine | 4.52±3.01 mo | Length of hospital days | 58.80±52.29 hr |
| Normal saline | 4.35±2.95 mo | 69.50±54.95 hr | ||||
| Van Woensel et al. (2003) [64] | Netherland | 82 | Dexamethasone | 5.9±0.9 wk | Length of hospital days | 15.9±1.5 days |
| Placebo | 9.8±1.6 wk | 14.9±1.2 days | ||||
| Mandelberg et al. (2003) [21] | Israel | 52 | Hypertonic salinea)+epinephrine | 2.6±1.9 mo | Length of hospital days | 3.0±1.2 days |
| Normal saline+epinephrine | 3.0±1.2 mo | 4.0±1.9 days | ||||
| Patel et al. (2002) [65] | Canada | 149 | Epinephrinec | 4.2±3.1 mo | Length of hospital days | 59.8±62.0 hr |
| Albuterol | 3.9±2.9 mo | 61.4±54.0 hr | ||||
| Normal saline | 4.7±2.9 mo | 63.3±47.0 hr | ||||
| Bertrand et al. (2001) [22] | Chile | 30 | Epinephrinec | 3.9±0.4 mo | Length of hospital days | 4.1±1.1 days |
| Salbutamol | 3.7±0.6 mo | 5.2±1.0 days | ||||
| Nasr et al. (2001) [66] | Canada | 75 | rhDNase | 5.43±6.26 mo | Length of hospital days | 3.33±2.0 days |
| Placebo | 4.53±4.56 mo | 3.43±2.30 days | ||||
| Guerguerian et al. (1999) [67] | Canada | 41 | Ribavirin | 62.5±35.9 days | Length of hospital days | 255.9±124.9 hr |
| Normal saline | 62.7±30.9 days | 295±120.4 hr | ||||
| Van Woensel et al. (1997) [68] | Netherlnad | 54 | Prednisolone | 3.3 mo | Length of hospital days | 7.3±1.2 days |
| Placebo | 3.9 mo | 8.3±0.9 days | ||||
| Klassen et al. (1997) [69] | Canada | 67 | Dexamethasone | 6 wk–15 mo | Length of hospital days | 57.00±54.46 hr |
| Placebo | 48.00±16.44 hr | |||||
| Chowdhury et al. (1995) [70] | Saudi Arabia | 89 | Salbutamol | 3.88±2.30 mo | Length of hospital days | 4.5±1.3 days |
| Ipratropium bromide | 4.16±2.40 mo | 4.4±4.3 days | ||||
| Salbutamol+ipratropium | 3.64±1.8 mo | 4.6±4.3 days | ||||
| Normal saline | 3.72±2.27mo | 4.3±1.1 days | ||||
| Meert et al. (1994) [71] | USA | 17 | Ribavirin | 5.2±7.5 mo | Length of hospital days | 10.5±4.0 days |
| Normal saline | 4.9±4.0 mo | 8.8±1.6 days | ||||
| Smith et al. (1992) [72] | USA | 21 | Ribavirin | 1.1±1.1 mo | Length of hospital days | 9.0±5.3 days |
| Placebo | 1.6±2.2 mo | 15.3±5.3 days |
Table 3.
Risk of bias of randomized controlled trials of treatment for bronchiolitis
| Study | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result |
|---|---|---|---|---|---|
| Jaquet-pilloud et al. (2020) [36] | High risk | Low risk | Low risk | Low risk | Low risk |
| Morikawa et al. (2018) [37] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Williamson et al. (2018) [38] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Alansari et al. (2017) [39] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Flores et al. (2016) [40] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Flores-González et al. (2016) [18] | Some concerns | Some concerns | Low risk | Some concerns | Low risk |
| Everard et al. (2015) [41] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Modaresi et al. (2015) [42] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Khanal et al. (2015) [43] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ojha et al. (2014) [44] | High risk | Some concerns | Low risk | High risk | Low risk |
| Everard et al. (2014) [45] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Jacobs et al. (2013) [46] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Alansari et al. (2013) [47] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sharma et al. (2012) [48] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Pinto et al. (2012) [49] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Miraglia Del Giudice et al. (2012) [19] | Some concerns | Some concerns | Some concerns | High risk | Low risk |
| Ipek et al. (2011) [50] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Anil et al. (2010) [51] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Gupta et al. (2010) [52] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Al-Ansari et al. (2010) [53] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Luo et al. (2010) [25] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zedan et al. (2010) [54] | Some concerns | Low risk | Low risk | Some concerns | Low risk |
| Luo et al. (2009) [23] | Low risk | Low risk | Low risk | Low risk | |
| Amirav et al. (2008) [55] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bar et al. (2008) [56] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Kneyber et al. (2008) [57] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Kuzik et al. (2007) [24] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Teeratakulpisarn et al. (2007) [58] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Boogaard et al. (2007) [59] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Tal et al. (2006) [20] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Bentur et al. (2005) [60] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Langley et al. (2005) [61] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zhang et al. (2003) [62] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Wainwright et al. (2003) [63] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Van Woensel et al. (2003) [64] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Mandelberg et al. (2003) [21] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Patel et al. (2002) [65] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Bertrand et al. (2001) [22] | Low risk | Low risk | Low risk | Some concerns | Low risk |
| Nasr et al. (2001) [66] | Low risk | Some concerns | Low risk | Some concerns | Low risk |
| Guerguerian et al. (1999) [67] | Some concerns | High risk | Low risk | Some concerns | Low risk |
| Van Woensel et al. (1997) [68] | Some concerns | High risk | Low risk | Low risk | Low risk |
| Klassen et al. (1997) [69] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Chowdhury et al. (1995) [70] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Meert et al. (1994) [71] | Low risk | Low risk | Low risk | Low risk | Low risk |
| Smith et al. (1992) [72] | Low risk | Low risk | Low risk | Low risk | Low risk |
Table 4.
P score ranking of length of hospital stay
- References
- 1. Jang MJ, Kim YJ, Hong S, Na J, Hwang JH, Shin SM, et al. Positive association of breastfeeding on respiratory syncytial virus infection in hospitalized infants: a multicenter retrospective study. Clin Exp Pediatr 2020;63:135–40.
[Article] [PubMed] [PMC]4. Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev 2017;12:CD006458.
[Article] [PubMed] [PMC]5. King VJ, Viswanathan M, Bordley WC, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children: a systematic review. Arch Pediatr Adolesc Med 2004;158:127–37.
[Article] [PubMed]6. Maguire C, Cantrill H, Hind D, Bradburn M, Everard ML. Hypertonic saline (HS) for acute bronchiolitis: systematic review and meta-analysis. BMC Pulm Med 2015;15:148.
[Article] [PubMed] [PMC]7. Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ 2011;342:d1714.
[Article] [PubMed] [PMC]8. Kellner JD, Ohlsson A, Gadomski AM, Wang EE. Efficacy of bronchodilator therapy in bronchiolitis. A meta-analysis. Arch Pediatr Adolesc Med 1996;150:1166–72.
[Article] [PubMed]9. House SA, Gadomski AM, Ralston SL. Evaluating the placebo status of nebulized normal saline in patients with acute viral bronchiolitis: a systematic review and meta-analysis. JAMA Pediatr 2020;174:250–9.
[Article] [PubMed] [PMC]10. Lee YJ, Kim CK. Montelukast use over the past 20 years: monitoring of its effects and safety issues. Clin Exp Pediatr 2020;63:376–81.
[Article] [PubMed] [PMC]11. Szupie ko S, Buczek A, Szyma ski H. Nebulised 3% hypertonic saline versus 0.9% saline for treating patients hospitalised with acute bronchiolitis: protocol for a randomised, double-blind, multicentre trial. BMJ Open 2023;13:e080182.
[Article] [PubMed] [PMC]12. Wang ZY, Li XD, Sun AL, Fu XQ. Efficacy of 3% hypertonic saline in bronchiolitis: a meta-analysis. Exp Ther Med 2019;18:1338–44.
[Article] [PubMed] [PMC]13. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130–7.
[Article] [PubMed]14. Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA 2012;308:1246–53.
[Article] [PubMed]15. Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis 1992;145:106–9.
[Article] [PubMed]16. Sterne JAC, Savovi J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898.
[Article] [PubMed]17. Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva (Engl Ed) 2018;42:444. –53. English, Spanish.
[Article] [PubMed]18. Flores-González JC, Matamala-Morillo MA, Rodríguez-Campoy P, Pérez-Guerrero JJ, Serrano-Moyano B, Comino-Vazquez P, et al. Epinephrine improves the efficacy of nebulized hypertonic saline in moderate bronchiolitis: a randomised clinical trial. PLoS One 2015;10:e0142847.
[Article] [PubMed] [PMC]19. Miraglia Del Giudice M, Saitta F, Leonardi S, Capasso M, Niglio B, Chinellato I, et al. Effectiveness of nebulized hypertonic saline and epinephrine in hospitalized infants with bronchiolitis. Int J Immunopathol Pharmacol 2012;25:485–91.
[Article] [PubMed]20. Tal G, Cesar K, Oron A, Houri S, Ballin A, Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Isr Med Assoc J 2006;8:169–73.
[PubMed]21. Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest 2003;123:481–7.
[Article] [PubMed]22. Bertrand P, Araníbar H, Castro E, Sánchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatr Pulmonol 2001;31:284–8.
[Article] [PubMed]23. Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatr Int 2010;52:199–202.
[Article] [PubMed]24. Kuzik BA, Al-Qadhi SA, Kent S, Flavin MP, Hopman W, Hotte S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr 2007;151:266. –70. 270.e1.
[Article] [PubMed]25. Luo Z, Fu Z, Liu E, Xu X, Fu X, Peng D, et al. Nebulized hypertonic saline treatment in hospitalized children with moderate to severe viral bronchiolitis. Clin Microbiol Infect 2011;17:1829–33.
[Article] [PubMed]26. Kini NM, Robbins JM, Kirschbaum MS, Frisbee SJ, Kotagal UR, Child Health Accountability Initiative. Inpatient care for uncomplicated bronchiolitis: comparison with Milliman and Robertson guidelines. Arch Pediatr Adolesc Med 2001;155:1323–7.
[Article] [PubMed]27. Pelletier JH, Au AK, Fuhrman D, Clark RSB, Horvat C. Trends in bronchiolitis ICU admissions and ventilation practices: 2010-2019. Pediatrics 2021;147:e2020039115.
[Article] [PubMed] [PMC]28. Hartling L, Wiebe N, Russell K, Patel H, Klassen TP. Epinephrine for bronchiolitis. Cochrane Database Syst Rev 2004;(1):CD003123.
[Article]29. Wohl ME, Chernick V. State of the art: bronchiolitis. Am Rev Respir Dis 1978;118:759–81.
[Article] [PubMed]30. Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev 2010;(10):CD004878.
[Article]31. Gadomski AM, Bhasale AL. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2006;(3):CD001266.
[Article]32. Zorc JJ. Inhaled epinephrine does not shorten hospital stay for infants with bronchiolitis destined to develop repeated bronchospasm. Lancet Respir Med 2015;3:665–7.
[Article] [PubMed]33. Zhang L, Gunther CB, Franco OS, Klassen TP. Impact of hypertonic saline on hospitalization rate in infants with acute bronchiolitis: a meta-analysis. Pediatr Pulmonol 2018;53:1089–95.
[Article] [PubMed]34. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58.
[PubMed] [PMC]35. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:79.
[Article] [PubMed] [PMC]36. Jaquet-Pilloud R, Verga ME, Russo M, Gehri M, Pauchard JY. Nebulised hypertonic saline in moderate-to-severe bronchiolitis: a randomised clinical trial. Arch Dis Child 2020;105:236–40.
[Article] [PubMed]37. Morikawa Y, Miura M, Furuhata MY, Morino S, Omori T, Otsuka M, et al. Nebulized hypertonic saline in infants hospitalized with moderately severe bronchiolitis due to RSV infection: a multicenter randomized controlled trial. Pediatr Pulmonol 2018;53:358–65.
[Article] [PubMed]38. Williamson K, Bredin G, Avarello J, Gangadharan S. A randomized controlled trial of a single dose furosemide to improve respiratory distress in moderate to severe bronchiolitis. J Emerg Med 2018;54:40–6.
[Article] [PubMed]39. Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. IV Magnesium sulfate for bronchiolitis: a randomized trial. Chest 2017;152:113–9.
[Article] [PubMed] [PMC]40. Flores P, Mendes AL, Neto AS. A randomized trial of nebulized 3% hypertonic saline with salbutamol in the treatment of acute bronchiolitis in hospitalized infants. Pediatr Pulmonol 2016;51:418–25.
[Article] [PubMed]41. Everard ML, Hind D, Ugonna K, Freeman J, Bradburn M, Dixon S, et al. Saline in acute bronchiolitis RCT and economic evaluation: hypertonic saline in acute bronchiolitis - randomised controlled trial and systematic review. Health Technol Assess 2015;19:1–130.
[Article] [PubMed] [PMC]42. Modaresi MR, Faghihinia J, Kelishadi R, Reisi M, Mirlohi S, Pajhang F, et al. Nebulized magnesium sulfate in acute bronchiolitis: a randomized controlled trial. Indian J Pediatr 2015;82:794–8.
[Article] [PubMed]43. Khanal A, Sharma A, Basnet S, Sharma PR, Gami FC. Nebulised hypertonic saline (3%) among children with mild to moderately severe bronchiolitis--a double blind randomized controlled trial. BMC Pediatr 2015;15:115.
[Article] [PubMed] [PMC]44. Ojha AR, Mathema S, Sah S, Aryal UR. A comparative study on use of 3% saline versus 0.9% saline nebulization in children with bronchiolitis. J Nepal Health Res Counc 2014;12:39–43.
[PubMed]45. Everard ML, Hind D, Ugonna K, Freeman J, Bradburn M, Cooper CL, et al. SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis. Thorax 2014;69:1105–12.
[Article] [PubMed] [PMC]46. Jacobs JD, Foster M, Wan J, Pershad J. 7% Hypertonic saline in acute bronchiolitis: a randomized controlled trial. Pediatrics 2014;133:e8–13.
[Article] [PubMed]47. Alansari K, Sakran M, Davidson BL, Ibrahim K, Alrefai M, Zakaria I. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics 2013;132:e810–6.
[Article] [PubMed]48. Sharma BS, Gupta MK, Rafik SP. Hypertonic (3%) saline vs 0.93% saline nebulization for acute viral bronchiolitis: a randomized controlled trial. Indian Pediatr 2013;50:743–7.
[Article] [PubMed]49. Pinto LA, Pitrez PM, Luisi F, de Mello PP, Gerhardt M, Ferlini R, et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: a randomized, double-blinded, and placebo-controlled clinical trial. J Pediatr 2012;161:1104–8.
[Article] [PubMed]50. Ipek IO, Yalcin EU, Sezer RG, Bozaykut A. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulm Pharmacol Ther 2011;24:633–7.
[Article] [PubMed]51. Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamolnormal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatr Pulmonol 2010;45:41–7.
[Article] [PubMed]52. Gupta N, Puliyel A, Manchanda A, Puliyel J. Nebulized hypertonic-saline vs epinephrine for bronchiolitis; proof of concept study of cumulative sum (CUSUM) analysis. Indian Pediatr 2012;49:543–7.
[Article] [PubMed]53. Al-Ansari K, Sakran M, Davidson BL, El Sayyed R, Mahjoub H, Ibrahim K. Nebulized 5% or 3% hypertonic or 0.9% saline for treating acute bronchiolitis in infants. J Pediatr 2010;157:630. –4. 634.e1.
[Article] [PubMed]54. Zedan M, Gamil N, El-Assmy M, Fayez E, Nasef N, Fouda A, et al. Montelukast as an episodic modifier for acute viral bronchiolitis: a randomized trial. Allergy Asthma Proc 2010;31:147–53.
[Article] [PubMed]55. Amirav I, Luder AS, Kruger N, Borovitch Y, Babai I, Miron D, et al. A double-blind, placebo-controlled, randomized trial of montelukast for acute bronchiolitis. Pediatrics 2008;122:e1249–55.
[Article] [PubMed]56. Bar A, Srugo I, Amirav I, Tzverling C, Naftali G, Kugelman A. Inhaled furosemide in hospitalized infants with viral bronchiolitis: a randomized, double-blind, placebo-controlled pilot study. Pediatr Pulmonol 2008;43:261–7.
[Article] [PubMed]57. Kneyber MC, van Woensel JB, Uijtendaal E, Uiterwaal CS, Kimpen JL, Dutch Antibiotics in RSV Trial (DART) Research Group. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr Pulmonol 2008;43:142–9.
[Article] [PubMed]58. Teeratakulpisarn J, Limwattananon C, Tanupattarachai S, Limwattananon S, Teeratakulpisarn S, Kosalaraksa P. Efficacy of dexamethasone injection for acute bronchiolitis in hospitalized children: a randomized, double-blind, placebo-controlled trial. Pediatr Pulmonol 2007;42:433–9.
[Article] [PubMed]59. Boogaard R, Hulsmann AR, van Veen L, Vaessen-Verberne AAPH, Yap YN, Sprij AJ, et al. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest 2007;131:788–95.
[Article] [PubMed]60. Bentur L, Shoseyov D, Feigenbaum D, Gorichovsky Y, Bibi H. Dexamethasone inhalations in RSV bronchiolitis: a doubleblind, placebo-controlled study. Acta Paediatr 2005;94:866–71.
[Article] [PubMed]61. Langley JM, Smith MB, LeBlanc JC, Joudrey H, Ojah CR, Pianosi P. Racemic epinephrine compared to salbutamol in hospitalized young children with bronchiolitis; a randomized controlled clinical trial [ISRCTN46561076]. BMC Pediatr 2005;5:7.
[Article] [PubMed] [PMC]62. Zhang L, Ferruzzi E, Bonfanti T, Auler MI, D'avila NE, Faria CS, et al. Long and short-term effect of prednisolone in hospitalized infants with acute bronchiolitis. J Paediatr Child Health 2003;39:548–51.
[Article] [PubMed]63. Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med 2003;349:27–35.
[Article] [PubMed]64. van Woensel JB, van Aalderen WM, de Weerd W, Jansen NJ, van Gestel JP, Markhorst DG, et al. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax 2003;58:383–7.
[Article] [PubMed] [PMC]65. Patel H, Platt RW, Pekeles GS, Ducharme FM. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. J Pediatr 2002;141:818–24.
[Article] [PubMed]66. Nasr SZ, Strouse PJ, Soskolne E, Maxvold NJ, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001;120:203–8.
[Article] [PubMed]67. Guerguerian AM, Gauthier M, Lebel MH, Farrell CA, Lacroix J. Ribavirin in ventilated respiratory syncytial virus bronchiolitis. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 1999;160:829–34.
[Article] [PubMed]68. van Woensel JB, Wolfs TF, van Aalderen WM, Brand PL, Kimpen JL. Randomised double blind placebo controlled trial of prednisolone in children admitted to hospital with respiratory syncytial virus bronchiolitis. Thorax 1997;52:634–7.
[Article] [PubMed] [PMC]69. Klassen TP, Sutcliffe T, Watters LK, Wells GA, Allen UD, Li MM. Dexamethasone in salbutamol-treated inpatients with acute bronchiolitis: a randomized, controlled trial. J Pediatr 1997;130:191–6.
[Article] [PubMed]70. Chowdhury D, al Howasi M, Khalil M, al-Frayh AS, Chowdhury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: a clinical trial. Ann Trop Paediatr 1995;15:77–84.
[Article] [PubMed]71. Meert KL, Sarnaik AP, Gelmini MJ, Lieh-Lai MW. Aerosolized ribavirin in mechanically ventilated children with respiratory syncytial virus lower respiratory tract disease: a prospective, double-blind, randomized trial. Crit Care Med 1994;22:566–72.
[PubMed]

 About
About Browse articles
Browse articles For contributors
For contributors