All issues > Volume 56(1); 2013
Immune reconstitution after allogeneic hematopoietic stem cell transplantation in children: a single institution study of 59 patients
- Corresponding author: Bin Cho, MD, PhD. Department of Pediatrics, Seoul Saint Mary's Hospital, The Catholic University of Korea College of Medicine, 222 Banpo-daero, Seocho-gu, Seoul 137-701, Korea. Tel: +82-2-2258-6187, Fax: +82-2-588-3589, chobinkr@catholic.ac.kr
- Received September 14, 2012 Revised October 02, 2012 Accepted October 08, 2012
- Abstract
-
- Purpose
- Purpose
- Lymphocyte subset recovery is an important factor that determines the success of hematopoietic stem cell transplantation (HSCT). Temporal differences in the recovery of lymphocyte subsets and the factors influencing this recovery are important variables that affect a patient's post-transplant immune reconstitution, and therefore require investigation.
- Methods
- Methods
- The time taken to achieve lymphocyte subset recovery and the factors influencing this recovery were investigated in 59 children who had undergone HSCT at the Department of Pediatrics, The Catholic University of Korea Seoul St. Mary's Hospital, and who had an uneventful follow-up period of at least 1 year. Analyses were carried out at 3 and 12 months post-transplant. An additional study was performed 1 month post-transplant to evaluate natural killer (NK) cell recovery. The impact of pre- and post-transplant variables, including diagnosis of Epstein-Barr virus (EBV) DNAemia posttransplant, on lymphocyte recovery was evaluated.
- Results
- Results
- The lymphocyte subsets recovered in the following order: NK cells, cytotoxic T cells, B cells, and helper T cells. At 1 month post-transplant, acute graft-versus-host disease was found to contribute significantly to the delay of CD16+/56+ cell recovery. Younger patients showed delayed recovery of both CD3+/CD8+ and CD19+ cells. EBV DNAemia had a deleterious impact on the recovery of both CD3+ and CD3+/CD4+ lymphocytes at 1 year post-transplant.
- Conclusion
- Conclusion
- In our pediatric allogeneic HSCT cohort, helper T cells were the last subset to recover. Younger age and EBV DNAemia had a negative impact on the post-transplant recovery of T cells and B cells.
- Introduction
- Introduction
Hematopoietic stem cell transplantation (HSCT) is now widely used for the treatment of children with blood diseases. An important factor in the prognosis of the patient post-transplant is host immune reconstitution (IR) which, if delayed, may increase the risk of infection, disease recurrence and secondary malignancies after transplant1). IR is affected by various treatment-related variables, such as the period of antibiotic use, routine administration of intravenous immunoglobulin (IVGV), and immunomodulatory treatment after transplant such as donor lymphocyte infusion.According past literature, IR occurs in the order of monocyte, granulocyte, macrophage, and natural killer (NK) cell, resulting in the restoration of a functional innate immune system. Recovery of the adaptive immune system, however, occurs over a considerably longer period of time, with B cell restoration requiring at least six months, and T cell recovery often taking two years for completion1).Factors influencing IR at the time of transplant include patient age, donor type, stem cell source, and method of T cell depletion, while prevention and treatment of graft-versus-host disease (GVHD) are significant factors influencing IR after transplant2).According to a recent Korean study on pediatric recipients of allogeneic HSCT, NK cells and cytotoxic T cells were rapidly restored after HSCT, with 92% and 76% of patients, respectively showing recovery at 3 months post-transplant. However, IR was slower for helper T cells and B cells which showed recovery in 85% and 69% of patients, respectively at 12 months post-transplant3). Important results that derive from this study are the negative effects of certain conditioning regimens, including the use of total body irradiation (TBI) and antithymocyte globulin (ATG), cord blood as the cell source, and diagnosis of chronic GVHD, on lymphocyte reconstitution.Despite this and other previous reports on IR4,5), studies on IR in pediatric recipients of allogeneic HSCT are few. Also, an important factor which has not yet received full analysis as a possible modulator of IR is post-transplant Epstein-Barr virus (EBV) infection.In this study, we evaluated the recovery of each lymphocyte subset in 59 recipients of allogeneic HSCT at our institution. In addition to the impact of well-established variables such as patient age, donor type, acute and chronic GVHD on IR, we also analyzed EBV infection for possible effects on lymphocyte recovery.
- Materials and methods
- Materials and methods
- 1. Patient cohort
- 1. Patient cohort
From January 2009 to December 2010, 90 patients received allogeneic HSCT at the Department of Pediatrics, The Catholic University of Korea Seoul St. Mary's Hospital. Out of this initial cohort, the following exclusions were made: 14 patients who relapsed within 1 year of transplant, 8 patients who died of transplant-related mortality within 1 year, 2 patients who experienced graft failure, and 7 patients with incomplete records concerning lymphocyte subset recovery. The final study cohort included 59 patients, the major characteristics of whom are summarized in Table 1.- 2. Transplant method
- 2. Transplant method
For infection prophylaxis, oral acyclovir was given from the start of conditioning to day 42, and oral trimethoprim-sulfamethoxazole was given from the start of conditioning to day -3, and from neutrophil engraftment to at least 6 months after transplant. Granulocyte-colony stimulating factor was given from day 5 to the time when the absolute neutrophil count surpassed 3.0×109/L. For antifungal prophylaxis, we administered intravenous (IV) micafungin (1 mg/kg/day) from the start of conditioning to neutrophil engraftment, followed by oral fluconazole for at least 2 months.GVHD prophylaxis consisted of IV cyclosporine from day -1 and mini-dose methotrexate (5 mg/m2) given at days 1, 3, 6, and 11.After transplant, EBV DNA titers were evaluated at fortnightly intervals for up till 3 months post-transplant using a real-time quantitative method. Rituximab was administered only with diagnosis of post-transplantation lymphoproliferative disease (PTLD).- 3. Immunophenotypic studies
- 3. Immunophenotypic studies
Bone marrow examination and peripheral blood lymphocyte subset analysis were done at intervals of 1, 3, 6, 9, and 12 months after HSCT.With peripheral blood, CD3+, CD3+/CD4+, CD3+/CD8+, CD19+ and CD16+/CD56+, the antigens of T cell, B cell, and NK cell, were analyzed through fluorescence-activated cell sorter system, and the absolute values of each subset were calculated using the percentage of each lymphocyte subset and the absolute lymphocyte counts (ALCs).- 4. Study endpoints
- 4. Study endpoints
The main study endpoints were as follows; first, we aimed to identify the number of patients with lymphocyte subset recovery, defined as the 25th percentile of normal value. Second, we analyzed for factors that may impact the recovery of each lymphocyte subset, including patient age, donor type, stem cell source, the use of either TBI or ATG in conditioning, diagnosis of acute or chronic GVHD, and EBV DNAemia. For this second analysis, both the 25th and 75th percentile of normal values were used. EBV DNAemia was defined as having a positive value when more than 500 copies were detected per 1 mL by real-time quantitative polymerase chain reaction. All analyses were done for 3 and 12 months post-transplant. In addition, analyses for NK cell recovery and risk factors for NK cell recovery were done at 1 month post-transplant.- 5. Statistical analysis
- 5. Statistical analysis
Logistic regression analysis was performed to determine whether the pre- and post-transplant independent variables had a significant impact on recovery of each lymphocyte subset. Factors with a P value<0.05 in univariate analysis were entered into a multivariate study. Statistical analysis was done using the SAS ver. 8 (SAS Institute Inc., Cary, NC, USA). The P value was considered significant when <0.05.
- Results
- Results
- 1. Recovery of each lymphocyte subset to normal value
- 1. Recovery of each lymphocyte subset to normal value
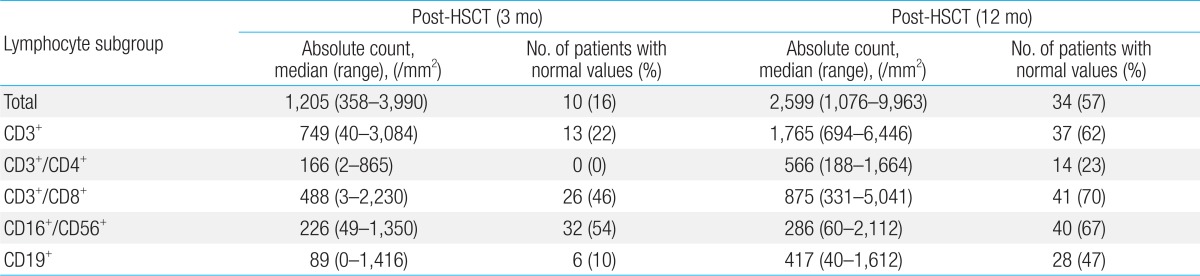
With regards to ALC, 34 patients (57%) in the overall cohort showed recovery at 12 months post-transplant (Table 2). CD3+/CD4+ was the slowest lymphocyte subset to show recovery, with 14 patients (23%) showing recovery at 12 months post-transplant, followed by CD19+ which showed recovery in 28 patients (47%).With regards to CD3+/CD8+ lymphocyte, 26 patients (46%) showed recovery at 3 months post-transplant, the number increasing to 41 patients (70%) at 12 months. Twenty-eight patients (47%) showed recovery of CD16+/CD56+ lymphocytes at 1 month post-transplant, and the number increased to 40 patients (67%) at 12 months.- 2. Factors influencing the recovery of lymphocyte subset (Table 3)
- 2. Factors influencing the recovery of lymphocyte subset (Table 3)
1) Total lymphocyte count recovery
1) Total lymphocyte count recovery
The use of ATG in conditioning significantly decreased the percentage of patients with total lymphocyte count recovery (25th percentile reference) at 12 months post-transplant (P=0.035). However, none of the other factors proved significant at any of the time points evaluated.2) CD16+/CD56+ subset recovery
2) CD16+/CD56+ subset recovery
With regards to CD16+/CD56+ cell recovery (75th percentile reference) at 1 month post-transplant, patient age, treatment with TBI in conditioning, and diagnosis of acute GVHD proved to be important in univariate study. However, in multivariate analysis, the presence of acute GVHD was most significant in terms of delaying CD16+/CD56+ cell recovery (odds ratio [OR], 24.3; 95% confidence interval [CI], 1.95 to 4,118.36; P=0.0076). In addition, diagnosis of EBV DNAemia, and unrelated transplant significantly delayed CD16+/CD56+ cell recovery at 3 months post-transplant for the 25th and 75th percentile reference levels respectively, on univariate analysis.3) CD3+ subset recovery
3) CD3+ subset recovery
At 12 months post-transplant, both EBV DNAemia and unrelated transplant significantly delayed overall CD3+ lymphocyte recovery (25th percentile reference). However, in multivariate study, only EBV DNAemia proved to be have significant impact (OR, 3.56; 95% CI, 1.16 to 10.87; P=0.026).4) CD3+/CD8+ subset recovery
4) CD3+/CD8+ subset recovery
At 12 months post-transplant, younger age (<10 years old), and the presence of either acute or chronic GVHD significantly delayed CD3+/CD8+ cell recovery (75th percentile reference). However, in multivariate analysis, only younger age had a significant impact on cytotoxic T cell recovery (OR, 3.72; 95% CI, 1.17 to 11.76; P=0.026).5) CD3+/CD4+ subset recovery
5) CD3+/CD4+ subset recovery
Diagnosis of EBV DNAemia significantly decreased CD3+/CD4+ recovery at 12 months post-transplant (P=0.033) (25th percentile reference). However, none of the other variables had a major impact on helper T cell subset recovery.6) CD19+ subset recovery
6) CD19+ subset recovery
Both younger patient age and EBV DNAemia had negative effects on CD19+ recovery at 12 months post-transplant (25th percentile reference). In multivariate study, however, younger patient age was most significant in terms of delaying B cell recovery (OR, 3.86; 95% CI, 1.15 to 12.99; P=0.029).
- Discussion
- Discussion
Previous studies have shown that among lymphocyte subsets, NK cells are the first to recover to normal levels after allogeneic HSCT. The recovery of T cells and B cells is much slower, and amongst T cells, cytotoxic T cells seem to show faster reconstitution than helper T cells3).The timing of B cell and T cell recovery has been a matter of controversy, with several previous studies concluding that the B cell recovered faster than the helper T cell, allowing the B cell to stimulate the thymus for T cell maturation and differentiation1). The results from our study also support the view that B cell recovery precedes helper T cell recovery, allowing for sequential lymphocyte maturation.Although the NK cell is known to repopulate rapidly, only 47% of the cohort showed normal levels by 1 month since transplant. Factors contributing to delayed early recovery of NK cells were younger patient age, the use of TBI in the conditioning regimen, and previous diagnosis of acute GVHD, with the last factor proving most significant in multivariate study.Several important points can be made regarding factors that influence the recovery of each lymphocyte subset.Although patient age has been reported to be an important factor, the age threshold with which the overall cohort has been divided, has varied from 10 to 16 years old2,4). Reports on the impact of patient age have also been conflicting. Some researchers have suggested that lymphocyte recovery occurs much faster in older patients2), while others have shown evidence that recovery is faster in the younger age group4). In our study, patients in the younger age group showed a significantly lower likelihood of both cytotoxic T cell and B cell recovery at 12 months post-transplant in multivariate analysis. One hypothesis for this result is that younger children may have less mature lymphoid organs that are more prone to damage from the HSCT conditioning regimen.Previous reports have shown that, although there is no difference in recovery of the innate immune system, related and unrelated transplants have shown discrepancies with regards to recovery of antigen-specific cellular immunity6). In our study, the effect of donor type in post-transplant IR was not significant in multivariate study, although univariate effects of delayed CD16+/CD56+ and CD3+ cell recovery were noted.Past studies have shown a faster rate of CD3+/CD4+ and CD3++/CD8+ lymphocyte recovery in recipients of peripheral blood stem cell transplantation, compared to bone marrow transplantation7). Our study did not show an advantage for either cell source with regards to IR, consistent with a recent domestic report on immune recovery3).The effect of ATG administered as part of the conditioning regimen on IR is a matter of controversy, with at least 1 study concluding that ATG has no significant effect on immunological recovery8). In our study, the use of ATG had a significantly detrimental effect on the recovery of ALC, as evidenced at 1 year post-transplant.The possible role of GVHD in IR has been studied considerably, with published data suggesting that acute GVHD and subsequent treatment may delay the recovery of CD3+/CD4+ lymphocytes9). GVHD is known to deter the activities of the thymus, and suppress both CD3+/CD4+ and CD3+/CD8+ lymphocytes, as well as CD19+ lymphocytes5). Our analysis showed that acute GVHD had a significant role in the delayed recovery of innate immunity, as represented by the CD16+/CD56+ subset in the early period after transplant. In contrast, diagnosis of chronic GVHD did not have a significant impact in multivariate analysis.Relatively little is known of the impact of EBV infection on the recovery of specific lymphocyte subsets. Past studies have shown that lymphocyte subset recovery was accelerated with cytomegalovirus infection5), and that EBV specific T lymphocytes increased with EBV infection10). The entity of PTLD would indicate that B cell lymphocyte proliferation is increased by EBV infection to the extent of gaining malignant potential11-13), and another study found that early infection by EBV or adenovirus delayed immune reconstruction because such early infections are linked to pathogenesis of chronic GVHD14). In our study, we found that EBV infection, as diagnosed by EBV DNA titers, significantly delayed recovery of CD3+ cells in multivariate study, was the only important factor in analysis of CD3+/CD4+ cell recovery, and was also significant in delayed recovery of CD19+ cells, although not in multivariate study. Whether EBV infection was actually responsible for delayed CD3+ recovery is problematic, as past literature supports the converse situation; CD3+/CD4+ cells and CD3+/CD8+ cells are known to prevent EBV infection 15-17), and in vivo T cell depletion, as was done for our unrelated donor transplant recipients, may have played a role in subsequent EBV infection. However, considering EBV infection was the independent factor and CD3+ recovery status the outcome, or dependent factor, in our regression analyses, our data also point to the possibility of delayed CD3+ recovery by EBV infection.In conclusion, the lymphocyte subsets in our study recovered in the following order: CD16+/CD56+, CD3+/8+, CD19, and CD3+/4+. In multivariate study, acute GVHD, EBV infection, and younger patient age had major effects on the delayed recovery of CD16+/56+, CD3+, and CD19+ cells, respectively. The results from our pediatric cohort should contribute to a greater understanding of IR after allogeneic HSCT in children, and may provide the basis for larger scale studies of the role of both debated and unrecognized factors such as patient age and EBV infection, in post-transplant immune recovery.
- References
- 1. Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol 2008;21:579–596.
[Article] [PubMed] [PMC]2. Hannet I, Erkeller-Yuksel F, Lydyard P, Deneys V, DeBruyere M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today 1992;13:215218
[Article] [PubMed]3. Bae KW, Kim BE, Koh KN, Im HJ, Seo JJ. Factors influencing lymphocyte reconstitution after allogeneic hematopoietic stem cell transplantation in children. Korean J Hematol 2012;47:44–52.
[Article] [PubMed] [PMC]4. Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol 2002;118:74–89.
[Article] [PubMed]5. de Vries E, van Tol MJ, van den Bergh RL, Waaijer JL, ten Dam MM, Hermans J, et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant 2000;25:267–275.
[Article] [PubMed]6. Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood 1999;93:467–480.
[Article] [PubMed]7. Heining C, Spyridonidis A, Bernhardt E, Schulte-Monting J, Behringer D, Grullich C, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transplant 2007;39:613–622.
[Article] [PubMed]8. Lynch BA, Vasef MA, Comito M, Gilman AL, Lee N, Ritchie J, et al. Effect of in vivo lymphocyte-depleting strategies on development of lymphoproliferative disorders in children post allogeneic bone marrow transplantation. Bone Marrow Transplant 2003;32:527–533.
[Article] [PubMed]9. Jimenez M, Martinez C, Ercilla G, Carreras E, Urbano-Ispizua A, Aymerich M, et al. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol 2005;33:1240–1248.
[Article] [PubMed]10. Kuzushima K, Kimura H, Hoshino Y, Yoshimi A, Tsuge I, Horibe K, et al. Longitudinal dynamics of Epstein-Barr virus-specific cytotoxic T lymphocytes during post-transplant lymphoproliferative disorder. J Infect Dis 2000;182:937–940.
[Article] [PubMed]11. Gartner BC, Schafer H, Marggraff K, Eisele G, Schafer M, Dilloo D, et al. Evaluation of use of Epstein-Barr viral load in patients after allogeneic stem cell transplantation to diagnose and monitor post-transplant lymphoproliferative disease. J Clin Microbiol 2002;40:351–358.
[Article] [PubMed] [PMC]12. Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of post-transplant lymphoproliferative disease in high-risk patients. Blood 2001;97:1165–1171.
[Article] [PubMed]13. Izumiya S, Ishida M, Hodohara K, Yoshida T, Okabe H. Epstein-Barr virus-associated lymphoproliferative disorder developed following autologous peripheral blood stem cell transplantation for relapsing Hodgkin's lymphoma. Oncol Lett 2012;3:1203–1206.
[Article] [PubMed] [PMC]14. Olkinuora H, von Willebrand E, Kantele JM, Vainio O, Talvensaari K, Saarinen-Pihkala U, et al. The impact of early viral infections and graft-versus-host disease on immune reconstitution following paediatric stem cell transplantation. Scand J Immunol 2011;73:586–593.
[Article] [PubMed]15. Wingate PJ, McAulay KA, Anthony IC, Crawford DH. Regulatory T cell activity in primary and persistent Epstein-Barr virus infection. J Med Virol 2009;81:870–877.
[Article] [PubMed]
Table 1

Values are presented as median (range) or number (%).
HSCT, hematopoietic stem cell transplantation; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; HLH, hemophagocytic lymphohistiocytosis; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; SAA, severe aplastic anemia; TBI, total body irradiation; ATG, antithymocyte globulin.
Table 3
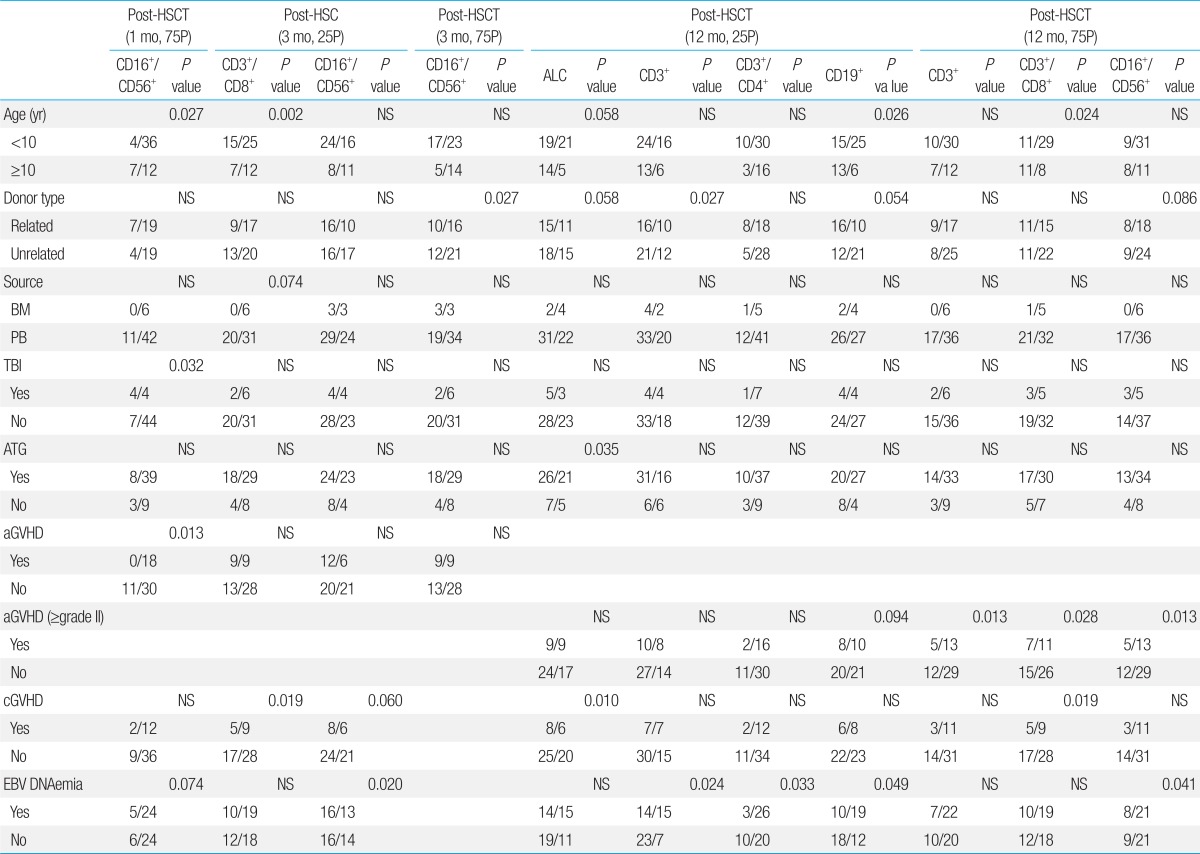
Data represented as number of patients with subset recovery within specified category/number of patients within specified category.
HSCT, hematopoietic stem cell transplantation; 75P, 75th percentile reference; 25P, 25th percentile reference; CD, cluster of differentiation; ALC, absolute lymphocyte count; BM, bone marrow; PB, peripheral blood; TBI, total body irradiation; ATG, anti-thymocyte globulin; aGVHD, acute graft versus host disease; cGVHD, chronic GVHD; EBV, Epstein-Barr virus; NS, not significant.

 About
About Browse articles
Browse articles For contributors
For contributors