All issues > Volume 55(8); 2012
Parental concerns about their premature infants' health after discharge from the neonatal intensive care unit: a questionnaire survey for anticipated guidance in a neonatal follow-up clinic
- Corresponding author: In Kyung Sung, MD, PhD. Department of Pediatrics, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, 222 Banpo-daero, Seocho-gu, Seoul 137-701, Korea. Tel:+82-2-2258-6184, Fax:+82-2-537-4544, sinky@catholic.ac.kr
- Received September 06, 2011 Revised December 20, 2011 Accepted March 20, 2012
- Abstract
-
- Purpose
- Purpose
- The aim of this study was to develop an appropriate nursing information guideline according to corrected age, after investigating parents' concerns about the growth, development, and diseases of their premature infants after discharge from the neonatal intensive care unit (NICU).
- Methods
- Methods
- The parents of premature infants (birth weight, <2,500 g; gestational age, <37 weeks) who went to a neonatal follow-up clinic after NICU discharge at Seoul St. Mary's Hospital from January 2005 to December 2009, were asked with regard to their concerns about their infants through a questionnaire survey. The results of physical examinations, including body measurements and neurodevelopmental status at 4, 8, 12, and 18 months of corrected age, were retrospectively reviewed in 390 infants.
- Results
- Results
- The most common parental concerns were developmental delay, poor growth, and feeding and nutritional problems. Parental concerns about developmental delay, growth failure in improvement in body weight and length, and overweightness were high in specificity but very low in sensitivity. After NICU discharge, 30% of premature infants experienced infectious diseases before 18 months of corrected age, the most common of which was respiratory tract infection.
- Conclusion
- Conclusion
- For guiding of premature infants in outpatient day clinics after NICU discharge, it is necessary to identify the parents' highest concerns, to educate them about the possibilities of growth and neurodevelopmental disabilities in their infants and to provide them with handouts containing guidelines on the management of infectious diseases, especially respiratory infections.
- Introduction
- Introduction
Premature infants in the neonatal intensive care unit (NICU) differ from healthy full-term infants. Due to complicated health problems, special care should be given to the premature infants even after hospital discharge. In terms of the health management of premature infants after NICU discharge, vaccination and individual disease management as well as general medical examinations including vision, hearing, and developmental screening tests are important. A wide variety of health problems can occur in premature infants before and after NICU discharge, including feeding problems, growth failure, respiratory morbidities including bronchopulmonary dysplasia, gastroesophageal reflux, anemia, eye diseases, neurodevelopmental disabilities, surgical problems, and dental diseases.It is not easy to care for premature infants after NICU discharge because caregivers must be extremely aware of their physical weaknesses and maintain a good nursing environment. Parents' stress, lack of confidence, or worry about raising their premature infant can have a negative effect on infant's growth and development. Parents' nursing behavior is closely correlated with their premature infant's development1). Since early intervention can help parents manage the related stress2), it is critical to teach them how to care for their premature baby after hospital discharge. The aim of this study was to create an appropriate nursing information guideline by corrected age after investigating the parents' concerns about their premature infants, and examine health problemsafter NICU discharge.
- Materials and methods
- Materials and methods
- 1. Subjects
- 1. Subjects
The parents of premature infants (birth weight, <2,500 g; gestational age, <37 weeks) who went to a neonatal follow-up clinic after NICU discharge at Seoul St. Mary's Hospital from January 2005 to December 2009 were asked about their concerns about their child using a questionnaire survey.All premature NICU graduates were scheduled to visit the neonatal follow-up clinic at the corrected ages of 0, 4, 8, 12, 18, and 24 months. After that point, the babies visited the hospital annually. Procedures undergoing at each follow-up visit consist of physical examination including body measurements, neurological examination using infant neurologic scoring system, and developmental test using Bayley Scales of Infant Development-II3).This study was performed with cross-sectional analysis. We examined 390 premature infants whose parents answered questionnaires at corrected ages of 4 months (n=127), 8 months (n=104), 12 months (n=85), and 18 months (n=74). Infants with chromosomal aberrations, congenital metabolic or endocrine disorders, major congenital anomalies in central nervous system, and who had surgery for gastrointestinal anomalies were excluded.- 2. Methods
- 2. Methods
The results of physical examinations including body measurements (weight, length, head circumference, etc.), growth percentiles, and neurodevelopmental findings were investigated through review of medical records. "Growth failure" was identified when body measurements were <10th percentile in terms of corrected age on the Korean children and adolescents' growth standard chart. Body measurement above the 10th percentile was categorized as "success in catch-up growth". Infants who weighed over the 90th percentile were classified as "overweight".The neurological opinions were divided into "normal," "suspect," and "abnormal." While hydrocephalus that led to cerebral palsy was categorized as "abnormal," tone, posture, reflex, and movement abnormalities that were not included in the other diagnostic criteria of cerebral palsy were categorized as "suspect". Those with Bayley Scales of Infant Development below mean -1 SD were defined as having "developmental delay".The questionnaires consisted of 7 questions (response to sound, motion symmetry, motion and tension of legs, family history of hearing impairment, concerns on eyesight, latest disease report, and others) for infants at 4, 8, and 12 months of corrected age and 8 questions (response to sound, language development level, accuracy in expression, gross motor development, family history of hearing impairment, concerns on eyesight, latest disease report, and others) for babies at 18 months of corrected age. This study evaluated any involvement of diseases, which were carefully monitored from one corrected age point to the next. Additionally, the parents were asked to reply whether they have any concerns regarding their children. If they have any, their concerns were categorically divided.Data were analyzed using the SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). Examined data were assessed using the t-test and analysis of variance. Data were expressed as the mean±SD, and P<0.05 was accepted as statistically significant.
- Results
- Results
- 1. Clinical characteristics
- 1. Clinical characteristics
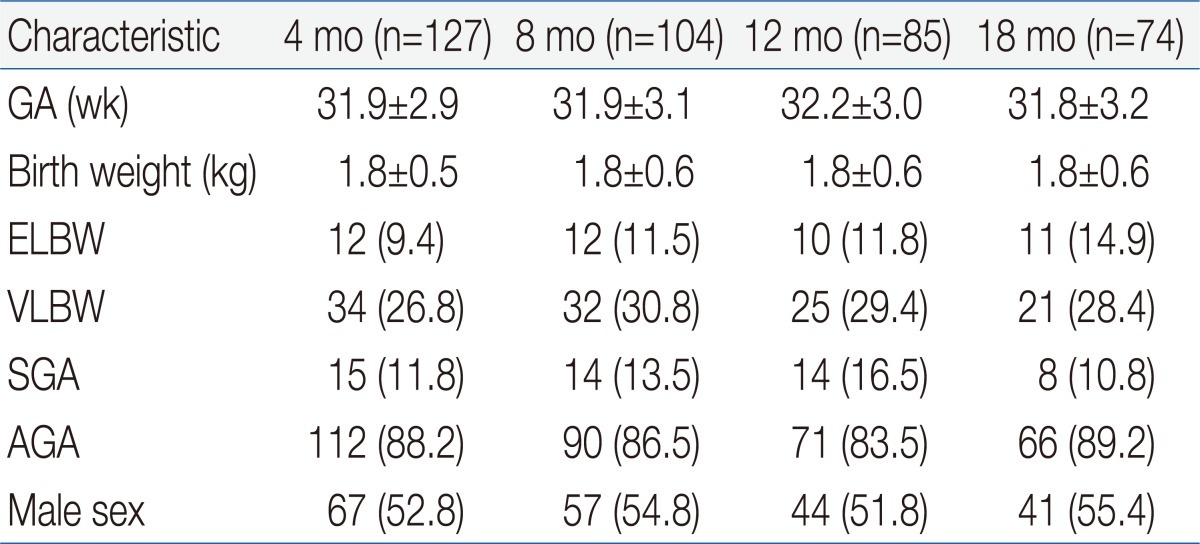
There was no age-dependent difference in gestational age or birth weight. In terms of gestational age, 61 infants were <28 weeks, 140 infants were with 28 to 31+6 weeks, and 189 infants were 32 to 36+6 weeks. Forty-five infants were extremely low birth weight (ELBW, <1,000 g), 112 had very low birth weight (VLBW, <1,500 g). In terms of composition ratio, there was no difference between ELBW and VLBW. In terms of weight appropriateness, 51 were small-for-gestational age (SGA), and 339 were appropriate for gestational age (AGA) at birth. Proportion of SGA infants at 12 months of corrected age was higher than the other ages, but the difference was not statistically significant. There were no significant differences in gender distribution between age groups (Table 1).- 2. Growth status
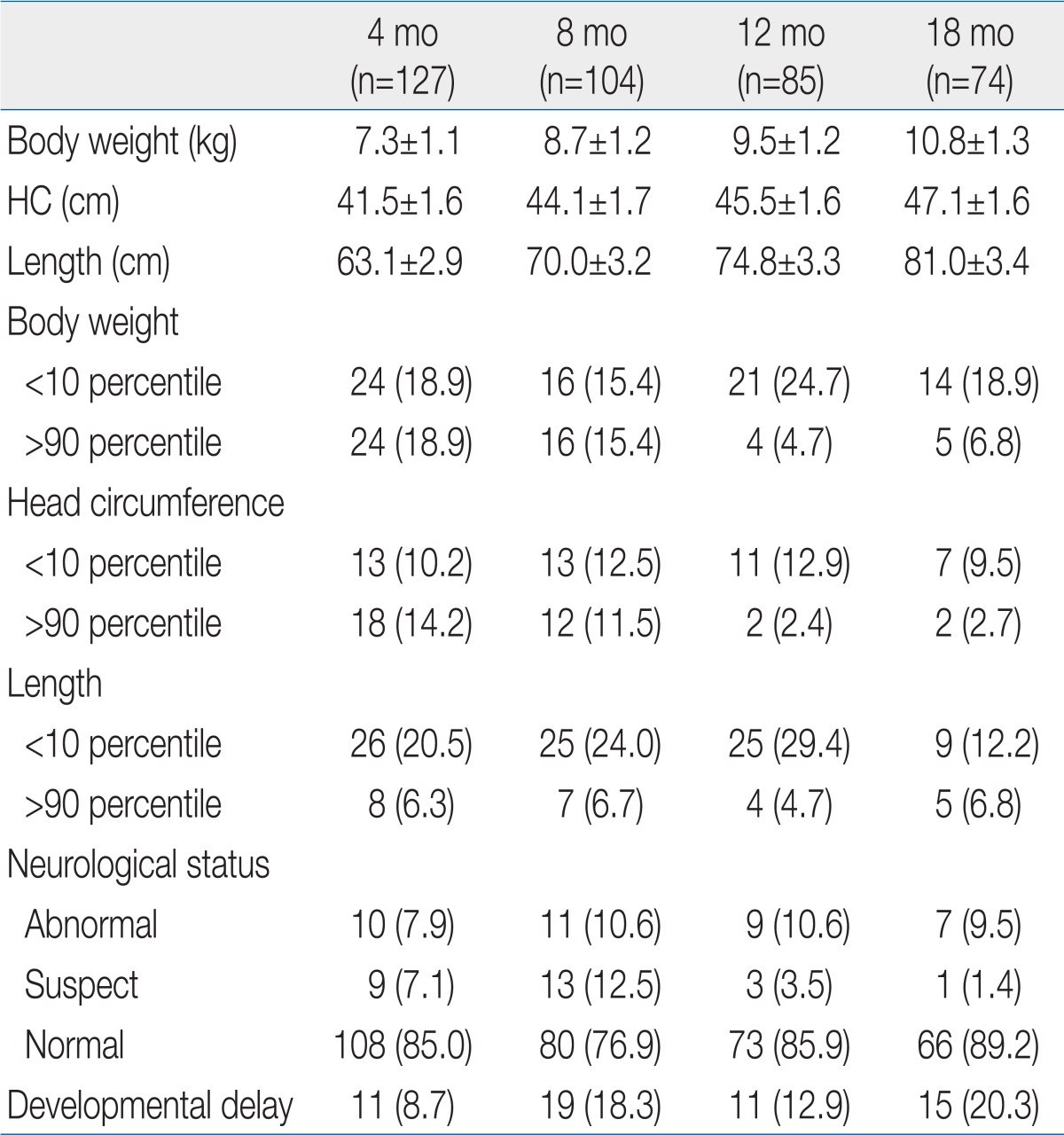
- 2. Growth status
The ratio of growth failure in body weight was highest at 12 months of corrected age, but age dependent difference was not significant (Table 2). Weight catch-up failure rate increased as birth weight decreased (P<0.05) and SGA infants showed higher growth failure rate than AGA infants (P<0.05) at 18 months of corrected age (Table 3). Most of the overweight infants were identified at 4 and 8 months of corrected age (Table 2).No significant difference in the ratio of infants who had head circumference <10th percentile was observed by corrected age. As birth weight decreased, head circumference catch-up failure rate increased (P<0.05) and SGA infants showed higher head circumference catch-up growth failure rate than AGA infants (P<0.05) at 18 months of corrected age (Table 3).The ratio of infants who had a length <10th percentile was lowerat 18 months than at 4, 8, and 12 months of corrected age (Table 2). Length catch-up growth failure rate was not different between infants <999 g and infants 1,000 to 1,499 g of birth weight, but the rates were significantly higher than infant 1,500 to 2,499 g. SGA infants showed higher length catch-up growth failure rate than AGA infants (P<0.05) at 18 months of corrected age (Table 3).- 3. Neurodevelpmental status
- 3. Neurodevelpmental status
There was no age-dependent difference in the rate of infant identified as neurologically "abnormal". Most of the infants rated as "suspect" were identified at 4 and 8 months of corrected age (Table 2). As birth weight decreased, abnormal neurological status rate increased (P<0.05). No significant difference in the rate of abnormal neurological status was observed between SGA and AGA infant at 18 months of corrected age (Table 3). The rate of abnormal neurological status was significantly higher in infant who failed to catch up head circumference than in infants with head circumference catch-up (29.5% vs. 8.7%, P<0.05).As birth weight decreased, developmental delay rate increased (P<0.05) and SGA infants showed higher developmental delay rate than AGA infants (P<0.05) at 18 months of corrected age (Table 3). Developmental delay was found in 40.9% among infants with head circumference <10th percentile, on the contrary, it was found in 10.9% of infants who succeed to catch up growth of head circumference (P<0.05).- 4. Parental concerns
- 4. Parental concerns
In terms of parents' concerns about their premature infants after NICU discharge, developmental delay was the highest, followed by feeding and nutrition, growth, ophthalmologic problems, skin problems, respiratory morbidity, and behavioral problems (Table 4).Parental concerns about motor development were the most common at 8 months of corrected age. Most of the respondents who worried about mental development focused on infants who were 12 or18 months of corrected age. Parents who worried about whether their child would suffer from developmental delay just because he/she was born prematurely were mainly at 4 months of corrected age. Among 109 parents who showed concern about a developmental delay, only 32 infants were confirmed as having a developmental delay. Of the 56 premature infants who were identified as having a developmental delay, only 32 parents showed concern about adevelopmental delay (sensitivity, 57.1% and specificity, 77.0%).Regarding feeding and nutrition, parents worried about gastrointestinal problems, poor oral intake, irregular or frequent feeding, weaning food problems.Regarding growth, most parents were worried about their infants being underweight. Among 44 parents who were concerned about growth failure or underweightness, body weight <10th percentile was observed in only 22 infants. On the contrary, among 75 infants with growth failure, only 22 parents actually showed concern (sensitivity, 29.3%; specificity, 93.0%). Among 49 premature infants whose body weight was >90th percentile, only 4 parents were concerned about over weightness (sensitivity, 8.2%; specificity, 100%). Among 6 respondents who worried about short length, only 3 were actually had length <10th percentile. Among 85 premature infants with length <10th percentile, only 3 respondents were actually concerned (sensitivity, 3.5%; specificity, 99.0%).- 5. Morbidity required medical care after discharge
- 5. Morbidity required medical care after discharge
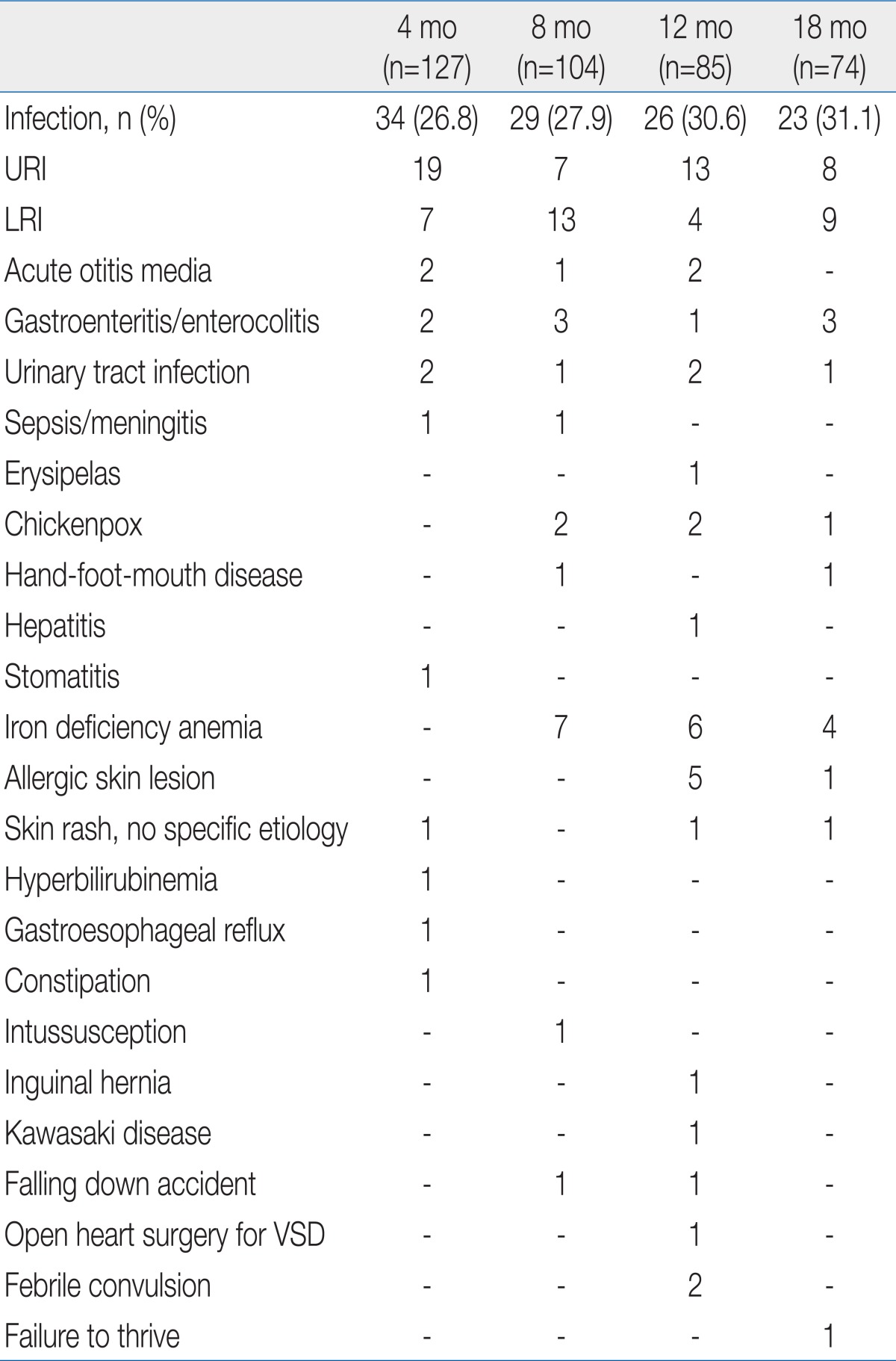
Infectious diseases were the most frequent issue for medical treatment in premature infants after NICU discharge. Upper and lower respiratory tract infections were the two leading causes of infectious diseases. Infants who received medical treatment for iron deficiency anemia were reported at 8, 12, and 18 months of corrected age (Table 5).
- Discussion
- Discussion
The mean body weights of premature infants at 4, 8, 12, and 18 months of corrected age were included within the 10th to 90th percentiles on the Korean children and adolescents' growth standard chart (2007). According to our investigation, 18.9% of the premature infants failed to catch up growing of body weight at 18 months of corrected age. Catch up growth failure rate was different by birth weight. In ELBW infant, 54.5% was failed to catch up weight while the rates were reported 30.0% and 9.4% in the infants whose birth weights were in the range of 1,000 to 1,499 g and 1,500 to 2,499 g. In other words, as birth weight decreased, catch-up growth failure rates increased. Compared to a study by Park et al.4), who insisted that 49.2% of ELBW babies failed to catch up growth until 12 months of corrected age, this study reached 66.7% in terms of catch-up growth failure rates. The time point at whichcatch-up growth is completed remains controversial, but many studies have agreed that it would not be completed until 2.5 to 3 years old. Therefore, it is necessary to perform an additional study on this matter over a longer period.The results of this study in which catch-up growth failed in 42.9% of VLBW infants was higher than the catch-up growth failure rates (32.9%) until 24 months of corrected age in VLBW infants in a study by Ma et al.5). In this study, the ratio of ELBW to VLBW was as high as 52.3%, much far higher than the figure (30.2%) reported by Ma et al.5). According to a study by Lim et al.6), the catch-up growth failure rates until 18 months of corrected age reached 12.4%. Because the catch-up success criterion ofbody weight was based on whether the 3rd percentile was reached in the study by Ma et al.5), it cannot be directly compared to our observation. The catch-up growth failure rates at 18 months of corrected age in ELBW infants in this study were similar to the rates mentioned in other studies such as a study by Jeon et al.7). However, it is difficult to compare catch-up growth because there are a variety of factors that have an effect on growth after discharge from the hospital, such as gestational age, birth weight, number of days hospitalized in the NICU, ratio of intrauterine growth retardation, and newborn morbidity.Catch-up growth failure is more common in SGA infants than in AGA babies8). In this study as well, 75.0% failed to catch up growth at 18 months of corrected age among the SGA infants, statistically significant compared to the failure rates (12.3%) in AGA infants.According to one study, growth failure in VLBW infants has a negative effect on neurodevelopmental outcomes9). In this study, frequencies of abnormal neurological status at 4, 8, 12, and 18 months of corrected age in infant who failed weight catch-up were 12.5%, 31.3%, 19.0%, and 21.4%, respectively. The figures were significantly higher than the neurological deficit frequencies (6.8%, 13.6%, 7.8%, and 6.7% at 4, 8, 12, and 18 months of corrected age, respectively) in premature infants who have had successful catch-up growth of weight. Meanwhile, according to a study by Ma et al.5), there were fewer VLBW infants who failed to catch up growth until 24 months of corrected age than VLBW babies who succeeded to catch-up growth in terms of the Psychomotor Developmental Index. However, the differences were not statistically significant. Even though an additional study needs to be conducted with more cases, appropriate nutritional evaluation and active nutritional care are important in the prevention or solving of growth failure among premature infants who received an intensive care.In this study, the body weight of 18.9% and 15.4% of infants at 4 and 8 months of corrected age, respectively, exceeded the 90th percentile. Since 12 months of corrected age, this kind of overweight frequency remained at 5 to 7%. Unlike parents' great concerns about growth failure, however, most parents were not concerned about overweightness of their premature infants. According to recent studies, rapid catch-up growth in the early stage could cause obesity, heart disease, and kidney disease10-13). Therefore, it is important to carefully watch overweightness as well as growth failure. It is also required to perform a study on how fast catch-up growth should proceed in premature infants after NICU discharge. At the same time, it is necessary to check nutritional guidelines for premature infants.The mean head circumferences of premature infants at 4, 8, 12, and 18 months of corrected age were included within the 10th to 90th percentiles on the Korean children and adolescents' growth standard chart (2007). The ratio of premature infants who failed to catch up growth in head circumference at 18 months of corrected age reached 10%. Among infants with head circumference <10th percentile, neurological deficits were observed in 29.5%, while developmental delay was found in 40.9%. Among infants with normal head circumference, on the contrary, neurological deficit was observed in 8.7%, while developmental delay was found in 10.9%. In addition, poor neurodevelopmental outcomes were observed among the infants who failed to catch up growth in head circumference due to frequent neurodevelopmental disorders. This kind of result is the same as the results of other papers in which catch-up growth in head circumference is was closely related with neurodevelopmental outcomes14-17).As mentioned above, it is important to check infant's growth in terms of body weight and head circumference. Therefore, this process must be included in observation at each visit to neonatal follow-up clinic.The mean length of premature infants at 4, 8, 12, and 18 months of corrected age were included within the 10th to 90th percentiles on the Korean children and adolescents' growth standard chart (2007). However, catch-up growth failure rates of length were 20.5%, 24.0 %, 29.4%, and 12.2% at 4, 8, 12, and 18 months of corrected age, respectively. According to a study by Wit et al.18), about 10% of premature infants stay in the low length category at age 5. In this study, 12.2 % of premature infants stayed at length <10th percentile at 18 months of corrected age. In SGA infants, the catch-up growth failure rates at 18 months of corrected age reached 37.5%, which was higher than the failure rate (17.5%) until age 2 reported by Hokken-Koelega et al.19).Studies about neurodevelopmental outcome of premature infants are mainly observation for major neurodevelopmental disabilities such as moderate to severe mental retardation, sensory impairment (hearing loss, vision loss), cerebral palsy, and seizure disorder. Major neurodevelopmental deficits account for 6 to 8% in LBW infants (birth weight<2,500 g), 14 to 17% in VLBW babies, and 20 to 25% in ELBW infants showing the evidence of increasing the major disabilities as birth weight decrease20-22). According to a study by Choi et al.23), 8.5% of VLBW infants and 19.2% of ELBW babies had cerebral palsy. Sensory nerve impairment, seizures, and hydrocephalus with a ventriculoperitoneal shunt have not been categorized as major neurological disabilities and not included in his study. According to a study by Jeon et al.7), ELBW infants' survival rates have improved since 2000. At the same time, the incidence rate of cerebral palsy until 18 months of corrected age decreased from 22.2 to 8.2%. In this study, major disabilities accounted for 11%, higher than the incidence rate of major disabilities among general LBW infants (6 to 8%). Among 120 VLBW infants, 31 babies were suffering from a major neurological deficit. Specifically, a major disability was detected in 18 (39.1%) among 48 ELBW infants and 13 (17.6%) among 74 infants with birth weight 1,000 to 1,499 g. In other words, the incidence rate of major disabilities increased as birth weight decreased. In general, neurological deficit was directly related with perinatal problems24-26). In this study, however, risk factors during the perinatal period were not analyzed. Hence, it was impossible to determine the effect of other risk factors such as increases in the incidence of neurological deficits.In addition, the proportion of premature infants categorized as 'suspect' with abnormal muscle tone (e.g., transient dystonia) and a problem in posture or reflex reached 6.7%. Among the premature infants, the incidence rate of dystonia was the highest (21 to 36%) at 7 months of corrected age27,28). In this study, the frequency of dystonia was the highest (12.5%) at 8 months of corrected age. However, the figure was lower than the level reported in earlier studies. It appears that this disparity occurred due to differences in gestational age, birth weight, and neonatal morbidity. A further study needs to be performed on various risk factors to elucidate this point.According to meta-analysis, the intelligence quotients (IQs) of LBW infants were usually lower than those of normal babies by 5 to 7 points29). In comparison, except for severe disorders, the IQ scores of premature infants were lower than those of normal babies by 3.8 to 9.8 points. The difference was reported to be up to 12 to 17 points in some studies30,31). In general, the IQ scores of the VLBW infants were lower than those of full-term infants by 8 to 11 points30-32). In this study, it was impossible to perform an IQ test on premature infants until 18 months of corrected age. However, 14.4% of premature infants categorized as having a developmental delay had developmental quotient (DQ) scores below mean -1 SD. No large difference was observed from 8 to 18 months of corrected age, and developmental delay rates stayed at about 20%. Because it is hard to predict IQ during the prepubertal period using the Bayley Scales of Infant Development17), it is unlikely that the DQ examined until 1s8 months of corrected age reflects the level of future cognitive development. In terms of the characteristics of cognitive development, because other factors such as nutrition and environment are influential as well, it is necessary to observe infants over a longer period.In Korea, no earlier study has examined parental concerns about their premature infants after NICU discharge. In this study, 27.9% of parents worried about the development of their premature baby. Therefore, it is necessary to educate and teach them how to care for their premature baby after NICU discharge. Until 8 months of corrected age, their concerns were mostly about motor development. Concerns about cognitive, sensory and language development were observed since 12 months of corrected age. In particular, the results of this study, which focused on the infants up to 18 months of corrected age, show that it is necessary to teach parents to consider their infant's corrected age with guidance in the follow-up clinic.Regarding nutrition and eating problems, 15.9% showed concerns. Specifically, they worried about inadequate eating and gastrointestinal functions at 4 months of corrected age, weaning food and gastrointestinal functions at 8 months of corrected age, no progress in weaning to food at 12 months of corrected age, and poor oral intake and failure to weaning to food at 18 months of corrected age. In particular, gastrointestinal problems such as indigestion, loose stools, frequent stool passage, constipation, and vomiting almost disappear at 8 months of corrected age. It is desirable to teach parents and guide them to consider their biggest concerns at each stage when they visit the follow-up clinic. If parents worry about poor food intake even though their child's growth is normal, in particular, it is necessary to evaluate growth based on corrected age and let them know the results of the evaluation.In terms of parents' concerns about their premature infants' growth, no difference was observed by corrected age. They were mostly concerned about low weight and slow weight increases. While 10 to 12% of parents were concerned about growth failure, almost no parents worried about overweight. This kind of result is very particular because the overweight infants (body weight>90th percentile) exceeded 15% up to 8 months of corrected age and 5 to 7% of whom had it at12 and 18 months of corrected age. Since the rapid catch-up growth in LBW infants could cause adult diseases such as obesity, heart disease, and kidney disease10-12,33), it is necessary to teach parents how to control rapid weight increases (e.g., nutrition, and breast feeding guidelines) in an early stage after NICU discharge.In this study, the parental concerns about developmental delay until 18 months of corrected age after NICU discharge had 57.1% sensitivity and 77.0% specificity. Concerns about growth failure in body weight and length had <10% sensitivity and >90% specificity. On the contrary, concerns about overweight had 8.2% sensitivity and 100% specificity. This kind of result differs from those of a study by Jensen and Harper34), who insisted that parental concerns about development, social skills, and disease of their high-risk child at age 5 were well matched with the developmental status of their infant. It has been impossible to figure out how this kind of difference has occurred. This study has attempted to point out that it is important to monitor and evaluate the development and growth of a child based on the standardized and objective evaluation tools by age instead of approaching the matter with parental complaints only.Regarding disease contraction after NICU discharge, the diseases treated at 4, 8, 12, and 18 months of corrected age were examined. According to the investigation, about 30% of premature infants suffered from an infectious disease (mostly upper and lower respiratory tract infections). In addition, iron deficiency anemia was observed in 5 to 7% of infants. In this study, however, hemoglobin and iron status and iron replacement therapy were not analyzed. Therefore, it was impossible to determine how much iron deficiency anemia was actually present. This study confirmed that it is essential to carefully watch respiratory infections as well as development, growth, and nutrition in the health management of premature infants after NICU discharge.
- References
- 1. Treyvaud K, Anderson VA, Howard K, Bear M, Hunt RW, Doyle LW, et al. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics 2009;123:555–561.
[Article] [PubMed]2. Kaaresen PI, Ronning JA, Ulvund SE, Dahl LB. A randomized, controlled trial of the effectiveness of an early-intervention program in reducing parenting stress after preterm birth. Pediatrics 2006;118:e9–e19.
[Article] [PubMed]3. Bayley N. Bayley Scales of Infant Development. 1993;2nd ed. San Antonio: Psychological Co..4. Park MR, Lee BS, Kim EA, Kim KS, Pi SY. Outcomes of extremely low birth weight infants at the Asan Medical Center between 2003 and 2006. J Korean Soc Neonatol 2008;15:123–133.5. Ma TH, Kim KA, Ko SY, Lee YK, Shin SM. Catch-up growth and development of very low birth weight infants. Korean J Pediatr 2006;49:29–33.
[Article]6. Lim JW, Jun NL, Kim KA, Kim AR, Kim KS, Pi SY. Postnatal catch-up growth of very low birth weight infants. J Korean Soc Neonatol 2002;9:1–11.7. Jeon GW, Kim MJ, Kim SS, Shim JW, Chang YS, Park WS, et al. Improved survival rate with decreased neurodevelopmental disability in extreme immaturity. Korean J Pediatr 2007;50:1067–1071.
[Article]8. Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW. Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 1998;152:1225–1231.
[PubMed]9. Kan E, Roberts G, Anderson PJ, Doyle LW. Victorian Infant Collaborative Study Group. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev 2008;84:409–416.
[Article] [PubMed]10. Demmelmair H, von Rosen J, Koletzko B. Long-term consequences of early nutrition. Early Hum Dev 2006;82:567–574.
[Article] [PubMed]11. Lucas A. Long-term programming effects of early nutrition: implications for the preterm infant. J Perinatol 2005;25(Suppl 2): S2–S6.
[Article] [PubMed]12. Syddall HE, Sayer AA, Simmonds SJ, Osmond C, Cox V, Dennison EM, et al. Birth weight, infant weight gain, and cause-specific mortality: the Hertfordshire Cohort Study. Am J Epidemiol 2005;161:1074–1080.
[Article] [PubMed]13. Simeoni U, Ligi I, Buffat C, Boubred F. Adverse consequences of accelerated neonatal growth: cardiovascular and renal issues. Pediatr Nephrol 2011;26:493–508.
[Article] [PubMed]14. Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 2008;121:e1534–e1540.
[Article] [PubMed]15. Gale CR, O'Callaghan FJ, Bredow M, Martyn CN. Avon Longitudinal Study of Parents and Children Study Team. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 2006;118:1486–1492.
[Article] [PubMed]16. Ghods E, Kreissl A, Brandstetter S, Fuiko R, Widhalm K. Head circumference catch-up growth among preterm very low birth weight infants: effect on neurodevelopmental outcome. J Perinat Med 2011;39:579–586.
[PubMed]17. Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child 1995;5:176–196.
[Article] [PubMed]18. Wit JM, Finken MJ, Rijken M, de Zegher F. Preterm growth restraint: a paradigm that unifies intrauterine growth retardation and preterm extrauterine growth retardation and has implications for the small-forgestational-age indication in growth hormone therapy. Pediatrics 2006;117:e793–e795.
[Article] [PubMed]19. Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res 1995;38:267–271.
[Article] [PubMed]20. Bennett FC, Scott DT. Long-term perspective on premature infant outcome and contemporary intervention issues. SeminPerinatol 1997;21:190–201.
[Article]21. Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics 2005;116:333–341.
[Article] [PubMed]22. Sung IK. Neurodevelopmental outcomes of very low birth weight infants and extremely low birth weight infants in Korea, 1984-2008. Korean J Pediatr 2009;52:14–21.
[Article]23. Choi KH, Chun CS, Kim HJ, Sung IY, Ha SB, Kim KS, et al. Prevalence of cerebral palsy in very low birth weight infants. J Korean Acad Rehabil Med 2000;24:432–438.24. Koo KY, Kim JE, Lee SM, Namgung R, Park MS, Park KI, et al. Effect of severe neonatal morbidities on long term outcome in extremely low birthweight infants. Korean J Pediatr 2010;53:694–700.
[Article] [PubMed] [PMC]25. Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics 2005;116:635–643.
[Article] [PubMed]26. Volpe JJ. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics 2005;116:221–225.
[Article] [PubMed]27. Breslau N, Chilcoat HD. Psychiatric sequelae of low birth weight at 11 years of age. Biol Psychiatry 2000;47:1005–1011.
[Article] [PubMed]28. Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Ment Retard Dev Disabil Res Rev 2002;8:241–248.
[Article] [PubMed]29. Aylward GP, Pfeiffer SI, Wright A, Verhulst SJ. Outcome studies of low birth weight infants published in the last decade: a metaanalysis. J Pediatr 1989;115:515–520.
[Article] [PubMed]30. Aylward GP. Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr 2005;26:427–440.
[Article] [PubMed]31. Staebler DL. Letter: binocularly induced motion of flicker patterns. J Opt Soc Am 1976;66:156–157.
[Article] [PubMed]32. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 2002;288:728–737.
[Article] [PubMed]
Table 3
Rates of Growth Failure and Neurodevelopmental Disabilities According to the Birth Weight and Appropriateness of Birth Weight for Gestational Age at 18 Months of Corrected Age

 About
About Browse articles
Browse articles For contributors
For contributors