All issues > Volume 55(6); 2012
Prevalence and risk factors for vitamin D deficiency in children with iron deficiency anemia
- Corresponding author: Moon Kyu Kim, MD. Department of Pediatrics, CHA Bundang Medical Center, CHA University, 59 Yatap-ro, Bundang-gu, Seongnam 463-712, Korea. Tel: +82-31-780-5235, Fax: +82-31-780-5239, mkkim929@gmail.com
- Received May 05, 2011 Revised September 09, 2011 Accepted November 30, 2011
- Abstract
-
- Purpose
- Purpose
- The increasing prevalence of breast feeding has led to concerns about vitamin D deficiency (VDD) and iron deficiency anemia (IDA) in children. We evaluated the prevalence of VDD in a population of Korean children with IDA and assessed the risk factors for VDD in these children.
- Methods
- Methods
- A total of 79 children who were diagnosed with IDA were prospectively surveyed from April 2010 to March 2011. Data were collected by questionnaire, medical assessment, and laboratory tests, including measurement of 25-hydroxyvitamin D (25OHD), hemoglobin, and wrist radiography.
- Results
- Results
- The median age was 22 months and 30% of the subjects were female. Over a half of subjects (58%) had subnormal vitamin D level (25OHD<30 ng/mL), and VDD (25OHD<20 ng/mL) was present in 39% of children. There was no difference in serum hemoglobin level between IDA patients with VDD and those without VDD. Most subjects (89%) were currently or had recently been breastfed and almost all subjects (97%) who had VDD received breastfeeding. Children with VDD were more likely to be younger than 2 years, to have been breastfed, and to have been tested in winter or spring. Multivariable analysis indicated seasonal variation was a significant independent risk factor for VDD in our IDA patients.
- Conclusion
- Conclusion
- Our results demonstrated that VDD has a high prevalence in Korean children with IDA. Primary care physicians should be aware of the possibility of VDD in children with IDA and should supplement the vitamin D as well as iron.
- Introduction
- Introduction
Iron deficiency is the most common nutritional deficiency in world-wide and vitamin D deficiency (VDD) is raised as important nutritional problem. Vitamin D affects calcium absorption and bone metabolism, and decreased vitamin D levels are associated with type I diabetes mellitus, hypertension, cancer, and altered immune function1-4). Several studies have reported an increasing incidence of VDD and nutritional rickets in children and adolescents from the United States5-10) and other countries11-14).There are several risk factors for VDD in infants and children, including limited sun exposure because of residence in northern latitudes, dark skin, excessive use of sun block, and reliance on breastfeeding without adequate supplementation5,6,15-19).In particular, a previous study reported that exclusive breastfeeding is a risk factor for iron deficiency anemia (IDA) in infants, that the risk of IDA at 9 months was higher in exclusively breastfed infants, and that infants given iron supplements had better visual acuity and higher psychomotor and developmental scores at 13 months than infants not given iron supplemen20). In recent years, breast feeding has become more common in Korea and other countries, prompting concern that IDA and VDD may also be increasing in infants and children21,22).Children in Korea may be vulnerable to IDA and VDD because of Korea's relatively northern latitude, skin pigmentation, and the increasing prevalence of breastfeeding. In the present study, we evaluated the prevalence of VDD in Korean children with IDA and assessed the risk factors of VDD.
- Materials and methods
- Materials and methods
- 1. Subjects and data collection
- 1. Subjects and data collection
Seventy-nine children, 4 months to 13 years in age, who were diagnosed with IDA at our institution (latitude 37.4° N) between April 2010 and March 2011 were prospectively enrolled. Data on demographics, birth history, feeding practices, micronutrient supplements, immunization status, and medical history were collected by questionnaire. The parent study also evaluated anemia and dietary supplementation. Feeding patterns were grouped into three categories with regard to use of breast milk, cow's milk, and formula.All subjects were examined by a pediatrician for evidence of illness. This examination included a full blood panel, and measurement of serum levels of reticulocytes, ferritin, iron, total iron-binding capacity (TIBC), 25-hydroxyvitamin D (25OHD), alkaline phosphatase (ALP), phosphate, and calcium. Wrist radiographs were performed to assess bone status.- 2. Definitions of anemia and VDD
- 2. Definitions of anemia and VDD
IDA was defined as hemoglobin (Hb) less than 11 g/dL and serum ferritin less than 12 ng/mL for both males and females23). Serum ferritin is an acute-phase reactant, so concentrations may be elevated in the presence of chronic inflammation, infection, or liver disease, and simultaneous measurement of C-reactive protein (CRP) is required to rule out inflammation. In this study, if serum ferritin was greater than 12 ng/mL, we considered the clinical symptoms and measured CRP and diagnosed for IDA if the mean corpuscular volume (MCV) was below than 70 fL, red blood cell distribution width (RDW) was elevated, and transferrin saturation was below 15%. There is no consensus on the measurement and definition of VDD and its functional outcomes in children24,25). In this study, VDD was defined as serum 25OHD less than 20 ng/mL8,10,26,27); vitamin D insufficiency (VDI) was defined as serum 25OHD between 20 and 30 ng/mL25,27); and vitamin D sufficiency was defined as 25OHD greater than 30 ng/mL. Serum 25OHD levels were measured by a radioimmunoassay kit from DiaSorin (Stillwater, MN, USA).Radiologic evidence of rickets was defined as the presence of fraying of the epiphyseal edges, cupping of the epiphyses, widening of the wrist, or angulation of the distal forearm bones. All radiographs were reviewed by a single radiologist who was blinded to serum vitamin D levels.- 3. Statistical analysis
- 3. Statistical analysis
Data are expressed as means±SDs. Statistical analysis was performed with the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The quantitative parameters of the groups were compared by t-tests. The chi-square and Fisher's exact tests were used to compare categorical variables. Multivariable logistic regression was used to examine the simultaneous influence of several variables on the risk for VDD. Significance was evaluated using a two-sided P value of less than 0.05 with a confidence interval (CI) of 95%.
- Results
- Results
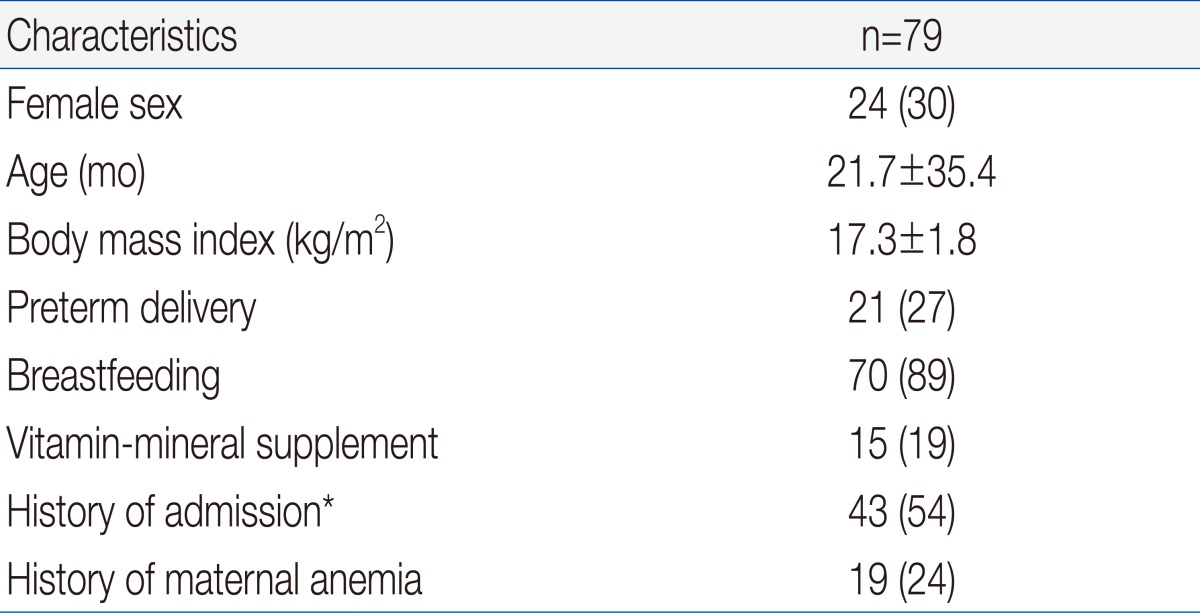
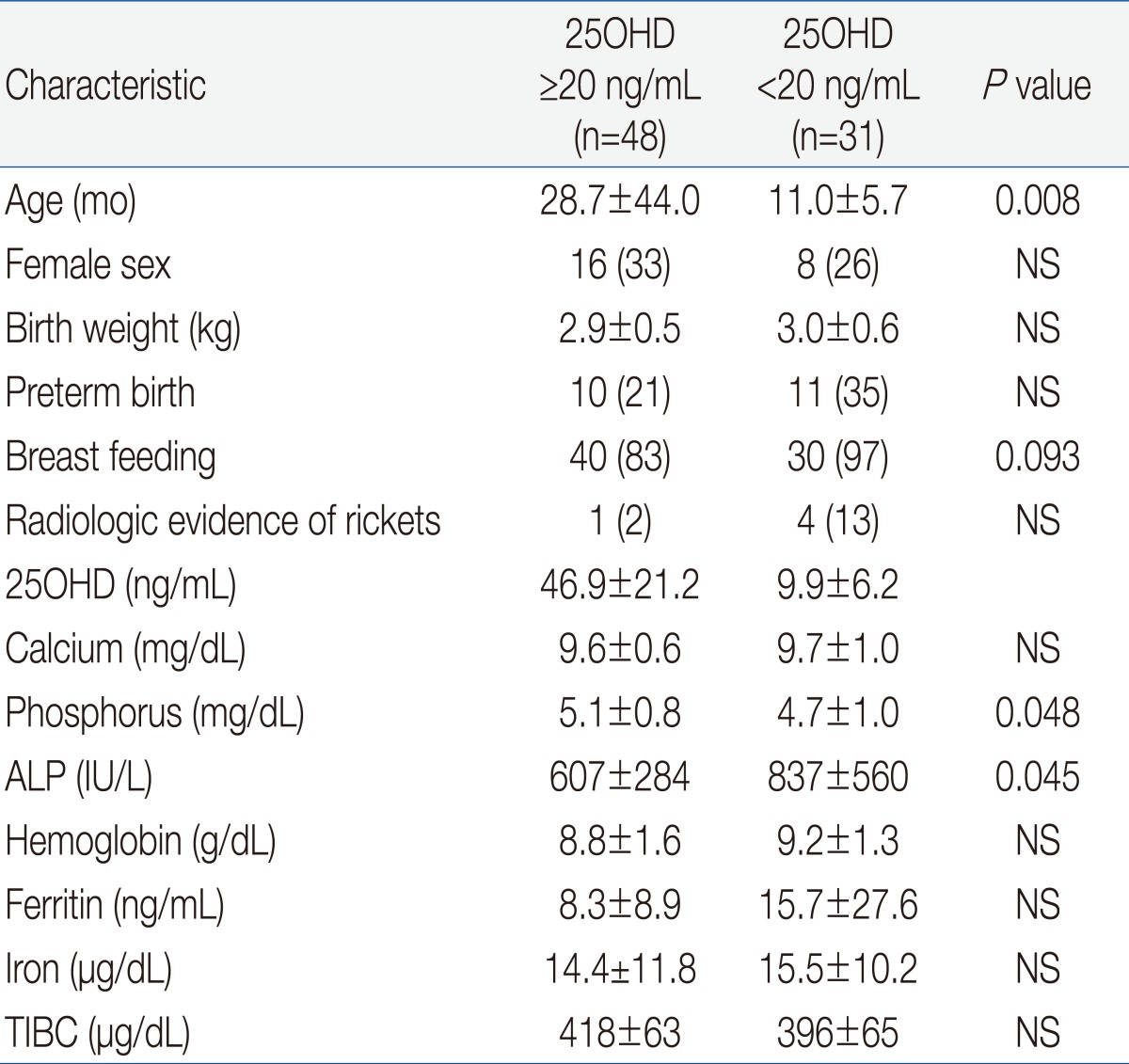
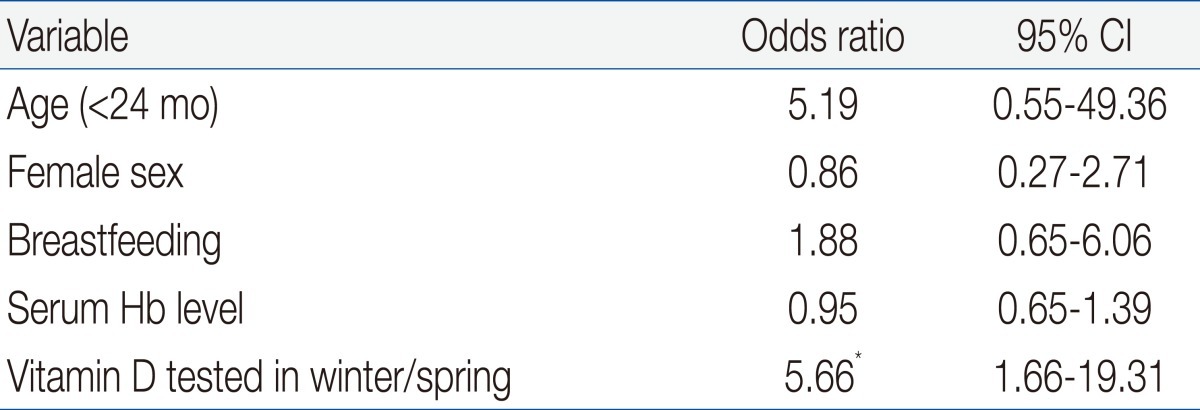
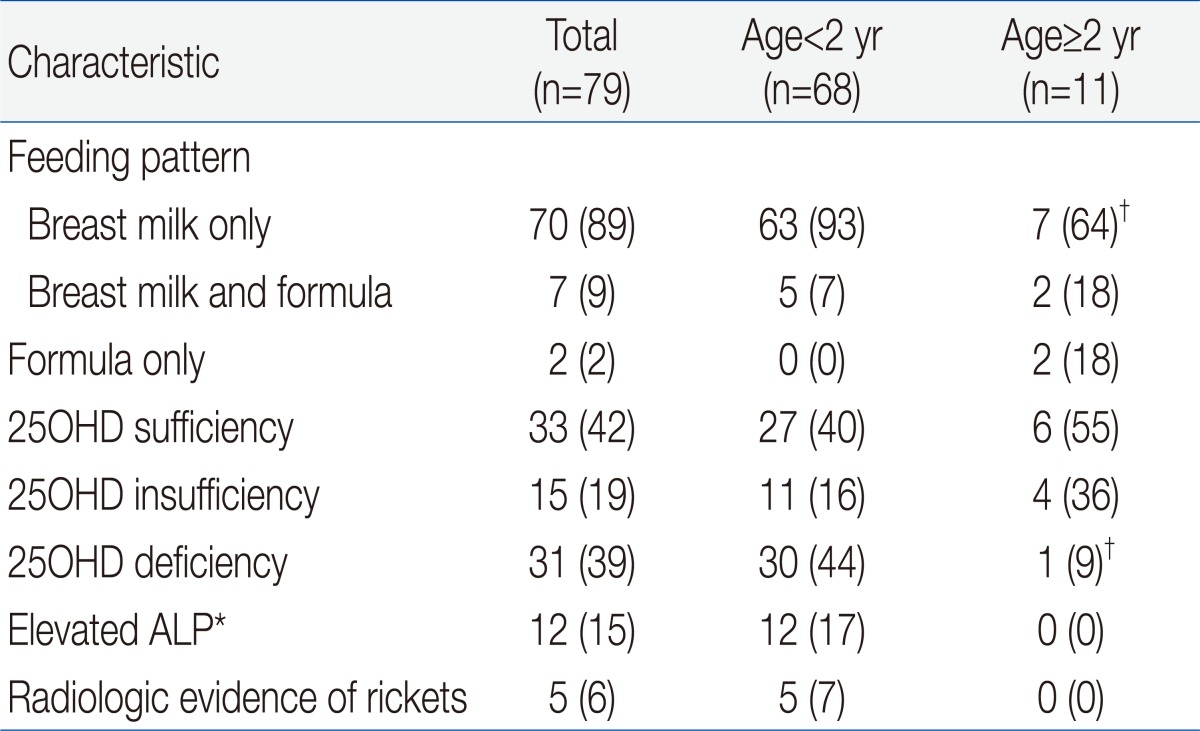
Table 1 shows the demographic and clinical characteristics of the 79 IDA children who were eligible for enrollment. The mean age at enrollment was 21.7±35.4 months and the mean body mass index was 17.3±1.8 kg/m2. One fourth of the patients had preterm birth and 24% of subjects had maternal anemia, but only 15 patients (19%) took vitamin-mineral supplements. More than half of the patients were hospitalized for diseases such as pneumonia, bronchiolitis, or gastroenteritis.We compared the etiology of IDA according to patient age. The prevalence of breast feeding was 93% in children younger than 2 years, and was 64% in children older than 2 years (P<0.05) (Table 2). Two patients older than 2 years ingested cow's milk over 1,000 mL per day and one patient with chronic gastro-intestinal bleeding was identified as having abdominal lymphangioma. Our measurement of vitamin D status indicated that 46 subjects (58%) had plasma 25OHD below 30 ng/mL, and that 31 of these subjects (39%) had VDD (25OHD<20 ng/mL). A total of 30 subjects (44%) younger than 2 years had VDD, but only 1 child older than 2 years (9%) had VDD (P=0.028). Twelve patients (15%) had elevated ALP and 5 patients (6%) had radiologic evidence of rickets, and all of these patients were younger than 2 years (P=0.159 and P=0.392, respectively). Except one patient, none of them had clinical signs of rickets. An 8 month-old patient who presented with hypocalcemic seizure at diagnosis had low levels of serum 25OHD and calcium but, did not have more severe IDA than the asymptomatic subjects. Subjects with radiologic evidence of rickets had lower levels of serum 25OHD, lower levels of phosphorus, and higher levels of serum ALP level than those with no evidence of rickets (P=0.04, P<0.001, and P<0.001, respectively). However, IDA parameters were similar between these two groups.Univariate analysis indicated that VDD was significantly associated with younger age, breastfeeding, phosphorus and ALP but not with gender, birth weight, preterm birth, radiologic evidence of rickets, serum calcium, or IDA variables (Hb, MCV, RDW, reticulocytes, ferritin, iron, and TIBC) (Table 3).We also analyzed the numbers of patients who were vitamin D sufficient, insufficient, and deficient, and the levels of vitamin D according to season (Fig. 1). The number of patients with VDI was 26 (76%) in spring (March to May), 12 (67%) in winter (December to February), 6 (33%) in summer (June to August), and 1 (22%) in autumn (September to November) (P<0.001). The mean level of serum 25OHD was significantly lower in spring/winter than summer/autumn (24±20 vs. 47±26 mg/dL, P<0.001). The spring season had the highest rate of VDD (56%). Serum Hb and ferritin concentrations were higher in winter than summer and autumn, and RDW levels were lowest in winter, but these differences were not significant (data not shown).By multivariable logistic regression the only independent risk factor for VDD was 25OHD tested in the winter or spring (odds ratio, 5.7; 95% CI, 1.7 to 19.3). Other variables including age and breastfeeding which were significant in the univariate analysis were not independently associated with VDD (Table 4).
- Discussion
- Discussion
In this study, 58% of the IDA children had subnormal vitamin D level and 39% had VDD. A total of 89% of the children were currently or had recently been breastfed, and 97% of the subjects who were VDD had been breastfed. However, subjects with VDD or VDI did not have lower serum Hb, ferritin, or iron than those with sufficient levels of 25OHD. Children with VDD tended to be younger than 2 years old, to have been breastfed, and to have been tested in winter or spring. In our study, seasonal variation in vitamin D production was the greatest risk factor for VDD. Among children who were vitamin D deficient, only 6% had radiographic evidence of rachitic changes and only 1 child had had clinical signs of hypocalcemia.There is currently no consensus on the prevalence of rickets and VDD in young children in Korea, so we compared our data with that of several other countries, although their definitions of VDD or VDI were somewhat different. VDD defined as serum 25OHD less than 20 ng/mL (50 nmol/L) was present in 12% of children aged between 8 to 24 months in Boston (latitude 42° N)8). VDD defined as serum 25OHD less than 25 ng/mL was present in 8.1% of children in California (latitude 38.5° N) and 31% of children in Alaska (over 58° N)5,7). Low level of 25OHD defined as less than 20 ng/mL was present in 87% of children in Australia (latitude 37.4° S)11). Relative to our results, the prevalence of VDD was lower in three of these studies and higher in one study. The populations of three studies are healthy children and the Australian study enrolled East African immigrant children with significant skin pigmentation, decreased daylight exposure and low socioeconomic status, so those results may not be comparable to ours because of differences of populations. However, anemia itself may predispose patients to VDD, because anemic patients are often fatigued and may be less likely to go outside to obtain adequate sun exposure for vitamin D synthesis28). And several observations suggested the role of vitamin D in erythropoiesis29-31) and the correlation of VDD and a greater risk of anemia28). Thus, although there was no significant association between the severity of anemia and low serum level of 25OHD in our study, anemia may be related to vitamin D production and further investigation needs to be conducted in healthy children.Our univariate analysis indicated that the breastfeeding is one of the risk factors for VDD. Previous studies have also reported that breastfeeding without supplementation was a risk factor for VDD5,6,9,19,32). Human breast milk has low levels of vitamin D; hence, the American Academy of Pediatrics recommended that all breastfeeding infants, regardless of whether they are given formula supplements, should be given 400 IU of vitamin D per day33). But in multivariate logistic analysis, we did not find that the breastfeeding is an independent risk factor for VDD in children with IDA.In addition, we also found a positive correlation between younger age and VDD. This correlation may be because of decreased vitamin D stores in this age group, prolonged breastfeeding, increased vitamin D requirements due to skeletal growth and/or insufficient sun exposure. However, these explanations are speculative, and were not formally assessed by this study. Thus we compared the prevalence of VDD or VDI according to age, in spite of the asymmetrical distribution of age in our patients. The prevalence of VDD was greater in children younger than 2 years than in children older than 2 years (P<0.05). All children with radiologic evidence of rickets were younger than 2 years. Interestingly almost all subjects who had VDD received breastfeeding and the prevalence of breast feeding was 93% in children younger than 2 years. Although we did not fully assess the reason of significant correlation between the younger age and VDD, higher prevalence of breastfeeding may affect the higher prevalence of VDD in children younger than 2 years. In addition prevalence of VDD is decreased in children over 2 year but, 36% still had VDI. Another study suggested that age younger than 5 years was a risk factor for VDD (P<0.001)11). We also found the positive correlation between the younger age than 5 years with VDD (P<0.001).We found that seasonal variation was an independent predictor of VDD in infants with IDA, in agreement with previous studies15-18). In particular, we found that VDD was more common and the level of serum 25OHD was significantly lower in spring/winter than summer/autumn. However, serum calcium, phosphorus, and ALP did not exhibit significant seasonal variations. Our multivariable logistic analysis indicated that seasonal variation in 25OHD was the only significant independent risk factor for VDD. Therefore we suggest the seasonal variation is the main risk factor of VDD in children with IDA.Interestingly serum Hb and ferritin concentrations were higher in winter than in summer, although these differences were not significant. A previous study of Nepali pregnant women reported that the prevalence of anemia was higher in summer/autumn than in winter/spring (P<0.001) and that Hb was lower during the monsoon period than in the winter34,35). We cannot fully reconcile these results with our own results, but they may be different due to differences in patient characteristics, economic status, and health care systems and status. Most of our subjects received breastfeeding and were younger than 24 months, so we assumed that maternal factors play a role in the Hb and ferritin levels of the children. This subject warrants further study.The limitations of our study are we restricted our patient population to children with IDA, and only a small number of children were over 2 years old. In addition, we did not examine a control group of healthy children, so our results on the prevalence of VDD in IDA cannot be generalized to other populations.In conclusion, our study of children with IDA indicated no correlation in the severity of anemia and VDD. However, our results also indicated that if a child is diagnosed with IDA, is or has been breastfed, is younger than 24 months, and the current season is winter or spring, then the primary physician should consider a diagnosis of VDD. Thus, physicians should measure the vitamin D level in such patients, a procedure that is not currently a part of routine care, and supplement the vitamin D when necessary.
- References
- 1. Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008;8:685–698.
[Article] [PubMed] [PMC]2. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80(6 Suppl): 1689S–1696S.
[Article] [PubMed]3. Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005;106:3490–3497.
[Article] [PubMed]4. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28.
[Article] [PubMed]5. Gessner BD, Plotnik J, Muth PT. 25-hydroxyvitamin D levels among healthy children in Alaska. J Pediatr 2003;143:434–437.
[Article] [PubMed]6. Ziegler EE, Hollis BW, Nelson SE, Jeter JM. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics 2006;118:603–610.
[Article] [PubMed]7. Liang L, Chantry C, Styne DM, Stephensen CB. Prevalence and risk factors for vitamin D deficiency among healthy infants and young children in Sacramento, California. Eur J Pediatr 2010;169:1337–1344.
[Article] [PubMed]8. Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med 2008;162:505–512.
[Article] [PubMed] [PMC]9. Kreiter SR, Schwartz RP, Kirkman HN Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African American breast-fed infants. J Pediatr 2000;137:153–157.
[Article] [PubMed]10. Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–44.
[Article] [PubMed]11. McGillivray G, Skull SA, Davie G, Kofoed SE, Frydenberg A, Rice J, et al. High prevalence of asymptomatic vitamin D and iron deficiency in East African immigrant children and adolescents living in a temperate climate. Arch Dis Child 2007;92:1088–1093.
[Article] [PubMed] [PMC]12. Callaghan AL, Moy RJ, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Arch Dis Child 2006;91:606–607.
[Article] [PubMed] [PMC]13. Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, et al. Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 2006;78:337–342.
[Article] [PubMed]14. Strand MA, Perry J, Jin M, Tracer DP, Fischer PR, Zhang P, et al. Diagnosis of rickets and reassessment of prevalence among rural children in northern China. Pediatr Int 2007;49:202–209.
[Article] [PubMed]15. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373–378.
[Article] [PubMed]16. Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxy-vitamin D concentrations of young American black and white women. Am J Clin Nutr 1998;67:1232–1236.
[Article] [PubMed]17. Du X, Greenfield H, Fraser DR, Ge K, Trube A, Wang Y. Vitamin D deficiency and associated factors in adolescent girls in Beijing. Am J Clin Nutr 2001;74:494–500.
[Article] [PubMed]18. Oliveri MB, Ladizesky M, Mautalen CA, Alonso A, Martinez L. Seasonal variations of 25 hydroxyvitamin D and parathyroid hormone in Ushuaia (Argentina), the southernmost city of the world. Bone Miner 1993;20:99–108.
[Article] [PubMed]19. Gessner BD, deSchweinitz E, Petersen KM, Lewandowski C. Nutritional rickets among breast-fed black and Alaska Native children. Alaska Med 1997;39:72–74.
[PubMed]20. Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003;143:582–586.
[Article] [PubMed]21. Meinzen-Derr JK, Guerrero ML, Altaye M, Ortega-Gallegos H, Ruiz-Palacios GM, Morrow AL. Risk of infant anemia is associated with exclusive breast-feeding and maternal anemia in a Mexican cohort. J Nutr 2006;136:452–458.
[Article] [PubMed]22. Korean Statistical Information Service [Internet]. Statistics Korea. c1996;cited 2011 Mar 20. Daejeon: Statistics Korea, Available from: http://kosis.kr.23. Cook JD, Lipschitz DA, Miles LE, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr 1974;27:681–687.
[Article] [PubMed]24. Greer FR. Defining vitamin D deficiency in children: beyond 25-OH vitamin D serum concentrations. Pediatrics 2009;124:1471–1473.
[Article] [PubMed]25. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009;19:73–78.
[Article] [PubMed]26. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–373.
[Article] [PubMed]27. Cole CR, Grant FK, Tangpricha V, Swaby-Ellis ED, Smith JL, Jacques A, et al. 25-hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics 2010;125:633–639.
[Article] [PubMed] [PMC]28. Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, et al. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol 2010;89:447–452.
[Article] [PubMed]29. Albitar S, Genin R, Fen-Chong M, Serveaux MO, Schohn D, Chuet C. High-dose alfacalcidol improves anaemia in patients on haemodialysis. Nephrol Dial Transplant 1997;12:514–518.
[Article] [PubMed]30. Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 2007;105:c132–c138.
[Article] [PubMed]31. Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med 1989;320:980–991.
[Article] [PubMed]32. Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ 2007;177:161–166.
[Article] [PubMed] [PMC]33. Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding. American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142–1152.
[Article] [PubMed]
Fig. 1
Changes in vitamin D status (bars, left axis) and mean vitamin D level (line, right axis) throughout the year. Vitamin D insufficiency+ deficiency was 76% in spring (March to May), 67% in winter (December to February), 33% in summer (June to August), and 22% in autumn (September to November) (P<0.001). The mean level of serum 25-hydroxyvitamin D (25OHD) was significantly lower in spring/winter than in summer/autumn (24±20 vs. 47±26 ng/mL, P<0.001).
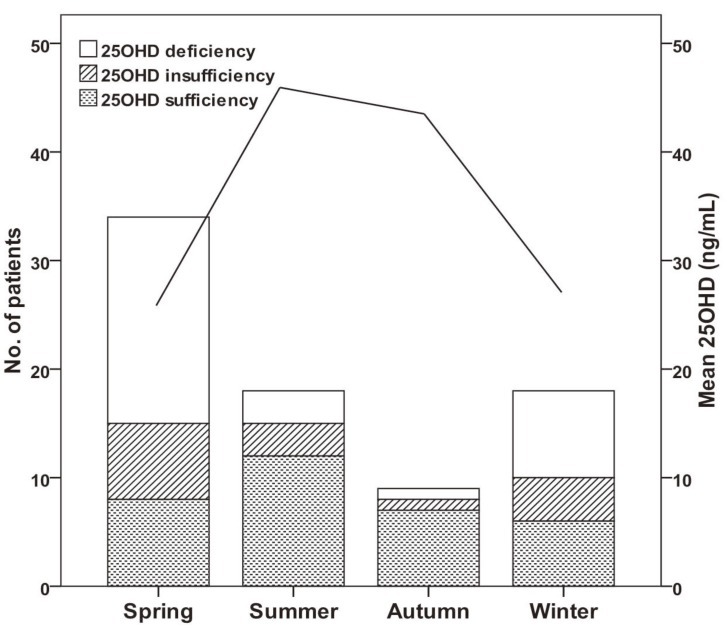
Table 2
Feeding Pattern, Vitamin D and Alkaline Phosphatase Status, and Radiological Evidence of Rickets in Children with Iron Deficiency Anemia

 About
About Browse articles
Browse articles For contributors
For contributors