All issues > Volume 55(8); 2012
Continuous renal replacement therapy in neonates weighing less than 3 kg
- Corresponding author: Dong-Kyu Jin, MD, PhD. Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-3539, Fax: +82-2-3410-0043, jindk@skku.edu
- Received November 01, 2011 Revised January 06, 2012 Accepted March 23, 2012
- Abstract
-
- Purpose
- Purpose
- Continuous renal replacement therapy (CRRT) is becoming the treatment of choice for supporting critically ill pediatric patients. However, a few studies present have reported CRRT use and outcome in neonates weighing less than 3 kg. The aim of this study is to describe the clinical application, outcome, and complications of CRRT in small neonates.
- Methods
- Methods
- A retrospective review was performed in 8 neonatal patients who underwent at least 24 hours of pumped venovenous CRRT at the Samsung Medical Center in Seoul, Korea, between March 2007 and July 2010. Data, including demographic characteristics, diagnosis, vital signs, medications, laboratory, and CRRT parameters were recorded.
- Results
- Results
- The data of 8 patients were analyzed. At the initiation of CRRT, the median age was 5 days (corrected age, 38+2 weeks to 23 days), and the median body weight was 2.73 kg (range, 2.60 to 2.98 kg). Sixty-two patient-days of therapy were reviewed; the median time for CRRT in each patient was 7.8 days (range, 1 to 37 days). Adverse events included electrolyte disturbances, catheter-related complications, and CRRT-related hypotension. The mean circuit functional survival was 13.9±8.6 hours. Overall, 4 patients (50%) survived; the other 4 patients, who developed multiorgan dysfunction syndrome, died.
- Conclusion
- Conclusion
- The complications of CRRT in newborns are relatively high. However, the results of this study suggest that venovenous CRRT is feasible and effective in neonates weighing less than 3 kg under elaborate supportive care. Furthermore, for using potential benefit of CRRT in neonates, efforts are required for prolonging filter survival.
- Introduction
- Introduction
The use of continuous renal replacement therapy (CRRT) has become expanded for the support of critically ill infants and pediatric patients with acute renal failure, fluid overload, or multiorgan dysfunction syndrome (MODS). CRRT mimics the effects of glomerular function with its continuous ultrafiltration and solute clearance even in hemodynamically unstable patients1-4). Owing to continuous fluid removal, fluid restrictions can be often minimized with the result of improved nutritional delivery5,6). Alteration of dialysate or replacement fluid is possible and can allow for control of the electrolyte levels within the target range5). CRRT in patients with sepsis has the additional benefit of removal of inflammatory cytokines7). In addition, CRRT is an effective adjuvant therapy for the acute treatment of inborn errors of metabolism; both ammonia and branched chain amino acids are effectively cleared8,9).Peritoneal dialysis (PD) continues to be the common choice of renal replacement therapy in the pediatric population. However PD is considered inappropriate in several cases (for example, after abdominal surgery). In addition, severe fluid overload cannot always be successfully treated by PD10). And complications associated with insertion of PD catheters including leakage and infections are more prone to occur in neonates11). Intermittent hemodialysis is hard to be applied for maintaining optimal fluid and electrolyte balance and circulatory stability in newborns with small blood volume12). Therefore, CRRT has advantages as treatment modality for neonatal patients with acute kidney injury and inborn errors of metabolism13,14).As CRRT technology advances, there have been attempts using CRRT on smaller and smaller patients. There are several reports that describe the use of CRRT in infants15-17) and neonates18-21). However, CRRT in neonatal patients, especially those weighing less than 3 kg remains a challenge because of their immature body temperature control, difficulties of vascular access, venous catheterization with large-caliber catheters and hemodynamic instability resulting from the large extracorporeal volume of the system (filters and lines), which predisposes the newborns to hypotension at the time of connection because the extracorporeal volume of the CRRT circuit should be less than 10% of the patient's blood volume4). Until recently, there have been limited reports performed indicating that CRRT can be an effective treatment modality for renal replacement therapy in neonates weighing less than 3 kg13,16,18). Therefore, the aim of this study was to describe the use of CRRT as a modality of renal replacement therapy in neonates weighing less than 3 kg and assess the outcome and feasibility. Here, the retrospective findings from the medical records of eight neonates treated with CRRT at a single center are reported.
- Materials and methods
- Materials and methods
A retrospective review was performed of eight neonatal patients that underwent at least 24 hours of pumped venovenous CRRT at Samsung Medical Center in Seoul, Korea between March 2007 and July 2010. All data was recorded including the demographic, diagnosis, vital signs, medications, laboratory, and CRRT data. MODS was defined as presence of at least three failed organs. Organ system failures were defined using the international pediatric sepsis consensus conference definitions22).CRRT data included the CRRT modality (continuous venovenous hemodialysis [CVVHD] and continuous venovenous hemodiafiltration [CVVHDF] as previously described4)), circuit prime, blood flow rate (BFR), anticoagulation, number of days on CRRT, complications associated with CRRT and outcome. Survival was defined successful discharge throughout entire hospitalization versus death.All CRRT was performed using Prisma (Gambro Healthcare, Lakewood, CO, USA) hemofiltration machines with M10 (Gambro Healthcare) filters. Priming of the extracorporeal circuit was performed with packed red blood cells (pRBC) diluted with either 5% albumin solution or 0.9% saline to achieve a hematocrit of 30 to 40%. For preventing bradykinin release phenomenon, heparin 100 unit, 250 mg of 3% calcium chloride, 30 mEq of sodium bicarbonate were added in the mixture of pRBC (50 mL) and saline or 5% albumin solution (50 mL).A 6.5 french diameter, dual lumen catheter (Gambro Healthcare) was used for all patients. Catheters were inserted percutaneously using the Seldinger technique of the subclavian veins or a cut-down of the internal jugular vein by a surgeon on the intensive care system (ICS) with a radiant warmer in neonatal intensive care unit (NICU).The BFR was determined as 5 to 10 mL/kg/min. Hypotension on connection to the filter was defined as a fall in the mean blood pressure of more than 20 mmHg over baseline during the first 60 minutes after connection to CRRT. For prevention of hypotension, the dose of inotropic agents was increased transiently before CRRT in patients receiving these agents. If a patient was hypotensive at initiation of CRRT, inotropic agents were administered or their dose augmented. Filter replacement fluid (prefilter) or counter-current dialysate was introduced at a rate of 2,000 mL/1.73 m2/hr. Commercially available bicarbonate-buffered hemofiltration fluid (Hemosol B0, Gambro Korea, Seoul, Korea) was used for the dialysate and replacement fluid. Electrolyte disturbance during CRRT was defined as the following: hypocalcemia (total calcium<8.4 mg/dL), hypophosphatemia (phosphorus<3.8 mg/dL), and hypokalemia (potassium<3.5 mmol/L). If the patient had hypokalemia, potassium chloride was added to the dialysate fluid to ensure a patient serum potassium level of 3.5 to 4.5 mmol/L. Hypocalcemia and hypophosphatemia were corrected by the administration of intravenous calcium gluconate or phosphate salt. The net fluid removal of patients was determined by the degree of fluid overload ranging from negative 5 to 0 mL/hr.Unfractionated heparin was continuously infused for anticoagulation in patients with a normal coagulation status using 10 U/kg/hr after a 20 U/kg initial bolus. The infusion rate was adjusted targeting the activated clotting time of 170 to 210 seconds. The patients were cared under a radiant warmer to prevent hypothermia.Pediatric risk mortality (PRISM) III score was calculated as previous report23). The comparison of PRISM III score in survival and death groups was performed using Mann-Whitney test. The cumulative proportion of survival of the filter was plotted according to time using Kaplan-Meier analysis. The data was assumed to be non-parametric. All statistical tests were performed with a P value less than 0.05 indicating statistical significance. SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
- Results
- Results
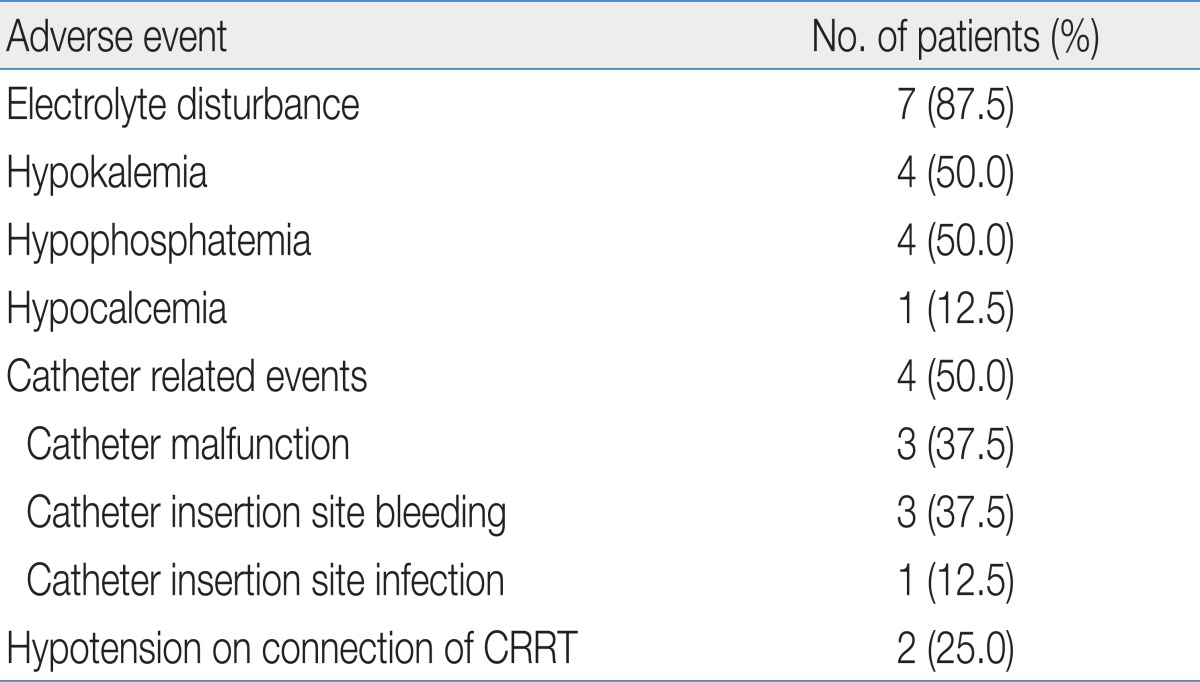
All eight neonatal patients (4 males and 4 females) were critically ill and in the NICU. Seven patients were on mechanical ventilation. Three of the patients were preterm <37 weeks gestational age. Among them, two patients were extremely low birth weight infants with birth weights less than 1,000 g. Clinical and laboratory datas at the initiation of CRRT are shown in Table 1. At the initiation of CRRT, the median days after birth was five days (corrected age, 38+2 weeks to 23 days), and median body weight was 2.73 kg (2.60 to 2.98 kg). Four patients required CRRT for control of volume overload and metabolic derangements related to acute renal dysfunction with sepsis and MODS (patient no. 1 to 4). One patient required CRRT for ARF resulting from atypical hemolytic uremic syndrome. Three patients required CRRT for the correction of acute metabolic abnormalities unrelated to acute renal failure; two patients for hyperammonemia and metabolic acidosis (patient no. 6, 7), and one patient for hyperammonemia (patient no. 8).Heparin was used for anticoagulation in four patients. In patients with disseminated intravascular coagulation or thrombocytopenia resulting from hemolytic uremic syndrome (HUS) or sepsis, CRRT was performed without anticoagulation. Median serum blood urea nitrogen was 78.7 mg/dL (52.4 to 81.3 mg/dL) at the start of CRRT and 42.1 mg/dL (27.2 to 51.8 mg/dL) in 24 hours of CRRT in five patients with uremia. Median urea reduction ratio was 47.4% (34.2 to 55.9%) in 24 hours of CRRT. In three patients with hyperammonemia, 50% reduction of ammonia was achieved within 12 hours of CRRT and non-toxic ammonia levels (<200 µmol/L) were within 24 hours or less after initiation of CRRT (Fig. 1).The adverse events that occurred during CRRT are shown in Table 2. Electrolyte disturbance was most frequently noted and occurred commonly 72 hours after the start of CRRT; however, it was easily corrected with replacement in all cases. Catheter related events occurred in four patients including catheter malfunction that required catheter replacement, as well as bleeding and infection at the catheter insertion site. Hypothermia was not noted during CRRT.Malfunction of the catheters, which required replacement, occurred on three occasions in two patients. The catheter was replaced twice (6 days, 17 days after insertion) in one patient. The catheter was replaced one day after CRRT in another patient due to venous thrombus formation at the catheter tip in the right atrium, which was found on echocardiogram. Facial venous congestion resulted from inadequate venous drainage of the superior vena cava in one preterm patient weighing 2.63 kg. The patient suddenly developed facial congestion which was relieved by replacement of the catheter. Catheter insertion site bleeding occurred in three patients, all of them had a coagulopathy resulting from MODS. Infection of the catheter insertion site was noted in one patient with MODS. Enterococcus feacalis was isolated on culture of purulent material.Hypotension soon after starting CRRT occurred in two patients (patients no. 7 and 8). Their blood pressure increased and maintained stably with administration of vasoactive drugs. Five patients (4 patients with MODS and 1 patient with a metabolic disorder) were already receiving vasoactive drugs before initiation of CRRT. For prevention of hypotension, the dose of drugs was increased upto twice before CRRT and they did not develop significant hypotension soon after connection.Review of 62 patient-days of therapy, showed a median time on CRRT of 7.8 days/patient (1 to 37 days). A total of 82 circuits were used. The mean circuit functional survival was 13.9±8.6 hours (range, 1 to 48 hours). Only 14.6% (12 of 82) of the circuits functioned for longer than 24 hours. Fig. 2 shows a Kaplan-Meier analysis of filter survival.Overall, four patients (50%) survived; three patients had inborn errors of metabolism and one patient had atypical HUS. All four patients with MODS died. Median PRISM III score of survival group was significantly lower in survival group than mortality group (9 vs. 14.5, P=0.03).
- Discussion
- Discussion
This study is a single-center report on venovenous CRRT using PRISMA M10 circuit in neonates weighing less than 3 kg. The estimated blood volume of neonates weighing 3 kg is 240 mL (patients weighing less than 10 kg have blood volumes of about 80 mL/kg). If the patient's circuit volume is in excess of 10% of the patient's total blood volume, blood priming is necessary4). Because the circuit volume of the M10 filter is 50 mL, all of the patients in this study needed blood priming. To overcome challenges, Cardio Renal Pediatric Dialysis Emergency Machine is newly developed. The system is miniaturized hemofiltration/dialysis equipment to be suitable for renal replacement therapy in newborns and children with a body weight less than 10 kg, operating hemofiltration circuit with a very low priming volume (15 mL for the whole circuit including the hemofilter)24). Hypotension at the initiation of CRRT may be due to dilution of vasoactive medications by the prime volume or binding of catecholamines to the surface of the extracorporeal circuit25). Santiago et al.26) reported that hypotension on connection to CRRT was detected in 30.4% (53/174) of children (mean age of 52.3 month). As shown in the results of this study, 25% of neonates weighing less than 3 kg developed hypotension; this is not much higher than in older and larger children, and can be readily treated by administrating vasoactive drugs or increasing the dose of medications. It could implicate that the hypotension did not limits the indication of CRRT in neonates.In small patients that require circuit blood priming, the use of the PRISMA and AN69 membrane might be associated with profound hypotension at the start of CRRT due to the bradykinin release phenomenon that occurs because the blood prime has a low pH (approximately 6.3), depending on the age of the blood4). The recommended methods for preventing the bradykinin release syndrome are either to buffer the blood prime, thereby increasing the pH to around 7.4, or to bypass the hemofilter by giving the blood post filter in synchrony with a saline filter prime along with judicious use of bicarbonate infusion during the initiation of these techniques; this approach has essentially eradicated this phenomenon in some reports4). To prevent hypotension at the start of CRRT, dose increments of vasoactive drugs, before initiation of CRRT or administration of vasoactive drugs when starting CRRT, can be helpful as it was in the patients reported here.Especially in patients with sepsis and MODS receiving CRRT, maintenance of optimal drug (ex. antibiotics) level is critical to maximize survival. Monitoring and adjustment of adequate drug dosing is essential in these patients because several factors including pharmacokinetics of drugs (volume of distribution, protein binding, residual renal elimination), mode of CRRT, dose of CRRT, BFR, filter material affect elimination of drugs during CRRT27).One of the most significant problems with CRRT is early coagulation of the filters, leading to blood loss, decreased efficacy of the technique, and a greater risk of hemodynamic instability with the connection. Because CRRT in newborns uses lower blood flows and smaller catheters, than in older patients, the circuits may consequently be more prone to clotting. In our study, the mean circuit functional survival was only 13.9±8.6 hours. There are many options for improving filter survival. Del Castillo et al.28) reported that the filter life can be increased by the use of hemodiafiltration, high heparin doses, and filters with a large surface area. Goldstein et al.29) demonstrated that the functional survival of the M10 circuit in patients weighing less than 15 kg was excellent for circuits that provided CRRT via a bigger catheter lumen. In their study, mean circuit life was 28.6±22.5 hours. They used more large catheters (5 to 8 french) than our study (6.5 french). Several studies in adults and the pediatric age group have validated the safety and efficacy of regional citrate anticoagulation in CRRT30-34). As it has been known that citrate can be used safely with calcium containing dialysate, citrate anticoagulation became available in Korea35,36). Despite the merits of regional citrate anticoagulation, citrate infusion can cause hypocalcemia, metabolic alkalosis and hypernatremia37). Moreover, citrate use in the neonate has not been well studied. Recently, nafamostat mesilate, a synthetic serine protease inhibitor, has become available with the advantage of a short half life. However, the appropriate dose for neonates has not yet been established and there are several side effects (anaphylaxis, agranulocytosis, hyperkalemia) reported with the use of nafamostat38). Moreover, nafamostat cannot be used with AN69 filter such as M10 because of adsorption in the membranes39). Although there are filters compatible with nafamostat such as the AN 69 ST or polyarylethersulfone, these filters are made for patients with a body weight of 8 kg or more. Further studies are needed on the use of nafamostat and additional efforts for developing new filters for children and newborn patients.Complications of catheterization include hematoma at the puncture site hemorrhage, altered venous drainage of the distal part of the catheter, incorrect position, pneumothorax, hemothorax and catheter related infections26). These complications are more common in infants with a weight of less than 10 kg26). In this study, malfunction of the catheter was frequent. The tip of the catheter has to be placed into the middle of the right atrium to provide adequate drainage of venous blood. The location of the catheter tip should be closely monitored during maintenance of CRRT. Hypothermia during CRRT was not observed in this study, possibly due to nursing patients in the ICS with a radiant warmer and warming of circuits.There is no consensus on the appropriate timing for the initiation of CRRT. Previous studies have suggested that earlier use improves survival in the pediatric population3,25). The outcomes of this study showed that the patients that died had already deteriorated (ex. hypotension needs for infusion of vasoactive drugs) prior to starting CRRT.Symons et al.2) reported a poorer outcome of venovenous CRRT in patients under 3 kg comparing with patients weighing between 3 kg and 10 kg: 25% of the patients (4 of 16 patients) weighing 3 kg or less survived, whereas 41% of patients (28 of 69 patients) weighing more than 3 kg survived. Also they demonstrated that patients with a metabolic disorder as the primary diagnosis had the highest rate of survival, 71%; however, survival was lowest for patients with MODS (15%)2). The survival data of our study also show better outcomes in neonates with metabolic disease; they had general systemic conditions that were better than the patients with MODS which were supported by lower PRISM III score in survival group (Table 1). Further study is needed to determine whether earlier use of CRRT can improve the outcome in neonatal patients with MODS.Although there is a report demonstrating the priority of HD over CRRT in rapid removal of toxic metabolites including ammonia in patients with inborn errors of metabolism40), CRRT can be quite effective treatment modality performed under setting with maximal dialysate fluid flow rate (2,000 mL/1.73m2/hr), CVVHD or CVVHDF mode in mild cases as shown in our results. Moreover, CRRT can be useful in some occasions including lack of local expertise of neonatal HD.In conclusion, complications in newborns on CRRT are relatively higher than in adults or older children2). However, the results of this study suggest that venovenous CRRT is feasible and effective in neonates weighing less than 3 kg under elaborate supportive care although our study population is small and heterogeneous. Furthermore, to take advantage of the potential benefit of the CRRT in neonates, effort to prolong filter survival is needed.
- References
- 1. Warady BA, Bunchman T. Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol 2000;15:11–13.
[Article] [PubMed]2. Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, et al. Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 2003;41:984–989.
[Article] [PubMed]3. Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005;67:653–658.
[Article] [PubMed]4. Warady BA, Schaefer FS, Fine RN, Alexander SR. Pediatric dialysis. 2004;Dordrecht: Kluwer Academic Publishers.5. Walters S, Porter C, Brophy PD. Dialysis and pediatric acute kidney injury: choice of renal support modality. Pediatr Nephrol 2009;24:37–48.
[Article] [PubMed] [PMC]6. Bellomo R, Ronco C. Continuous haemofiltration in the intensive care unit. Crit Care 2000;4:339–345.
[Article] [PubMed] [PMC]7. Ronco C, Bonello M, Bordoni V, Ricci Z, D'Intini V, Bellomo R, et al. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif 2004;22:164–174.
[Article] [PubMed]8. Jouvet P, Jugie M, Rabier D, Desgres J, Hubert P, Saudubray JM, et al. Combined nutritional support and continuous extracorporeal removal therapy in the severe acute phase of maple syrup urine disease. Intensive Care Med 2001;27:1798–1806.
[Article] [PubMed]9. Thompson GN, Butt WW, Shann FA, Kirby DM, Henning RD, Howells DW, et al. Continuous venovenous hemofiltration in the management of acute decompensation in inborn errors of metabolism. J Pediatr 1991;118:879–884.
[Article] [PubMed]10. Ponikvar R, Kandus A, Urbancic A, Kornhauser AG, Primozic J, Ponikvar JB. Continuous renal replacement therapy and plasma exchange in newborns and infants. Artif Organs 2002;26:163–168.
[Article] [PubMed]11. Bonilla-Felix M. Peritoneal dialysis in the pediatric intensive care unit setting. Perit Dial Int 2009;29(Suppl 2): S183–S185.
[Article] [PubMed]12. Teehan GS, Liangos O, Jaber BL. Update on dialytic management of acute renal failure. J Intensive Care Med 2003;18:130–138.
[Article] [PubMed]13. Westrope C, Morris K, Burford D, Morrison G. Continuous hemofiltration in the control of neonatal hyperammonemia: a 10-year experience. Pediatr Nephrol 2010;25:1725–1730.
[Article] [PubMed]14. Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E. Dialysis in neonates with inborn errors of metabolism. Nephrol Dial Transplant 1999;14:910–918.
[Article] [PubMed]15. Ronco C, Parenzan L. Acute renal failure in infancy: treatment by continuous renal replacement therapy. Intensive Care Med 1995;21:490–499.
[Article] [PubMed]16. Reeves JH, Butt WB, Sathe AS. A review of venovenous haemofiltration in seriously ill infants. J Paediatr Child Health 1994;30:50–54.
[Article] [PubMed]17. Jaing TH, Hsueh C, Tain YL, Hung IJ, Hsia SH, Kao CC. Tumor lysis syndrome in an infant with Langerhans cell histiocytosis successfully treated using continuous arteriovenous hemofiltration. J Pediatr Hematol Oncol 2001;23:142–144.
[Article] [PubMed]18. Schröder CH, Severijnen RS, Potting CM. Continuous arteriovenous hemofiltration (CAVH) in a premature newborn as treatment of overhydration and hyperkalemia due to sepsis. Eur J Pediatr Surg 1992;2:368–369.
[Article] [PubMed]19. Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, et al. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol 2001;16:862–867.
[Article] [PubMed]20. Leyh RG, Notzold A, Kraatz EG, Sievers HH, Bernhard A. Continuous venovenous haemofiltration in neonates with renal insufficiency resulting from low cardiac output syndrome after cardiac surgery. Cardiovasc Surg 1996;4:520–525.
[Article] [PubMed]21. Jouvet P, Poggi F, Rabier D, Michel JL, Hubert P, Sposito M, et al. Continuous venovenous haemodiafiltration in the acute phase of neonatal maple syrup urine disease. J Inherit Metab Dis 1997;20:463–472.
[Article] [PubMed]22. Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8.
[Article] [PubMed]23. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24:743–752.
[Article] [PubMed]24. Ronco C, Davenport A, Gura V. The future of the artificial kidney: moving towards wearable and miniaturized devices. Nefrologia 2011;31:9–16.
[PubMed]25. Maclaren G, Butt W. Controversies in paediatric continuous renal replacement therapy. Intensive Care Med 2009;35:596–602.
[Article] [PubMed]26. Santiago MJ, Lopez-Herce J, Urbano J, Solana MJ, del Castillo J, Ballestero Y, et al. Complications of continuous renal replacement therapy in critically ill children: a prospective observational evaluation study. Crit Care 2009;13:R184
[Article] [PubMed] [PMC]27. Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med 2009;37:2268–2282.
[Article] [PubMed]28. del Castillo J, Lopez-Herce J, Cidoncha E, Urbano J, Mencia S, Santiago MJ, et al. Circuit life span in critically ill children on continuous renal replacement treatment: a prospective observational evaluation study. Crit Care 2008;12:R93
[Article] [PubMed] [PMC]29. Goldstein SL, Hackbarth R, Bunchman TE, Blowey D, Brophy PD. Prospective Pediatric Crrt Registry Group Houston. Evaluation of the PRISMA M10 circuit in critically ill infants with acute kidney injury: a report from the Prospective Pediatric CRRT Registry Group. Int J Artif Organs 2006;29:1105–1108.
[Article] [PubMed]30. Monchi M, Berghmans D, Ledoux D, Canivet JL, Dubois B, Damas P. Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med 2004;30:260–265.
[Article] [PubMed]31. Kreuzer M, Ahlenstiel T, Kanzelmeyer N, Ehrich JH, Pape L. Management of regional citrate anticoagulation in pediatric high-flux dialysis: activated coagulation time versus post-filter ionized calcium. Pediatr Nephrol 2010;25:1305–1310.
[Article] [PubMed]32. Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 2005;20:1416–1421.
[Article] [PubMed]33. Bunchman TE, Maxvold NJ, Barnett J, Hutchings A, Benfield MR. Pediatric hemofiltration: Normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol 2002;17:150–154.
[Article] [PubMed]34. Ricci Z, Guzzo I, Picca S, Picardo S. Circuit lifespan during continuous renal replacement therapy: children and adults are not equal. Crit Care 2008;12:178
[Article] [PubMed] [PMC]35. Hahn H, Park YS. Regional citrate anticoagulation for continuous renal replacement therapy in children. J Korean Soc Pediatr Nephrol 2005;9:76–82.36. Shin JA, Choi YS, Jung HW, Lee YJ, Kang NR, Yang S, et al. Regional citrate anticoagulation in continuous venovenous hemodiafiltration: report of two cases. Korean J Nephrol 2006;25:447–451.37. Park JS, Kim GH, Kang CM, Lee CH. Regional anticoagulation with citrate is superior to systemic anticoagulation with heparin in critically Ill patients undergoing continuous venovenous hemodiafiltration. Korean J Intern Med 2011;26:68–75.
[Article] [PubMed] [PMC]38. Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial 2009;22:141–145.
[Article] [PubMed]
Fig. 1
Changes in serum ammonia levels after continuous renal replacement therapy in patients with hyperammonemia. Pt, patient; ARR, ammonia reduction ratio.
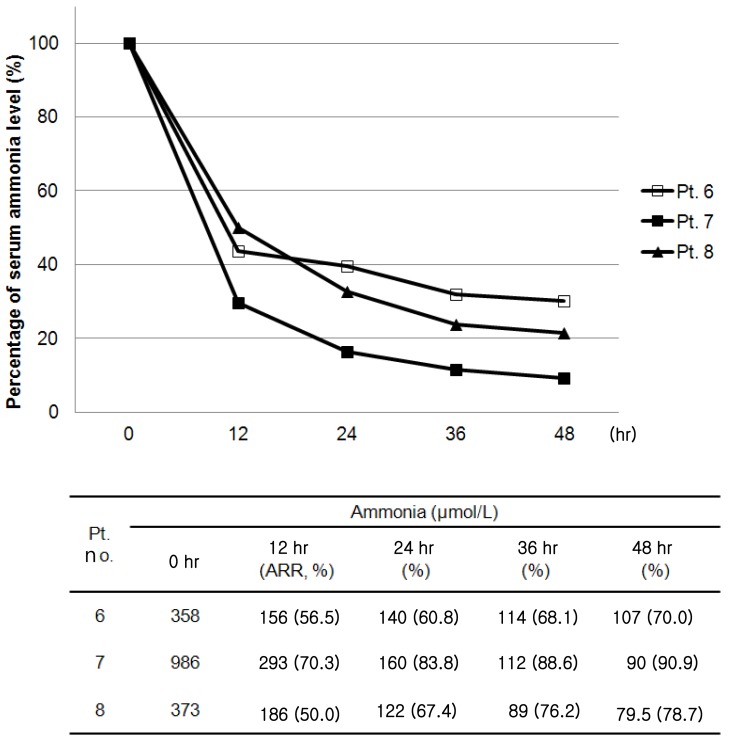
Table 1
Clinical and Laboratory Findings at CRRT Initiation
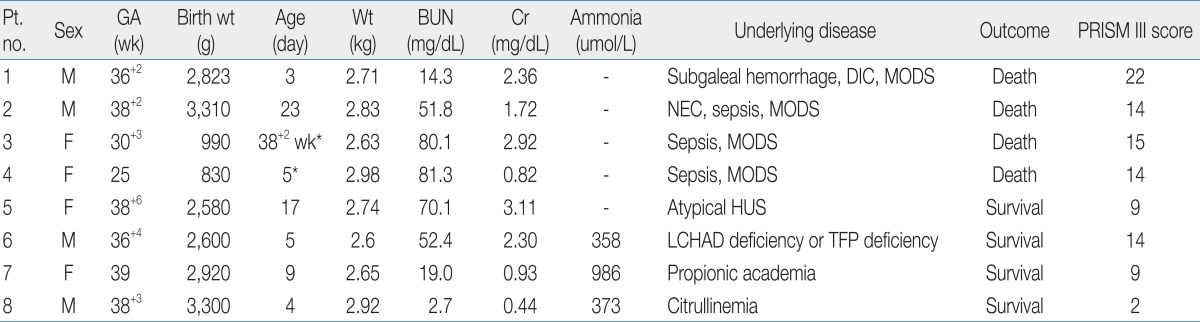
CRRT, continuous renal replacement therapy; Pt, patient; GA; gestational age, Wt, weight; BUN, blood urea nitrogen; Cr, creatinine; PRISM, pediatric risk of mortality; DIC, disseminated intravascular coagulation; MODS, multiorgan dysfunction syndrome; NEC, necrotizing enterocolitis; HUS, hemolytic uremic syndrome; LCHAD, long chain L-3-hydroxyacyl CoA dehydrogenase; TFP, trifunctional protein; PRISM III, pediatric risk of mortality III23).
*Corrected age.

 About
About Browse articles
Browse articles For contributors
For contributors