All issues > Volume 55(11); 2012
Indirect revascularization surgery for moyamoya disease in children and its special considerations
- Corresponding author: Kyu-Chang Wang, MD, PhD. Division of Pediatric Neurosurgery, Department of Neurosurgery, Seoul National University Children's Hospital, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea. Tel: +82-2-2072-3489, Fax: +82-2-2072-0274, kcwang@snu.ac.kr
- Received June 29, 2012 Accepted August 18, 2012
- Abstract
-
Moyamoya disease (MMD) is the most common pediatric cerebrovascular disease in Far Eastern countries. In children, MMD frequently manifests as ischemic symptomatology. Cerebral perfusion gradually decreases as the disease progresses, which often leads to cerebral infarction. The benefits of revascularization surgery, whether direct or indirect, have been well established in MMD patients with ischemic symptoms. In adults, the increase in cerebral blood flow achieved with indirect revascularization is often unsatisfactory, and direct revascularization is usually feasible. In children, however, direct revascularization is frequently technically not feasible, whereas the response to indirect revascularization is excellent, although 1 or 2 weeks are required for stabilization of symptoms. The authors describe surgical procedures and perioperative care in indirect revascularization for MMD. In addition, special considerations with regard to very young patients, patients with recent cerebral infarction, and patients with hyperthyroidism are discussed.
- Introduction
- Introduction
Moyamoya disease (MMD) is the most common pediatric cerebrovascular disease in Far Eastern countries. In children, MMD frequently manifests itself as ischemic symptomatology, especially transient ischemic attack (TIA) that is provoked by hyperventilation. Cerebral perfusion gradually decreases as the disease progresses, often leading to cerebral infarction. The benefits of revascularization surgery, whether direct or indirect, have been well established in MMD patients with ischemic symptoms.The main goal of surgical treatment in MMD is reversal of cerebral ischemia and protection of the brain from infarction. Even though there is a theoretical background for this, the role of surgery in the prevention of hemorrhage has not been well proven. Nonetheless, some investigators insist on revascularization surgery for preventing hemorrhage. Reduction of hemodynamic stress on the moyamoya vessels, especially at the basal ganglia, by providing widespread additional cortical collaterals via surgery may be beneficial in preventing recurrent hemorrhage.Although the primary pathology is located at the distal internal carotid artery and the proximal middle and anterior cerebral arteries, these vessels are not the targets of surgical or endovascular treatment because of associated risks and the relative safety and effectiveness of alternative surgical methods.The main surgical treatment in MMD is revascularization through the cerebral cortical vessels. A direct (direct anastomosis of scalp arteries and cerebral cortical arteries) or indirect (insertion of a scalp or muscle layer onto the surface of the brain to promote ingrowth of blood vessels into the ischemic brain) revascularization method, or a combination of both is performed to increase the cerebral blood flow (CBF). In children, direct revascularization is frequently technically not feasible, whereas the response to indirect revascularization is excellent, although 1 or 2 weeks are required for stabilization of symptoms. In contrast, in adults, the increase in CBF achieved by indirect revascularization is often unsatisfactory and direct revascularization is usually feasible. However, a sudden increase of CBF in the brain that has suffered from longstanding chronic severe ischemia may cause hemorrhagic phenomenon and/or neurological deterioration (hyperperfusion syndrome). A combined method may increase the revascularization effect although only to a small extent.
- Surgery
- Surgery
- 1. Types of operation
- 1. Types of operation
1) Direct revascularization
1) Direct revascularization
Most commonly, the superficial temporal artery (STA) is anastomosed to a cortical branch of the middle cerebral artery. Although it is less common, other scalp arteries may be used for different areas of cerebral ischemia; for example, the occipital artery may be used for ischemia of the posterior cerebral artery territory.2) Indirect revascularization
2) Indirect revascularization
When the galea, with or without scalp arteries, muscles, periosteum, or a combination of these, is inserted and placed on the surface of ischemic but live brain, blood vessels grow from the donor tissue into the ischemic brain. The name of the operation depends on the tissue inserted: encephaloduroarteriosynangiosis (EDAS), encephalogaleo[periosteo]synangiosis, encephalomyosynangiosis, or encephaloduroarteriomyosynangiosis.
- Preparation
- Preparation
During the perioperative period, hypovolemia, anemia, hyperventilation, or hypotension should be avoided. Adequate monitoring and routes for infusion of fluid and blood should be installed. Seizures that occur during the perioperative period may cause or aggravate cerebral infarction. Therefore, preventive anticonvulsant medication is recommended. Antibiotic coverage is the usual for craniotomies.
- Operation
- Operation
For direct revascularization, detailed descriptions of intracranial-extracranial arterial bypass such as STA-middle cerebral artery bypass should be referred to. The same procedures used for other causes of cerebral ischemia are applied in MMD. However, sometimes the recipient vessels in MMD are more fragile compared with those found in other diseases.Various procedures for indirect revascularization surgery can be adapted according to the tissues inserted into the cranial cavity. In the present discussion, representative procedures, STA EDAS, bifrontal encephalogaleosynangiosis (EGS), and multiple burr hole trephination are described.In children, no evident difference in surgical outcome has been noted among the various operative methods, although a slightly better outcome has been reported by "combined" (direct+indirect) surgery. However, because of technical limitations, combined surgery is not widely applied to pediatric MMD1). In adults, the revascularization outcome of indirect surgery alone is poorer than that of direct bypass surgery2).
- STA EDAS
- STA EDAS
The STA and surrounding galeal tissue are inserted and placed over the cerebral convexity of the middle cerebral artery territory3) (Fig. 1).During the perioperative period, hyperventilation, hypoventilation, hypovolemia, anemia, hypotension, and hyperthermia should be avoided. Of these, continuous hyperventilation is especially injurious to the brain. Surgeons must repeatedly check the status of anesthesia, especially the level of PaCO2, during the operation.After induction of anesthesia, the course of the parietal branch of the STA is palpated or traced with a Doppler device and is marked. When the STA cannot be palpated or traced, insertion of galeal tissue without the STA is a good alternative in pediatric MMD cases.If the operation is combined with simultaneous bifrontal EGS, both areas are prepared. The scalp layer is superficially cut to the layer of galeal tissue where the STA is located. The galeal tissue is harvested with anterior and posterior galeal incisions parallel to the STA as wide as the segment that is to be inserted onto the brain. The continuity of the STA should be preserved. Even in cases where the STA is injured, however, neovascularization is frequently satisfactory in pediatric cases.During the operation, scalp tissue should be handled with care. The scalp layer in young children is thin, and the vascularized layers are inserted on the brain while only the less vascularized portion remains. Therefore, the remaining scalp tissue is vulnerable to ischemic necrosis. Reflection of the scalp with an acute angle should be avoided, coagulation and retraction should be minimized, and the tissue should be kept moist.The muscles and periosteum are incised parallel to the STA, and craniotomy is performed. During craniotomy, massive venous bleeding may be encountered. In MMD patients, profuse collaterals can be formed through the cranium. They are cut during the craniotomy. Rapid craniotomy and bone waxing is performed to minimize blood loss.On opening the dura, the main branches of middle meningeal artery are saved. On gross examination, it is difficult to differentiate dural arteries from veins. Preoperative angiography is useful for salvaging branches of the MMA. The base of the dural flaps is the anterior or posterior margin of the craniotomy. After dural incision, the dural flaps are reflected inward and inserted into the subdural space adjacent to the craniotomy. This maneuver increases the area of contact between the vascularized surface of the dura and the brain.Many surgeons recommend incision of the arachnoid membrane to promote neovascularization from the STA and galeal tissue to the ischemic brain. However, the effect of this is not clear. Under the surgical microscope, the arachnoid membrane is incised to expose the cerebrospinal fluid (CSF) in the subarachnoid space.After the arachnoid incision, the galeal flap is sutured to the anterior and posterior margins of the dural opening. The adventitia of the STA may be sutured to the pial membrane4). Pial synangiosis may be valuable, especially when the brain is atrophic and the gap between the donor tissue and the brain surface is of significant distance. To minimize CSF leak through the area around the proximal and distal ends of the STA, pieces of Gelfoam are used to pack the gaps.The bone flap is replaced. Attention is given to preventing compression of the proximal and distal ends of the STA and the galeal tissue by the bone margins. When the brain is atrophic, the bone flap may be left floating to promote contact between the donor tissue and the brain surface. The depressed skull flap becomes less prominent as the child grows older.The scalp layers are closed in the usual fashion. As described earlier, the profuse development of collaterals through the cranial bone causes oozing of blood in the subdural and epidural spaces. Meticulous hemostasis with dural tenting (including the central portion of the bone flap) and placement of a drainage catheter into the epifascial or epiperiosteal space is helpful. To promote healthy healing of the less vascularized portion of the remaining scalp tissue, meticulous subcutaneous sutures are desirable.
- Bifrontal EGS
- Bifrontal EGS
For ischemia of the anterior cerebral artery territory, bifrontal EGS is recommended, frequently simultaneously with STA EDAS5,6) (Fig. 2). It may prevent progression of ischemia to the contralateral cerebral hemisphere from the STA EDAS side even though the surgery is limited to the anterior cerebral artery area.For bifrontal EGS, arterial branches of the STA are not used. Galeal tissue with or without periosteum is inserted into each side of the frontal area. A curvilinear scalp incision and bifrontal craniotomy crossing midline are designed. The posterior margin of the craniotomy is placed at the coronal sutures, and the anterior margin is located posterior to the hair line. A posteriorly placed craniotomy may be associated with profuse bleeding during craniotomy or dural incision. The arterial and venous phases of preoperative angiography are helpful for avoidance of large draining parasagittal veins. When collateral arterial branches of the STA that already supply the brain are seen at the area of craniotomy and dural incision, multiple burr hole trephination with insertion of galeoperiosteal flaps is a good alternative.The galeal tissue is harvested using anterior and posterior incisions and a midline galeal incision as wide as the segment that is to be inserted onto the brain. The galeal flap of each side is reflected laterally. Some reflect and insert the periosteum with galeal tissue as 1 layer, whereas others insert galeal tissue only and leave the periosteum in situ. For very young children, the scalp is thin, and the vascularized part is inserted on the brain surface, and therefore, only the less vascularized part remains, which leads to a high possibility of postoperative ischemic scalp necrosis. Leaving the vascularized periosteum in situ may be helpful to avoid this complication.Craniotomy is performed as usual. Often there is profuse bleeding during craniotomy and dural incision because of the collateral formation that occurs with MMD and because of the proximity of the surgical field to parasagittal draining veins. Dural incision is rarely impossible and frequently, the area of dural incision is limited because of prominent venous lakes with associated draining veins at the site of the dural incision. The dural flaps are reflected into the subdural space of the lateral frontal convexity. Some surgeons insert the medial dural flap into the interhemispheric fissure with the arachnoid membrane incision at the same region, but this is sometimes not feasible because of parasagittal veins. Others do not recommend such medial frontal procedures because of their tedious nature and because of the lack of evidence of superior outcome of the procedure. A generous arachnoid incision, galea dural suture, and closure are performed as for STA EDAS.
- Multiple burr hole trephination
- Multiple burr hole trephination
When there are collateral channels already formed or if neovascularization is present but not satisfactory after revascularization with craniotomy, multiple burr hole trephination with insertion of galeoperiosteal flaps at points where no collateral channels have formed is a good alternative7).
- Postoperative recovery
- Postoperative recovery
As was stated earlier, it is important to avoid hyperventilation, hypoventilation, hypovolemia, anemia, hypotension, and hyperthermia to prevent postoperative ischemia-related complications. Young children who easily become irritable and cry when in postoperative pain and separated from parents are vulnerable to postoperative hyperventilation and subsequent ischemic damage of the brain. To detect the most common postoperative complications of ischemic brain damage, postoperative epidural or subdural hemorrhage, and scalp necrosis, attention should be paid to changes in neurologic and wound status.
- Postoperative management
- Postoperative management
In the immediate postoperative period, the general hemodynamic status is very important. Hypovolemia, anemia, and hypotension, if any, should be corrected. Hyperventilation should be avoided. For young children who need close parental care, parents are recommended to stay at the bedside even when the patient is observed in the intensive care unit. Sometimes sedation or intermittent rebreathing using a plastic bag is helpful for maintenance of the PaCO2 level.Rapid correction of postoperative coagulation abnormalities is useful for preventing postoperative epidural or subdural hematoma formation.Prophylactic anticonvulsants are recommended to prevent postoperative seizures, which may aggravate ischemic brain injury. If there is no postoperative seizure for 1 to 4 weeks, anticonvulsants are stopped.Fluid challenge with volume expanders is effective for reversal of TIA or minimization of cerebral infarct. In children older than 3 to 5 years, new cerebral infarction at the surgical area is very rare after 1 week postoperative, even though symptoms of TIA may persist. However, in young children, new cerebral infarction may occur more than 1 week after the operation.Often the drainage bag is filled with fluid mixed with CSF. In this case, the drainage tube is clamped, and intermittent opening of drainage tube is recommended.When patients show focal neurologic deficits that correspond to the surgical area, the possibility of cerebral infarction and postoperative epidural or subdural hemorrhage should be ruled out by urgent neuroimaging studies. When new cerebral infarction occurs, mild elevation of blood pressure is one of the treatment options. However, conversion to hemorrhagic infarction may occur.The hyperperfusion syndrome is absent in cases with indirect revascularization. However, it may occur after direct revascularization, in cases of chronic profound brain ischemia8,9). In these cases, hypertension should be strictly avoided (mean arterial blood pressure should be maintained within 20 to 30 mm.Hg from the preoperative level, for 7 days following surgery) as should hypotension9).
- Postsurgical morbidity requiring management
- Postsurgical morbidity requiring management
Major postoperative complications include cerebral ischemia-related events (aggravated TIA or cerebral infarction), epidural or subdural hematoma formation, scalp necrosis, and hyperperfusion syndrome (in cases of direct revascularization surgery). Seizure, involuntary movement, alopecia, or headache may appear after surgery, sometimes in a delayed fashion.
- Postoperative outcome
- Postoperative outcome
After indirect revascularization surgery, new cerebral infarction at the site of surgery is rare more than 1 week after the operation in children older than 3 years. Symptomatic improvement can be noted as early as 2 weeks after surgery and usually occurs within a few months, although the speed of increase in cerebral perfusion is much slower than in the direct method.Nowadays, revascularization surgery is indicated in virtually all children with MMD, except in cases with previous widespread cerebral infarction or serious medical illness. The progressive nature of pediatric MMD and the fact that approximately 90% of pediatric cases are asymptomatic or in an improved state after surgery of any kind, and that the main predictor of poor long-term prognosis is cerebral infarction, suggest the importance of early diagnosis and surgical treatment of MMD in childhood.
- Hemodynamic and neurological outcome
- Hemodynamic and neurological outcome
An improvement in hemodynamics is reported in 83 to 96% of cases. Although cerebral hemodynamics are more improved in direct revascularization or combined direct and indirect surgery cases than in cases using indirect revascularization alone, however, the difference is not significant nor is there a difference in functional status in children with MMD10). Contrary to what is found with pediatric patients, direct revascularization with or without indirect surgery is the mainstream of treatment in adults because of their poor response to indirect revascularization.
- Functional Outcome
- Functional Outcome
The long-term outcome of children with MMD largely depends on the presence or extent of cerebral infarction. In a review of a large number of pediatric cases (1,156), 69% of patients are independent and 23% partially dependent10).
- Special considerations
- Special considerations
- 1. Young children
- 1. Young children
In children younger than 3 years, MMD frequently manifests with cerebral infarction without preceding TIA. The progression of cerebral ischemia is much faster, and the frequency of perioperative ischemia-related complications is higher than in older children. Within a few months, contralateral cerebral infarction may occur, leading to a devastated state with poor intelligence and labile emotions. Urgent revascularization surgery is recommended. When the area of initial cerebral infarction is wide, saving the contralateral intact hemisphere is of the utmost concern11). New infarction may occur around the surgical area even more than 1 week postoperatively, which is very rare in older children. However, the size of postoperative infarction is much smaller than in cases without previous operation.- 2. Recent cerebral infarction
- 2. Recent cerebral infarction
In cases with recent cerebral infarction, the timing of revascularization surgery is one of the issues. For indirect revascularization procedures, early operation after resolution of mass effect is a good option. For direct bypass surgery, some recommended delayed surgery to avoid hemorrhage, but many do not recommend delay for such a low-flow bypass surgery.- 3. Hyperthyroidism
- 3. Hyperthyroidism
Hyperthyroidism provokes symptoms of MMD. Treatment of hyperthyroidism leads to amelioration of MMD symptoms and may reduce the perioperative risk of ischemia-related complications12).
- References
- 1. Veeravagu A, Guzman R, Patil CG, Hou LC, Lee M, Steinberg GK. Moyamoya disease in pediatric patients: outcomes of neurosurgical interventions. Neurosurg Focus 2008;24:E16
[Article]2. Lee SB, Kim DS, Huh PW, Yoo DS, Lee TG, Cho KS. Long-term follow-up results in 142 adult patients with moyamoya disease according to management modality. Acta Neurochir (Wien) 2012;154:1179–1187.
[Article] [PubMed]3. Matsushima Y, Fukai N, Tanaka K, Tsuruoka S, Inaba Y, Aoyagi M, et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol 1981;15:313–320.
[Article] [PubMed]4. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004;100(2 Suppl Pediatrics): 142–149.
[Article] [PubMed]5. Kinugasa K, Mandai S, Tokunaga K, Kamata I, Sugiu K, Handa A, et al. Ribbon enchephalo-duro-arterio-myo-synangiosis for moyamoya disease. Surg Neurol 1994;41:455–461.
[Article] [PubMed]6. Kim SK, Wang KC, Kim IO, Lee DS, Cho BK. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal) synangiosis in pediatric moyamoya disease. Neurosurgery 2002;50:88–96.
[Article] [PubMed]7. Endo M, Kawano N, Miyaska Y, Yada K. Cranial burr hole for revascularization in moyamoya disease. J Neurosurg 1989;71:180–185.
[Article] [PubMed]8. Fujimura M, Kaneta T, Mugikura S, Shimizu H, Tominaga T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg Neurol 2007;67:273–282.
[Article] [PubMed]9. Kim JE, Oh CW. In: Cho BK, Tominaga T,Moyamoya disease in adult: post-bypass symptomatic hyperperfusion. editors. Moyamoya disease update. 2010Tokyo: Springer, :306–317.10. Fung LW, Thompson D, Ganesan V. Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst 2005;21:358–364.
[Article] [PubMed]
Fig. 1
Operative photo of right superficial temporal artery (STA) encehaloduroarteriosynangiosis. The galeal flap containing the STA was retracted with a rubber band. A branch of the middle meningeal artery was saved. Dural flaps were reflected inward into the adjacent subdural space. After this phase of the operation, the arachnoid membrane was incised under a surgical microscope, and the galeal flap was sutured to the margin of the dural opening.
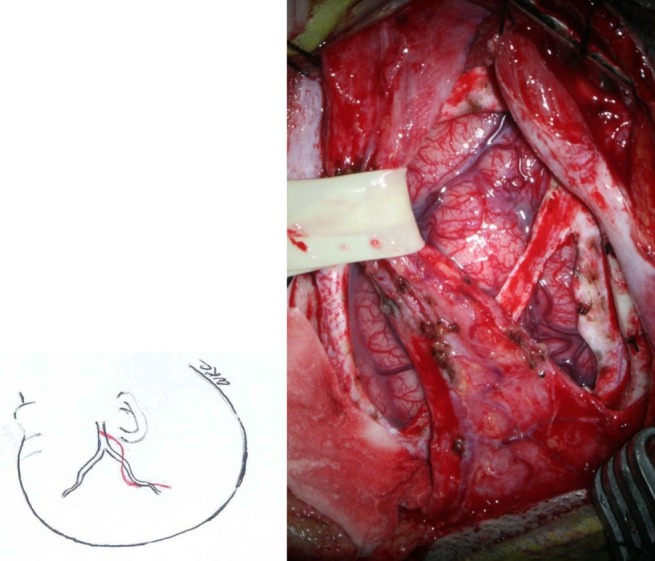
Fig. 2
Operative photo of bifrontal encephalogaleosynangiosis. The galeal flaps of both sides, which do not contain large superficial temporal artery branches, were reflected laterally, and bifrontal craniotomy was performed. The dura was opened, saving the superior sagittal sinus. The dural flaps were reflected inward into the lateral subdural space. After this phase of the operation, the arachnoid membrane was incised under a surgical microscope, and the galeal flap of each side was sutured to the margin of the dural opening.


 About
About Browse articles
Browse articles For contributors
For contributors
