All issues > Volume 67(11); 2024
Protecting our future: environmental hazards and children’s health in the face of environmental threats: a comprehensive overview
- Corresponding author: Young Yoo, MD, PhD. Department of Pediatrics, Korea University Medical College, 73 Goryeodae-ro, Seongbuk-gu, Seoul 02841, Korea Email: yoolina@korea.ac.krCo-corresponding Author: Yunkoo Kang, MD. Department of Pediatrics, Yonsei University Wonju College of Medicine, 20, Ilsanro, Wonju 26426, Korea Email: monkeydluffy@yonsei.ac.kr*
- Received November 23, 2023 Revised June 10, 2024 Accepted June 23, 2024
- Abstract
-
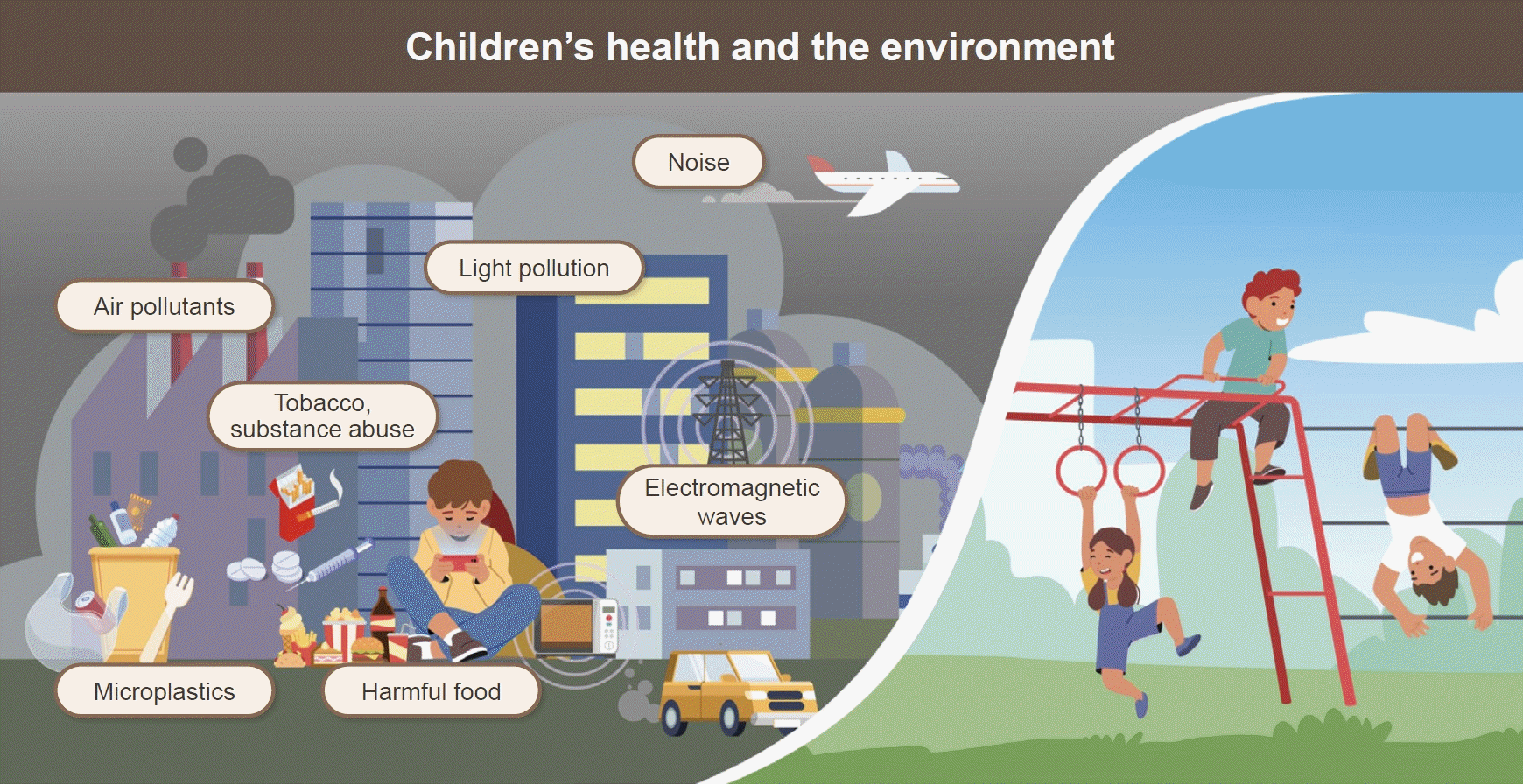
Children face the excitement of a changing world but also encounter environmental threats to their health that were neither known nor suspected several decades ago. Children are at particular risk of exposure to pollutants that are widely dispersed in the air, water, and food. Children and adolescents are exposed to chemical, physical, and biological risks at home, in school, and elsewhere. Actions are needed to reduce these risks for children exposed to a series of environmental hazards. Exposure to a number of persistent environmental pollutants including air pollutants, endocrine disruptors, noise, electromagnetic waves (EMWs), tobacco and other noxious substances, heavy metals, and microplastics, is linked to damage to the nervous and immune systems and affects reproductive function and development. Exposure to environmental hazards is responsible for several acute and chronic diseases that have replaced infectious diseases as the principal cause of illnesses and death during childhood. Children are disproportionately exposed to environmental toxicities. Children drink more water, eat more food, and breathe more frequently than adults. As a result, children have a substantially heavier exposure to toxins present in water, food, or air than adults. In addition, their hand-to-mouth behaviors and the fact that they live and play close to the ground make them more vulnerable than adults. Children undergo rapid growth and development processes that are easily disrupted. These systems are very delicate and cannot adequately repair thetional development in children’s environmental health was the Declaration of the Environment Leaders of the Eight on Children’s Environmental Health by the Group of Eight. In 2002, the World Health Organization launched an initiative to improve children’s environmental protection effort. Here, we review major environmental pollutants and related hazards among children and adolescents.
Graphical abstract.
- Introduction
- Introduction
Environmental problems can significantly impact children’s health and cause many diseases, even in adulthood. With the advent of new industries, we are faced with new environmental health problems owing to exposure to various new environmental contaminants [1]. The risks to children in their everyday environments are numerous. In addition to air pollutants, various environmental hazards including dietary supplements, electromagnetic fields (EMFs), endocrine disruptors (EDs), and heavy metals, can influence environment related diseases. Outdoor air pollution problems caused by industrialization, such as the Great Smog of London in 1952, and lead poisoning caused by contained in automobile exhaust gas or paint, have become major health, environmental and social issues [2,3]. In addition, environmental diseases caused by heavy metals, such as Minamata disease in Japan, resulted from Hg and Cd poisoning originating from factory wastewater during the industrialization of the 19th century [4]. However, as the use of Pb and Cd is legally restricted, the problem of environmental pollution is gradually disappearing [5].In recent years, indoor environmental pollution, including particulate matter (PM), gases, vapors, and biological materials, has been increasingly recognized as pertinent to children’s health alongside outdoor pollution [6]. Given that children spend a significant proportion of their time indoors, factors such as their smaller size (and hence a relatively higher dose of ingested toxins), closer proximity to the ground, dirt, indoor dust, boundless curiosity, oral exploratory behaviors, and habits like pica may put them at greater risk of exposure to environmental pollutants [7].Environmental problems can affect a fetus and cause problems into adulthood [8]. Environmental problems are commonly encountered and although children and parents do not consider them dangerous, they gradually affect their health [9]. People are exposed to air pollution as well as other harmful substances, such as dietary supplements, without knowing the level of risk [10,11]. Owing to the consumption of food or other substances by the mother during pregnancy, the fetus can encounter harmful substances before birth [12]. It may seem trivial, but the accumulation of such exposures can lead to health problems and, eventually diseases [13]. In this review, we list, identify and summarize various environmental problems that can affect infants, children, and adolescents (Table 1).
- Environment and children’s health
- Environment and children’s health
- 1. Air pollution and respiratory health
- 1. Air pollution and respiratory health
The prevalence of allergic diseases in children has gradually increased and is considered associated with modern Westernized lifestyles and increasing air pollutants. Trafficrelated air pollution consists of a complex of PM, nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone and is associated with reduced lung growth, decreased lung function, and the development and exacerbation of allergic diseases [14]. Moreover, exposure itself as well as its timing increasingly recognized as crucial, and recent evidence supports that air pollution exposure during prenatal and early-life contributes to the development of allergic diseases in later life [15].Several mechanisms are involved in the development and exacerbation of allergic diseases associated with air pollution, including oxidative stress, airway injury, and inflammation. These mechanisms are complex and based on host genetics, host immunological milieu, and exposure [16]. Air pollution affects the bronchial epithelium by promoting oxidative stress, which can damage the integrity of epithelial barriers, induce airway inflammation and hyperresponsiveness, and enhance allergic sensitization. Epigenetics was recently recognized as a key mechanism for the development of allergic diseases associated with exposure to air pollution, which alters gene expression without directly altering the DNA sequence [17]. Early-life exposure to air pollution is associated with epigenetic changes that can increase the risk of asthma later in life. Moreover, the epigenetic effects of air pollution can be direct or indirect through the induction of oxidative stress. An advanced understanding of the complex mechanisms between modifiable environmental factors and the development of allergic diseases will help prevent allergic diseases.To manage allergic diseases, we must first improve air quality. In the Children’s Health Study, long-term improvements in air quality were significantly associated with lung development [18]. Reducing outdoor air pollution requires considerable effort and should be monitored and politically controlled. However, children spend most of their time indoors because their lifestyles have changed. Indoor air pollution, which shares many characteristics with outdoor air pollution, may be relatively easily modifiable by using air cleaners and improving ventilation. A recent report showed that the increased medical costs of allergic rhinitis were significantly associated with the monthly concentrations of PM10, NO2, and carbon monoxide in Korea [19]. Monitoring and managing outdoor and indoor air quality are essential for establishing effective inter-ventions to decrease exposure and reduce the risk and burden of allergic diseases.- 2. Air pollution and kidney disease
- 2. Air pollution and kidney disease
Environmental pollutants, including metals, air, phthalates and melamine can potentially increase the risk of chronic kidney disease (CKD) development or accelerate its progression [20]. Kuzma et al. [21] reported that short-term exposure to elevated air pollution levels was associated with a decreased estimated glomerular filtration rate and that the main pollutants affecting the kidneys were PM and SO2. In the midterm, an increase in annual concentration of PM2.5 (odds ratio [OR] for interquartile range [IQR] increase, 1.07; 95% confidence interval [CI], 1.01–1.15; P=0.037) and NO2 (OR for IQR increase1.05; 95% CI, 1.01–1.10; P=0.047) increased the number of patients with CKD. Several studies demonstrated evidence of the relationship between exposure to environmental factors such as bisphenol A (BPA), heavy metals including Pb, Cd, and Cr, and cigarette smoking and direct kidney damage causing albuminuria in the pediatric population [22].Inhaled airborne particles may cause vascular injury, intraglomerular hypertension, or glomerulosclerosis through multifactorial interactions with hemodynamic and nonhemodynamic factors. The mechanisms linking air pollutant exposure to CKD include elevated blood pressure, worsening oxidative stress and inflammatory responses, DNA damage and abnormal metabolic changes that aggravate renal damage [23]. Clinicians should be aware that environmental exposure is relatively common and should be vigilant about screening for it. Maintaining air quality, promoting education, improving health, and promoting targeted nephroprotective measures through effective policies and research are required to address this global public health problem [24].- 3. Obesity and dietary supplements
- 3. Obesity and dietary supplements
Over the past few decades, the prevalence of pediatric obesity has increased among Korean adolescents [25]. As obesity increases, the prevalence of various complications, such as nonalcoholic steatohepatitis, and diabetes mellitus also increases [26]. The National School Health Exami-nation showed that 19% of adolescents in the United States are obese while the prevalence of obesity in children aged 6–18 years in Korea increased from 8.7% in 2007 to 15.0% in 2017 [25]. The prevalence varies depending on the data being analyzed; however, it has increased globally over time. Several risk factors have been identified; however, they differ by age and sex. An increased consumption of sugary beverages and snacks and decreased activity levels are also cause of pediatric obesity. Socio-cultural factors such as the use of food as a reward and family food preferences also contribute to the obesity epidemic. Finally, as social safety deteriorates, the number of students who walk or ride bicycles to school decreases, whereas the number of children who ride in their parents’ cars increases [27].Owing to the lock down during the coronavirus disease 2019 pandemic, the prevalence of obesity in children and adolescents has been increasing globally [28]. This has affected lifestyle choices and led to decreased physical activity levels, thereby causing obesity. To lead a healthy life, activity must eventually be increased along with healthy diet control; but this was not observed. Instead, the consumption of dietary supplements was observed. Such dietary supplements that are intended for adults can easily be administered to children, resulting in side effects. Previous studies reported that up to 64% of parents administer dietary supplements to their children in an effort to keep them healthy [11,29].According to the Korea National Health and Nutrition Examination Survey (KNHANES), 34% of Korean children take dietary supplements, including vitamin and mineral supplements (34.4%), omega-3 fatty acid (28.8%), ginseng (18.3%), colostrum (14.1%), and Chlorella/Spirulina (10%) [10]. Dietary supplements are used by many adolescents without medical guidance, and a lack of knowledge can cause serious adverse events such as dizziness, nausea, and vomiting. The importance of adequate activity and a healthy diet should be emphasized.- 4. Health effects of EMF exposure
- 4. Health effects of EMF exposure
When electricity flows, electric and magnetic signals are simultaneously generated around it, called an EMF; the waves generated therein are called EMW. Sunlight and the Earth's magnetic field are natural electromagnetic environments, while electricity, telecommunications, home appliances, transmission towers, mobile phones, Wi-Fi signals, and base stations create artificial ones. EMF exposure is categorized into extremely-low-frequency (ELF; 3–3,000 Hz) and radiofrequency (RF; 30 kHz–300 GHz) exposure. Television, computers, air conditioners, refrigerators, electric ranges, bidets, electric massagers, electric pads, and low-frequency household medical devices all generate ELF. When the products come in close contact with the body, greater EMW exposure occurs. Most electronic devices are safe when used approximately 30 cm away from the body. However, for children, it is better to avoid using multiple electronic devices for a long time. Exposure to ELF can also occur in schools and on public transportation [30].Radio and television broadcasts, mobile phones, and base stations are major sources of RF exposure. Infants and toddlers can be indirectly exposed to RF in their living environments, including indoor Wi-Fi and by nearby mobile phones, even if they do not directly use them. According to the Stewart Report 2000, children are more vulnerable to EMF than any other age group, as they are exposed to EMW for a longer period of time than adults, their nervous systems are under development, and their conductivity is higher because of higher moisture and ion content than adults [31]. For these reasons, the developing brains of children and adolescents can absorb more EMW, making them more vulnerable than adults to exposure, including those produced by cell phones. However, the carcinogenicity of EMF associated technologies remains under debate [30].Scientific results on the safety of EMF in children and adolescents required to avoid excessive anxiety or optimism. Although results to date on the effects of EMF are inconsistent, it is true that children's nervous systems are more vulnerable than those of adults [30,31]. Therefore, the precautionary principle should be applied when introducing new EMW technology, and EMF exposure in children and adolescents should be minimized until safety is ensured. In cases in which it is difficult to adhere to the precautionary principle, laws protecting children and adolescents must be enacted. Stricter standards are required for facilities in which children stay for long periods, such as schools. Thus, exposure to EMF in children and adolescents should be minimized, especially in children under 6 years of age.- 5. Noise and blue-light hazards
- 5. Noise and blue-light hazards
Noise is defined as an “unwanted or unpleasant sound,” and with recent rapid global modernization, various detrimental environmental noise effects are seen in adults as well as infants, children, and adolescents. According to a survey in the United States, 12.5% of children and adolescents (aged 6–19 years) show noise-induced hearing threshold shifts, indicating exposure to high noise levels [32]. Owing to the increase in the number of people exposed to noise and the use of personal sound-creating devices, Koreans are also highly vulnerable to noise pollution; therefore, appropriate measures are crucial. Continuous or even intermittent exposure to loud noise affects one’s health and daily life through non-auditory effects such as sleep disturbance, discomfort, increased blood pressure, and impaired cognitive performance.Because of auditory effects, high-intensity noise signals (>130-dB sound pressure level), excessive noise exposure or prolonged exposure (>1 hour) to low-intensity noise signals can cause temporary hearing loss or, in severe cases, permanent noise-induced hearing loss [33]. In the fetal or neonatal period, sounds >90 dB outside the mother can irritate the fetus or cause hearing difficulties in early life. Many toys and products create excessive noise for children who have difficulty expressing themselves. Additionally, young children are defenselessly exposed to excessive noise caused by audio devices used by or actions of adults. The most problematic factor for school-aged children is the use of personal sound-creating devices. In a 2007 survey of 490 Korean teenagers (aged 12–18 years), 94.3% used personal sound-creating devices, 46.7% used them for 1–3 hr/day, and 14.1% used them for 3–5 hr/day [34]. In addition, the KNHANES V (2010–2012) tested 1,845 adolescents (aged 12–19 years) and found that the prevalence rates of unilateral and bilateral hearing loss were 8.56% and 1.03%, respectively. The incidence of hearing loss in the high–frequency region related to noise was 32.7% on one side and 5.53% on both sides, significantly higher than those reported in other countries [35]. Therefore, continuous efforts toward public education on the harmful effects of noise pollution through actions such as broadcasting in schools, transportation, and television are urgently required. Moreover, medical professionals should be familiar with the basic knowledge of environmental noise, educate patients to minimize its impact, and strive to detect and treat patients early through screening tests.Children and adolescents are more sensitive to light than adults. The enhanced transmission rate in young individuals is especially prominent for the short wavelengths of light-emitting electronic devices (all computers and smartphones currently on the market) [36]. The effects of light on melatonin secretion depend on the time of day, intensity, duration, and spectral properties of the exposure. Exposure to light in the morning results in a phase advance, with peak melatonin secretion occurring earlier. Exposure at the end of the afternoon results in a phase delay. Exposure between midnight and 4 am (the normal timing of peak melatonin secretion) completely inhibits secretion for the full duration of the exposure [37].Melatonin secretion was inhibited by moderately bright light (580 lx) in children at almost twice the rate that observed in adults (88% and 46%, respectively). Under domestic lighting conditions (120–140 lx), melatonin secretion was significantly suppressed in children versus adults (51% and 26%, respectively).Increased sensitivity to evening light was noted in the prepuberty group versus adolescents at all tested light levels (low [15 lx], moderate [150 lx], and bright [5,000 lx]) [38]. The spectral composition of light produced by many electronic devices is enriched for short-wavelength light (~450 nm) in the blue-light range. Short-wavelength light is generally more effective than longer-wavelength light at suppressing melatonin levels, phase shifting the circadian clock, and altering subsequent sleep [39,40]. Our current understanding of the effects of artificial light on sleep and circadian rhythms in children and adolescents is based on only a handful of well-controlled studies. Individual efforts to minimize the damage caused by noise and blue-light pollution remain crucial.- 6. EDs and the endocrine system
- 6. EDs and the endocrine system
EDs are ubiquitous and exogenous chemicals that interfere with hormone action [41]. EDs are inhaled, consumed orally, and ingested transdermally and act like estrogens or androgens through various mechanisms [41]. During early development, infants are more sensitive to EDs than adults due to immaturity of their metabolic enzymes, which they are unable to remove. In addition, children are exposed to more EDs than adults because of their lower metabolic capacity, higher intake of water and food, higher inhalation rate per unit body mass, higher intestinal absorption rate, frequent hand-to-mouth activities, and higher dermal permeation rate due to their thinner skin and larger skin surface area per unit of body weight [42]. During this period, ED-induced epigenetic changes permanently modify the germline epigenome and can be transmitted to the next generation. With the rapid changes in endocrine-dependent organ systems, childhood and puberty have been recognized as additional sensitive periods [41]. Studies on EDs have shown inconsistent findings for distinct reasons. First, EDs exert their actions in nonmonotonic dose-response curves [41]. Second, exposure timing and duration significantly affect outcomes. Finally, humans are simultaneously exposed to many chemicals, which cause extreme difficulty interpreting their effects [43].Fetal growth restriction and premature birth are associated with ED exposure and an increased risk of short stature, metabolic syndrome, high blood pressure, and obesity. Prenatal BPA exposure reduces birth weight because of decreased insulin-like growth factor availability [44]. No significant evidence exists of the association of polybrominated diphenyl ethers, phenols, and phthalates with birth weight. There is evidence of a relationship between phthalates and premature birth. The associations between premature birth and per- and polyfluoroalkyl substances (PFAS) and phenol vary among studies [45]. A meta-analysis of 10 cohort studies found a 25% increase in child adiposity (95% CI, 4%–50%; I2=40.5%) and 0.1-unit increase in body mass index z score per 1 ng/mL of PFAS in the maternal blood (95% CI, 0.03–0.15 ng/mL; I2=27.9%). However, prenatal exposure to phthalates and bisphenols has not been consistently associated with child adiposity [45].The onset of puberty tends to occur earlier, while the completion of puberty (menarche and testicular volume ≥12 mL) shows no significant change in the median age but a positively skewed distribution [43,44]. The incidence of central precocious puberty in girls is increasing partly due to ED exposure [43,44]. EDs act at any level of the hypothalamicpituitary-gonadal-peripheral tissue-endocrine axis. A significant relationship was noted between postnatal exposure to phthalates and earlier thelarche and later pubarche in a qualitative trend synthesis of 52 studies, but no consistent associations were found between the timing of pubertal onset in boys and girls and exposure to any of the studied xenobiotic EDs in a meta-analysis of 23 studies [46]. Exposure to BPA during puberty increases the risk of breast cancer in women. EDs may reprogram normal progenitor cells in the breast, which are subsequently transformed upon hormone exposure. In vitro, Ak strain transformation increases proliferation and apoptosis resistance, and is upregulated by BPA [44].- 7. Impact of heavy metal exposures on children’s health
- 7. Impact of heavy metal exposures on children’s health
Pregnant women are more vulnerable to environmental exposure than nonpregnant women because of dynamic metabolic, neurological, and immunological changes that occur during pregnancy. Moreover, prenatal exposure to Pb, Cd, and Hg are associated with low birthweight, preterm birth, and delayed neurologic development in children [47]. According to the data from the Ko-CHENS (Korean Children's ENvironmental health Study), a national prospective birth cohort that explored the association between environmental exposure and health outcomes in mothers and their children [48], higher Pb and Cd exposure during late pregnancy were associated with lower birthweight (LBW) [49]. Studies have shown a significant inverse association between prenatal Pb exposure and newborn birth weight as well [50]. However, Hg exposure has been associated with reduced fetal growth in multiple studies, whereas other studies reported no association [51]. This can be explained by variations in exposure level and timing. In addition to prenatal exposure to heavy metals, there are numerous exposure routes,including ingestion,inhalation, and dermal absorption, subsequently feature some health effects [52]. The harmful consequences for child and adolescent health include mental retardation, neurocognitive disorders, behavioral disorders, respiratory problems, cancer, and cardiovascular diseases [53].- 8. Tobacco and substance use
- 8. Tobacco and substance use
The negative effects of tobacco use have been well-documented. Cigarette smoking causes diseases in almost all organs of the body and worsens an individual’s health conditions [54]. Children can be exposed to tobacco through maternal smoking during gestation, exposure to secondhand smoke (SHS) after birth, and active smoking. Maternal prenatal smoking interferes with the oxygen supply to the fetus, alters fetal development, and exposes the fetus to toxic substances. Maternal prenatal smoking been associated with numerous adverse pregnancy outcomes, including spontaneous pregnancy loss, placental abruption, preterm premature rupture of the membranes, placenta previa, preterm labor and delivery, LBW, and ectopic pregnancy [55]. Prenatal tobacco exposure is associated with postnatal morbidities including sudden infant death syndrome, respiratory infection, asthma, atopy, otitis media, attention-deficit hyperactivity disorder (ADHD), childhood obesity, and decreased school performance [56,57]. Prenatal exposure is more strongly associated with the development of ADHD than childhood SHS exposure [58]. Ip et al. [59] reported telomere shortening in children with prenatal tobacco exposure associated with cancer, type 2 diabetes mellitus, cardiovascular disease, Alzheimer disease, and premature death.Childhood exposure to tobacco occurs through passive inhalation of tobacco smoke. With the ban on smoking in public places, children are more likely to be exposed to SHS in their homes. Third-hand smoke refers to exposure to tobacco residues in the hair, skin, clothes, furniture, or automobiles. It is difficult to evaluate the risks of SHS separately from the effects of prenatal exposure since children exposed to tobacco during the prenatal period are often continuously exposed to SHS after birth. Cotinine, a nicotine metabolite, is a biomarker of an individual’s exposure to tobacco smoke. Children are at higher risk of the effects of environmental tobacco smoke than adults, with 70% higher cotinine levels after similar exposures [60]. According to the data provided by the Korea Youth Risk Behavior Web-based Survey in 2005–2021, the prevalence of ever-smokers was 27.7% (95% CI, 27.3%–28.1%) in 2005–2008 but decreased to 9.8% (95% CI, 9.3%–10.3%) in 2021 [61]. A consistent trend was found in daily smokers, as the estimates decreased from 5.4% (95% CI, 5.2%–5.6%) between 2005 and 2008 to 2.3% (95% CI, 2.1%–2.5%) in 2021 [61].However, the rate of e-cigarette smoking has increased among adolescents. According to the Korea Centers for Disease Control and Prevention, the rate of youth smoking e-cigarettes in 2023 was 3.8%, an increase of 1% in 3 years. Although e-cigarettes are relatively safe since they do not contain tobacco or combustible compounds, they adversely affect on adolescents by inducing nicotine dependence and acting as a gateway to tobacco smoking [62]. The adolescent brain is more sensitive to nicotine, causing youth to develop nicotine dependence more easily than adults [62]. A considerable percentage of individuals who start smoking during adolescence continue into adulthood, and one-third of these individuals die prematurely due to smoking-related diseases [63].The use of substances for nontherapeutic purposes has recently become a significant issue in Korea. According to the 17th Korea Youth Risk Behavior Web-Based Survey of 2021, the identified risk factors for substance use among children and adolescents were anxiety, loneliness, living separately from family, suicidal ideation, use of e-cigarettes, and high stress levels. Educational plans to prevent substance abuse and programs that promote safe substance use based on adolescent characteristics should are required [64].- 9. Microplastics
- 9. Microplastics
Plastics have been created through advances in modern science and technology and contributed significantly to the development of human civilization. However, plastics are also a major issue that must be addressed. Recently, small pieces of plastic that are difficult to identify with the naked eye have been referred to as microplastics and recognized as pollutants from plastics. A global definition of microplastic size has yet to be established. The International Organization for Standardization defines microplastics as solid plastic particles 1–1,000 µm in size that are insoluble in water [65], while the United Nations Environment Programme defines microplastics as those solid plastic particles ≤5 mm. Microplastics are categorized based on their generation: pri-mary microplastics are those contained in intentionally manufactured products, while secondary microplastics are those generated when plastic products and fragments are discharged into the environment and decomposed by weathering and wear. Most microplastics found in nature are secondary. Major sources of terrestrial and marine microplastics include laundry, tire wear, urban dust, road and ship paints, and cleaning products. Microplastics have been detected in tap water, drinking water, rivers, lakes, oceans, seafood, and marine products worldwide.The major routes through which microplastics are ingested include fish, shellfish, honey, sugar, salt, beer, bottled water, canned sardines, and tap water [66]. The impact of microplastics on the human body has not yet been clearly identified, but the number of related studies has increased with the growing interest in recent years. Among their potential effects on the human body, damage to internal organs and inflammatory response due to the physical properties of ingested microplastic particles are evident. Particles ≤0.2 µm in size can be absorbed into other tissues and organs, potentially causing localized immune system abnormalities and inflammation. Nanoplastics ≤0.1 µm in size can be absorbed into all organs and pass through the blood-brain barrier and placental barrier, potentially leading to adverse events such as compromised immunity, a hypersensitive immune response, and an abnormal inflammatory response [67]. The impact may be more severe in fetuses and growing children than in adults. Another impact is the chemical effects of additives found in microplastics, such as BPA and phthalates. These compounds are known EDs. BPA interferes with the thyroid hormone activity and causes reproductive toxicity, developmental disorders, and cardiovascular diseases. In recent studies, phthalates, which are known to cause reproductive developmental disorders and birth defects, are highly associated with depression, whereas intrauterine exposure significantly contributes the development of autism and ADHD symptoms in children [68]. And finally, microplastics play a role as carriers of other harmful substances. Polypropylene particles, the most abundant substances in microplastics, easily bind to persistent organic pollutants and carcinogens such as dichlorodi-phenyltrichloroethane, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls. Moreover, microplastics can easily adsorb heavy metals such as Pb, Cd, and Ni. Pb in particular can cause cognitive impairment, neurobehavioral abnormalities, and developmental disorders in children [69]. Humans are the sources and the victims of microplastic pollution.Once discharged into nature, microplastics cannot be collected using existing science and technology methods because, even when broken down, they remain microplastics. Although microplastics have low toxicity, humans continuously ingest and inhale them daily, leading to the conclusion that chronic exposure to microplastics is likely to become a significant issue for humans in the future. Therefore, in the absence of a clear understanding of the health impacts of microplastics at the present time, the best approach is to minimize their discharge into the environment.
- Conclusions
- Conclusions
Environmental risks affect the health and development of children from conception through childhood and adolescence to adulthood. A child’s environment determines their future: early-life exposure impacts adult health, as fetal programming and early growth may be altered by environmental risk factors. Recent initiatives by organizations such as the World Health Organization and the United Nations have highlighted the pressing need to address environmental pollution and protect of children's health globally. These entities play significant roles in advocating policies and actions that promote environmental health among children worldwide.
- Footnotes
-
Conflicts of interest No potential conflict of interest relevant to this article.
Funding This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution Conceptualization: JL, HBK, HJJ, MC, SEP, KHL, WSK, JHM, JWL, JWS, SSL, YK, Yoo YY; Data curation: JL, HBK; Formal analysis: JHM, JWL, JWS, LS, MC, SEP, WSK; Methodology: HJJ, KHL; Project administration: YK, YY; Visualization: YY; Writing-original draft: JL, HBK, HJJ, MC, SEP, KHL, WSK, JHM, JWL, JWS, SSL, YK, YY; Writing-review & editing: JL, Kim H, HJJ, MC, SEP, KHL, WSK, JHM, JWL, JWS, SSL, YK, YY.
-
Table 1.
Exposure to environmental pollutants and children’s health
- References
- 1. Mastorci F, Linzalone N, Ait-Ali L, Pingitore A. Environment in children's health: a new challenge for risk assessment. Int J Environ Res Public Health 2021;18:10445.
[Article] [PubMed] [PMC]3. Le QH, Tran DD, Chen YC, Nguyen HL. Risk of lead exposure from transport stations to human health: a case study in the highland province of Vietnam. Toxics 2019;7:48.
[Article] [PubMed] [PMC]4. Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 1995;25:1–24.
[Article] [PubMed]5. Michalek IM, Benn EKT, Dos Santos FLC, Gordon S, Wen C, Liu B. A systematic review of global legal regulations on the permissible level of heavy metals in cosmetics with particular emphasis on skin lightening products. Environ Res 2019;170:187–93.
[Article] [PubMed]6. Gabriel MF, Felgueiras F, Batista R, Ribeiro C, Ramos E, Mourao Z, et al. Indoor environmental quality in households of families with infant twins under 1 year of age living in Porto. Environ Res 2021;198:110477.
[Article] [PubMed]7. Hauptman M, Woolf AD. Childhood ingestions of environmental toxins: what are the risks? Pediatr Ann 2017;46:e466–71.
[Article] [PubMed] [PMC]8. Zheng T, Zhang J, Sommer K, Bassig BA, Zhang X, Braun J, et al. Effects of environmental exposures on fetal and childhood growth trajectories. Ann Glob Health 2016;82:41–99.
[Article] [PubMed] [PMC]9. Deguen S, Zmirou-Navier D. Social inequalities resulting from health risks related to ambient air quality-A European review. Eur J Public Health 2010;20:27–35.
[Article] [PubMed]10. Yoon JY, Park HA, Kang JH, Kim KW, Hur YI, Park JJ, et al. Prevalence of dietary supplement use in Korean children and adolescents: insights from Korea National Health and Nutrition Examination Survey 2007-2009. J Korean Med Sci 2012;27:512–7.
[Article] [PubMed] [PMC]11. Stierman B, Mishra S, Gahche JJ, Potischman N, Hales CM. Dietary supplement use in children and adolescents aged ≤19 years - United States, 2017-2018. Morb Mortal Wkly Rep 2020;69:1557–62.
[Article] [PubMed] [PMC]12. Koletzko B, Cremer M, Flothkotter M, Graf C, Hauner H, Hellmers C, et al. Diet and lifestyle before and during pregnancy - Practical recommendations of the Germany-wide Healthy Start - Young Family Network. Geburtshilfe Frauenheilkd 2018;78:1262–82.
[Article] [PubMed] [PMC]13. Mori C, Todaka E. For a healthier future: a virtuous cycle for reducing exposure to persistent organic pollutants. J Epidemiol Community Health 2017;71:660–2.
[Article] [PubMed] [PMC]14. Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M, et al. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol 2017;139:122–9.
[Article] [PubMed]15. Yang SI, Lee SY, Kim HB, Kim HC, Leem JH, Yang HJ, et al. Prenatal particulate matter affects new asthma via airway hyperresponsiveness in schoolchildren. Allergy 2019;74:675–84.
[Article] [PubMed]16. Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 2019;129:1504–15.
[Article] [PubMed] [PMC]17. Ho SM. Environmental epigenetics of asthma: an update. J Allergy Clin Immunol 2010;126:453–65.
[Article] [PubMed] [PMC]18. Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Eng J Med 2015;372:905–13.
[Article] [PubMed] [PMC]19. Kim JY, Park Y, Kim SH, Kim SP, Park SW, Yoon HJ. Effect of ambient air pollutants on the medical costs of allergic rhinitis in Seoul, Korea. Laryngoscope 2023;133:1828–33.
[Article] [PubMed]20. Zheng LY, Sanders AP, Saland JM, Wright RO, Arora M. Environmental exposures and pediatric kidney function and disease: a systematic review. Environ Res 2017;1589:625–48.
[Article] [PubMed] [PMC]21. Kuzma L, Malyszko J, Bachorzewska-Gajewska H, Kralisz P, Dobrzycki S. Exposure to air pollution and renal function. Sci Rep 2021;11:11419.
[PubMed] [PMC]22. Sierra-Diaz E, Celis-de la Rosa AJ, Lozano-Kasten F, BravoCuellar A, Garcia-Gutierrez M, Georgina HF. Non-traditional risk factors of albuminuria in the pediatric population: a scoping review. Int J Environ Res Public Health 2017;14:1231.
[Article] [PubMed] [PMC]23. Chen Y, Cao F, Xiao JP, Fang XY, Wang XR, Ding LH, et al. Emerging role of air pollution in chronic kidney disease. Environ Sci Pollut Res Int 2021;28:52610–24.
[Article] [PubMed]24. Shubham S, Kumar M, Sarma DK, Kumawat M, Verma V, Samartha RM, et al. Role of air pollution in chronic kidney disease: an update on evidence, mechanisms and mitigation strategies. Int Arch Occup Environ Health 2022;95:897–908.
[Article] [PubMed]25. Kim JH, Moon JS. Secular trends in pediatric overweight and obesity in Korea. J Obes Metab Syndr 2020;29:12–7.
[Article] [PubMed] [PMC]26. Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J Hepatol 2014;6:263–73.
[Article] [PubMed] [PMC]27. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care 2015;4:187–92.
[Article] [PubMed] [PMC]28. Gwag SH, Oh YR, Ha JW, Kang E, Nam HK, Lee Y, et al. Weight changes of children in 1 year during COVID-19 pandemic. J Pediatr Endocrinol Metab 2022;35:297–302.
[Article] [PubMed]29. Mohsen H, Ibrahim C, Bookari K, Saadeh D, Al-Jawaldeh A, Sacre Y, et al. Prevalence of essential nutrient supplement use and assessment of the knowledge and attitudes of Lebanese mothers towards dietary supplement practices in maternal, infancy and preschool ages: findings of a national representative cross-sectional study. Foods 2022;11:3005.
[Article] [PubMed] [PMC]30. Moon JH. Health effects of electromagnetic fields on children. Clin Exp Pediatr 2020;63:422–8.
[Article] [PubMed] [PMC]31. Stewart W. Mobile phones and health, report of independent expert group on mobile phones. Chilton (UK): IEGMP Secretariat, 2000.32. Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics 2001;108:40–3.
[Article] [PubMed]33. Harrison RV. The prevention of noise induced hearing loss in children. Int J Pediatr 2012;2012:473541.
[Article] [PubMed] [PMC]34. Kim MG, Hong SM, Shim HJ, Kim YD, Cha CI, Yeo SG. Hearing threshold of Korean adolescents associated with the use of personal music players. Yonsei Med J 2009;50:771–6.
[Article] [PubMed] [PMC]35. Kim SH, Cha ES, Cha HE, Song JJ, Chae SW. Prevalence and clinical aspects of hearing loss among the South Korean adolescent: data from a population-based study. Int J Pediatr Otorhinolaryngol 2020;128:109698.
[Article] [PubMed]36. Nose Y, Fujinaga R, Suzuki M, Hayashi I, Moritani T, Kotani K, et al. Association of evening smartphone use with cardiac autonomic nervous activity after awakening in adolescents living in high school dormitories. Childs Nerv Syst 2017;33:653–8.
[Article] [PubMed]37. Touitou Y, Touitou D, Reinberg A. Disruption of adolescents' circadian clock: the vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. J Physiol Paris 2016;110(4 Pt B): 467–79.
[Article] [PubMed]38. Lee SI, Matsumori K, Nishimura K, Nishimura Y, Ikeda Y, Eto T, et al. Melatonin suppression and sleepiness in children exposed to blue-enriched white LED lighting at night. Physiol Rep 2018;6:e13942.
[Article] [PubMed] [PMC]39. Shechter A, Kim EW, St-Onge MP, Westwood AJ. Blocking nocturnal blue light for insomnia: a randomized controlled trial. J Psychiatr Res 2018;96:196–202.
[Article] [PubMed] [PMC]40. Heo JY, Kim K, Fava M, Mischoulon D, Papakostas GI, Kim MJ, et al. Effects of smartphone use with and without blue light at night in healthy adults: a randomized, double-blind, cross-over, placebo-controlled comparison. J Psychiatr Res 2017;87:61–70.
[Article] [PubMed]41. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2:the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:e1–150.
[Article] [PubMed] [PMC]42. Ghassabian A, Vandenberg L, Kannan K, Trasande L. Endocrine-disrupting chemicals and child health. Annu Rev Pharmacol Toxicol 2022;62:573–94.
[Article] [PubMed]43. Livadas S, Chrousos GP. Molecular and environmental mechanisms regulating puberty initiation: an integrated approach. Front Endocrinol (Lausanne) 2019;10:828.
[Article] [PubMed] [PMC]44. Iughetti L, Lucaccioni L, Street ME, Bernasconi S. Clinical expression of endocrine disruptors in children. Curr Opin Pediatr 2020;32:554–9.
[Article] [PubMed]45. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 2020;8:703–18.
[Article] [PubMed] [PMC]46. Uldbjerg CS, Koch T, Lim YH, Gregersen LS, Olesen CS, Andersson AM, et al. Prenatal and postnatal exposures to endocrine disrupting chemicals and timing of pubertal onset in girls and boys: a systematic review and meta-analysis. Hum Reprod Update 2022;28:687–716.
[Article] [PubMed] [PMC]47. Shah-Kulkarni S, Lee S, Jeong KS, Hong YC, Park H, Ha M, et al. Prenatal exposure to mix tures of heavy metals and neurodevelopment in infants at 6 months. Enviro Res 2020;182:109122.
[Article]48. Jeong KS, Kim S, Kim WJ, Kim HC, Bae J, Hong YC, et al. Cohort profile: beyond birth cohort study - The Korean CHildren's ENvironmental health Study (Ko-CHENS). Environ Res 2019;172:358–66.
[Article] [PubMed]49. Choe SA, Yoo Y, The Korean CHildren's ENvironmental health Study (Ko-CHENS) study Group. Effect modification by gestational weight gain in the association between maternal exposure to heavy metals and birthweight [abstract]. ISEE 2023: 35th Annual Conference of the International Society of Environmental Epidemiology. Durham (NC): EHP Publishing; 2023.50. Goto Y, Mandai M, Nakayama T, Yamazaki S, Nakayama S, Isobe T, et al. Association of prenatal maternal blood lead levels with birth outcomes in the Japan Environment and Children's Study (JECS): a nationwide birth cohort study. Int J Epidemiol 2021;50:156–64.
[Article] [PubMed]51. Takatani T, Eguchi A, Yamamoto M, Sakurai K, Takatani R, Taniguchi Y, et al. Individual and mixed metal maternal blood concentrations in relation to birth size: an analysis of the Japan Environment and Children's Study (JECS). Environ Int 2022;165:107318.
[Article] [PubMed]52. Al Osman M, Yang F, Massey IY. Exposure routes and health effects of heavy metals on children. Biometals 2019;32:563–73.
[Article] [PubMed]53. Patra RC, Rautray AK, Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011;2011:457327.
[Article] [PubMed] [PMC]54. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress. A report of the surgeon general. The health consequences of smoking: 50 years of progress. Atlanta (GA): Centers for Disease Control and Prevention (US), 2014.55. Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 2016;184:87–97.
[Article] [PubMed] [PMC]56. Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 1999;38:892–9.
[Article] [PubMed]57. Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res 2011;12:5.
[Article] [PubMed] [PMC]58. Zhu JL, Olsen J, Liew Z, Li J, Niclasen J, Obel C. Parental smoking during pregnancy and ADHD in children: the Danish national birth cohort. Pediatrics 2014;134:e382–8.
[Article] [PubMed]59. Ip P, Chung BH, Ho FK, Chan GC, Deng W, Wong WH, et al. Prenatal tobacco exposure shortens telomere length in children. Nicotine Tob Res 2017;19:111–8.
[Article] [PubMed]60. Chiswell C, Akram Y. Impact of environmental tobacco smoke exposure on anaesthetic and surgical outcomes in children: a systematic review and meta-analysis. Arch Dis Child 2017;102:123–30.
[Article] [PubMed] [PMC]61. Shin H, Park S, Yon H, Ban CY, Turner S, Cho SH, et al. Estimated prevalence and trends in smoking among adolescents in South Korea, 2005-2021: a nationwide serial study. World J Pediatr 2023;19:366–77.
[Article] [PubMed] [PMC]62. Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb Perspect Med 2012;2:a012120.
[Article] [PubMed] [PMC]63. Ellickson PL, McGuigan KA, Klein DJ. Predictors of late-onset smoking and cessation over 10 years. J Adolesc Health 2001;29:101–8.64. Park SY. Predictive factors of substance misuse and abuse in South Korean adolescents: a secondary data analysis of the 2021 Youth Risk Behavior Web-based Survey. Child Health Nurs Res 2024;30:67–74.
[Article] [PubMed] [PMC]65. ISO/TR 21960:2020, Plastics - environmental aspects - state of knowledge and methodologies. Geneva (Switzerland): International Organization for Standardization (ISO), 2020.66. Toussaint B, Raffael B, Angers-Loustau A, Gilliland D, Kestens V, Petrillo M, et al. Review of micro- and nanoplastic contamination in the food chain. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2019;36:639–73.
[Article] [PubMed]67. Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and bi omarker responses suggest widespread health risks of exposure. Sci Rep 2017;7:46687.
[Article] [PubMed] [PMC]

 About
About Browse articles
Browse articles For contributors
For contributors