All issues > Volume 56(2); 2013
The control of invasive Candida infection in very low birth weight infants by reduction in the use of 3rd generation cephalosporin
- Corresponding author: Jang Hoon Lee, MD, PhD. Department of Pediatrics, Ajou University School of Medicine, 206 Worldcup-ro, Yeongtong-gu, Suwon 443-721, Korea. Tel: +82-31-219-4487, Fax: +82-31-219-5169, neopedlee@gmail.com
- Received July 24, 2012 Revised August 24, 2012 Accepted October 24, 2012
- Abstract
-
- Purpose
- Purpose
- To evaluate the effectiveness of new management policies on the incidence of invasive Candida infections
- Methods
- Methods
- This observational study involved a retrospective analysis of the patients' medical records. In total, 99 very low birth weight infants, who were admitted to the neonatal intensive care unit at Ajou University Hospital from January 2010 to December 2011, were enrolled for the study. Period I, defined as the period before the revision of management policies, comprised 57 infants; whereas, period II, defined as the period after the implementation of new management policies, comprised 42 infants. The new management policies entailed a reduction in antibiotic and histamine type 2 receptor blocker (H2 blocker) use, duration of central venous catheterization, and duration of endotracheal intubation.
- Results
- Results
- There was a significant overall decrease in the use of antibiotics including 3rd generation cephalosporin and H2 blockers (P<0.05), and a significantly lower incidence of invasive Candida infections in period II as compared to period I (0/42 vs. 6/57, respectively; P=0.037). Comparison between infants with invasive Candida infections (n=6) and those without (n=93) showed that gestational age (odds ratio [OR], 0.909; 95% confidence interval [CI], 0.829 to 0.996; P=0.042) and the duration of 3rd generation cephalosporin use (OR, 1.093; 95% CI, 1.009 to 1.183; P=0.029) were statistically significant risk factors.
- Conclusion
- Conclusion
- The new management policies effectively decreased overall use of antibiotics, especially 3rd generation cephalosporin, and H2 blockers, which led to a significantly lower incidence of invasive Candida infections.
- Introduction
- Introduction
Despite recent advances in neonatal intensive care and an increase in survival of very preterm infants, sepsis due to nosocomial infection has been a common problem that result in significant morbidity and mortality1,2).Fungi are the third most frequent etiologic organisms of late onset sepsis in preterm infants3,4), with an incidence of 1.6% to 3% in a very low birth weight (VLBW) infants, and up to 15% to 20% in extremely low birth weight (ELBW) infants4-6) and mortality ranges from 25% to 55%1,6). Invasive fungal infection in the neonatal period is associated with early gestational age, low birth weight, central venous catheterization, mechanical ventilation, use of broad spectrum antibiotics, especially the 3rd generation cephalosporin, delayed enteral feeding and the use of histamine type 2 receptor blocker (H2 blocker)5,7). However, there have been only few studies that deal with the effect of clinical interventions on risk factors in a neonatal intensive care unit (NICU) setting.Invasive Candida infection is an important and critical problem that is often encountered in our NICU. Therefore, authors reviewed the overall management policies that is associated with nosocomial infection in our NICU, and revised it in order to prevent invasive Candida infection.This study was done to compare the management and outcomes that are associated with invasive Candida infection and overall nosocomial sepsis before and after revising the management policies, and thus, evaluate the effectiveness of the new management policies.
- Materials and methods
- Materials and methods
- 1. Study design and setting
- 1. Study design and setting
1) Reduction in the use of antibiotics, especially the 3rd generation cephalosporin
1) Reduction in the use of antibiotics, especially the 3rd generation cephalosporin
Initial empirical antibiotics used in period I were ampicillin plus cephalosporin and in period II, ampicillin plus gentamicin8). Administration of extended period of antibiotics was suppressed by early discontinuation of antibiotics after considering laboratory results and symptom changes on 3rd and 7th day of treatment9). Vancomycin was prescribed only in symptomatic infants with confirmed methicillin resistant Staphylococci growth in culture studies10). Use of carbapenem without evidence of necrotizing enterocolitis (NEC, >stage Ib) or multidrug resistant bacteria was suppressed11).2) Reduction in the use of H2 blocker
2) Reduction in the use of H2 blocker
In period I, H2 blocker was used when more than 3 days of fasting was required in infants. However in period II, H2 blocker was administrated only in infants who had a risk of stress-related mucosal damage that can occur rapidly, including conditions such as sepsis or after surgery12).3) Reduction in the duration of central venous catheterization and endotracheal intubation
3) Reduction in the duration of central venous catheterization and endotracheal intubation
This was achieved by encouraging early enteral feeding and disuse of invasive mechanical ventilation by early weaning and removal of endotracheal tube.Through this study, authors tried to compare the management and outcomes associated with an invasive Candida infection and the overall nosocomial sepsis before and after the revision of management policies, and evaluate the effectiveness of new management policies, regarding invasive Candida infections.
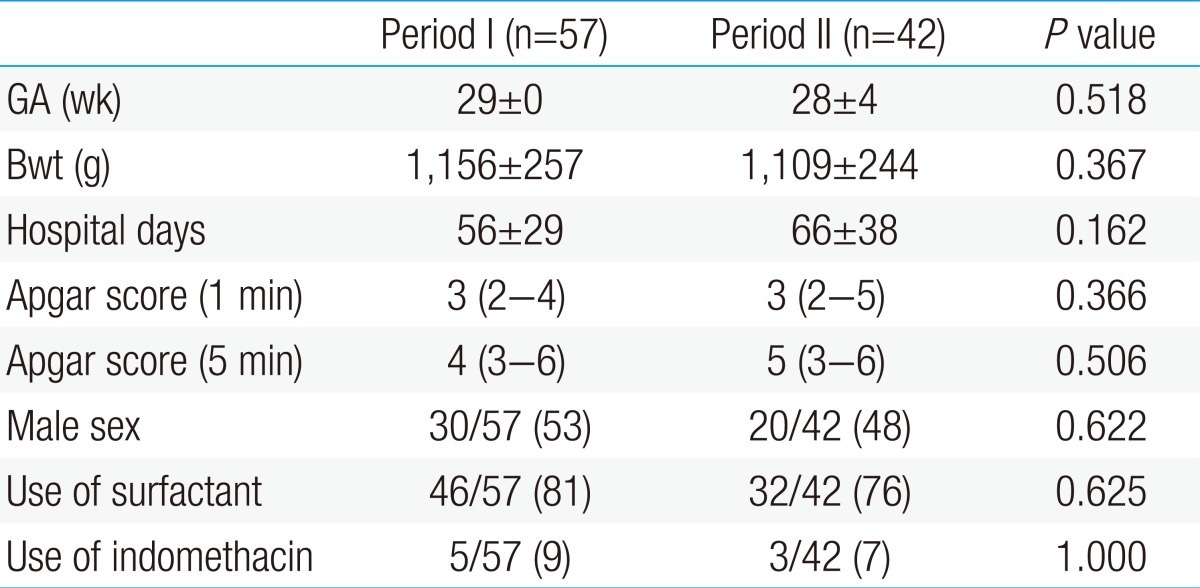
This study was an observational study, which incorporated the reviewing of medical records in a retrospective manner. Among the 108 VLBW infants admitted to the NICU, at the Ajou University Hospital from January 2010 to December 2011, a total of 99 VLBW infants were enrolled in this study. Of those, 57 infants born from January 2010 to December 2010 were included in period I (before revising management policies), and 42 infants born from January 2011 to December 2011 were included in period II (after revising management policies). Nine infants were excluded in this study; six infants in period I were excluded due to deaths within the postnatal one week (n=4) and temporal overlap with period II (n=2). Three infants in period II were excluded due to deaths within postnatal one week (Fig. 1).Demographic data were collected, including the gestational age, birth weight, hospital days, gender, Apgar score (1 minute, 5 minutes) and the use of surfactant and indomethacin. This study was approved by the Institutional Review Board at the Ajou University Hospital.After reviewing the medical records of infants admitted to the NICU in 2010 and the associated studies, we revised the management policies as follows.- 2. Definitions and blood culture method
- 2. Definitions and blood culture method
Nosocomial sepsis was defined on the basis of Center for Disease Control and Prevention criteria13). Even when a positive blood culture was identified in our hospital microbiology laboratory with automated BACTEC system, the infant's systemic instability such as apnea, bradycardia and feeding intolerance, and laboratory values such as C-reactive protein at the time of sampling, was considered to exclude possible contamination and colonization. Only when such systemic instability and increase in C-reactive protein was accompanied with positive blood cultures or when the same bacteria or fungus was recultured, it was defined as true sepsis11,14).Bronchopulmonary dysplasia was defined as when there is a need for supplemental oxygen support at the 36 weeks' post-menstrual age15), and the NEC was defined as the Bell's stage II or greater16) and stage III or IV intraventricular hemorrhage as Papile's classification17). Full enteral feeding was defined as feeding of more than 100 kcal/kg/day.- 3. Statistical analysis
- 3. Statistical analysis
Categorical data are presented as numbers (%), and continuous data as the mean±standard deviation or the median (25-75%). The χ2 or Fisher's exact test was used to compare the categorical variables and the Student's t-test, paired t-test, Mann-Whitney's rank sum U test were also used. Data were analyzed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). A P value of <0.05 was considered significant. Multiple logistic regression with forward conditional variable selection was performed with the risk factors identified as significant in the univariate analysis.
- Results
- Results
- 1. Summary of nosocomial sepsis in period I and II
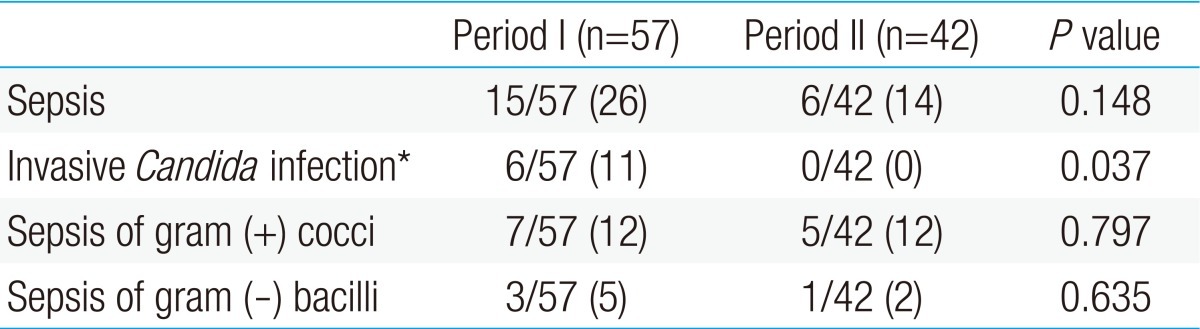
- 1. Summary of nosocomial sepsis in period I and II
There were a total of 15 infants with nosocomial sepsis in period I (26%, 15/57). Of these, 6 of them had invasive Candida infections in whole blood cultures. Candida infection was found on average of 26.8 days of birth (range, 11 to 50 days). Liposomal amphotericin B (5 mg/kg/dose) was administrated on average of 19.3 days. Three of the treated infants expired and in one case, voriconazole was used due to treatment failure with liposomal amphotericin B. There were 7 infants with gram-positive cocci sepsis and 3 infants with gram-negative bacilli sepsis with proven Klebsiella oxytoca and Enterobacter cloacae growths. One infant had experienced two episodes of nosocomial sepsis with Candida and gram-positive coccus, respectively.In period II, there were 6 infants with nosocomial sepsis (14%, 6/42). There were no invasive Candida infections in period II. Out of these 6 infants, 5 infants had gram-positive cocci sepsis, all with proven Staphylococcus epidermidis growth, and one infant had gram-negative bacilli sepsis with proven Pseudomonas aeruginosa growth. All the cultured S. epidermidis in period I and period II were methicillin resistant S. epidermidis. Summary of comparison of nosocomial sepsis in period I and II are shown in Table 1.- 2. Comparisons of demographic data between period I and period II
- 2. Comparisons of demographic data between period I and period II
There were no significant differences in the gestational age, birth weight and hospital days between the period I and II. Total number of infants admitted to the NICU in period I and II were 755 and 725, respectively. Total inpatients service day for period I and II were 8,159 and 8,092, respectively, and there were no statistical significant difference between these two periods. There were no changes in the members of the nursing team. Guidelines for hand-washing, infant handling and central venous catheter management were constantly regulated and maintained. The demographic data of these two groups are shown in Table 2.- 3. Comparisons of morbidity and mortality between period I and period II
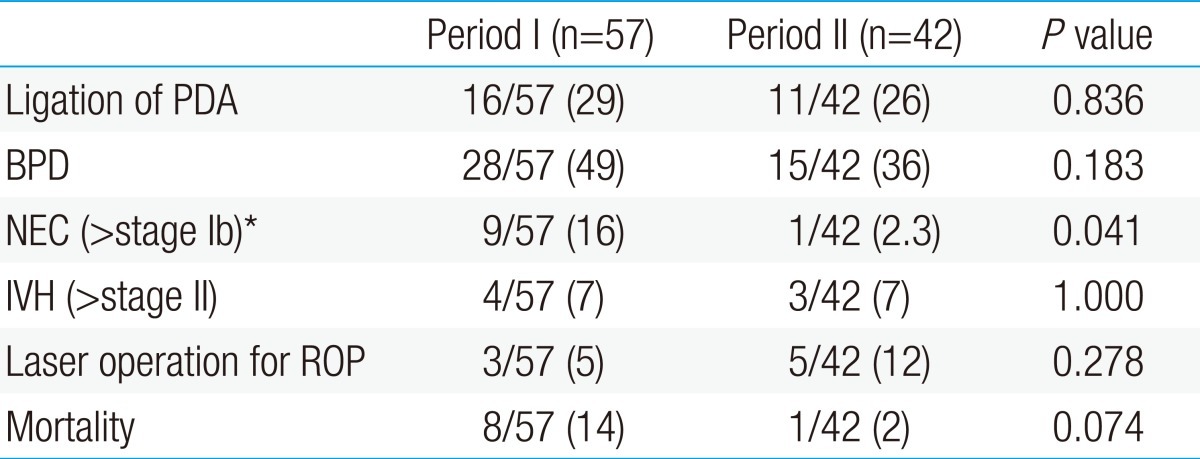
- 3. Comparisons of morbidity and mortality between period I and period II
Although most of the perinatal morbidities and mortality were not different between the two periods, incidence of NEC decreased significantly in period II, in comparison to period I (period I, 16% [9/57] vs. period II, 2.3% [1/42]; P=0.041). The demographic data of these two groups are shown in Table 3.- 4. Comparisons of incidence of sepsis and associated factors between period I and period II
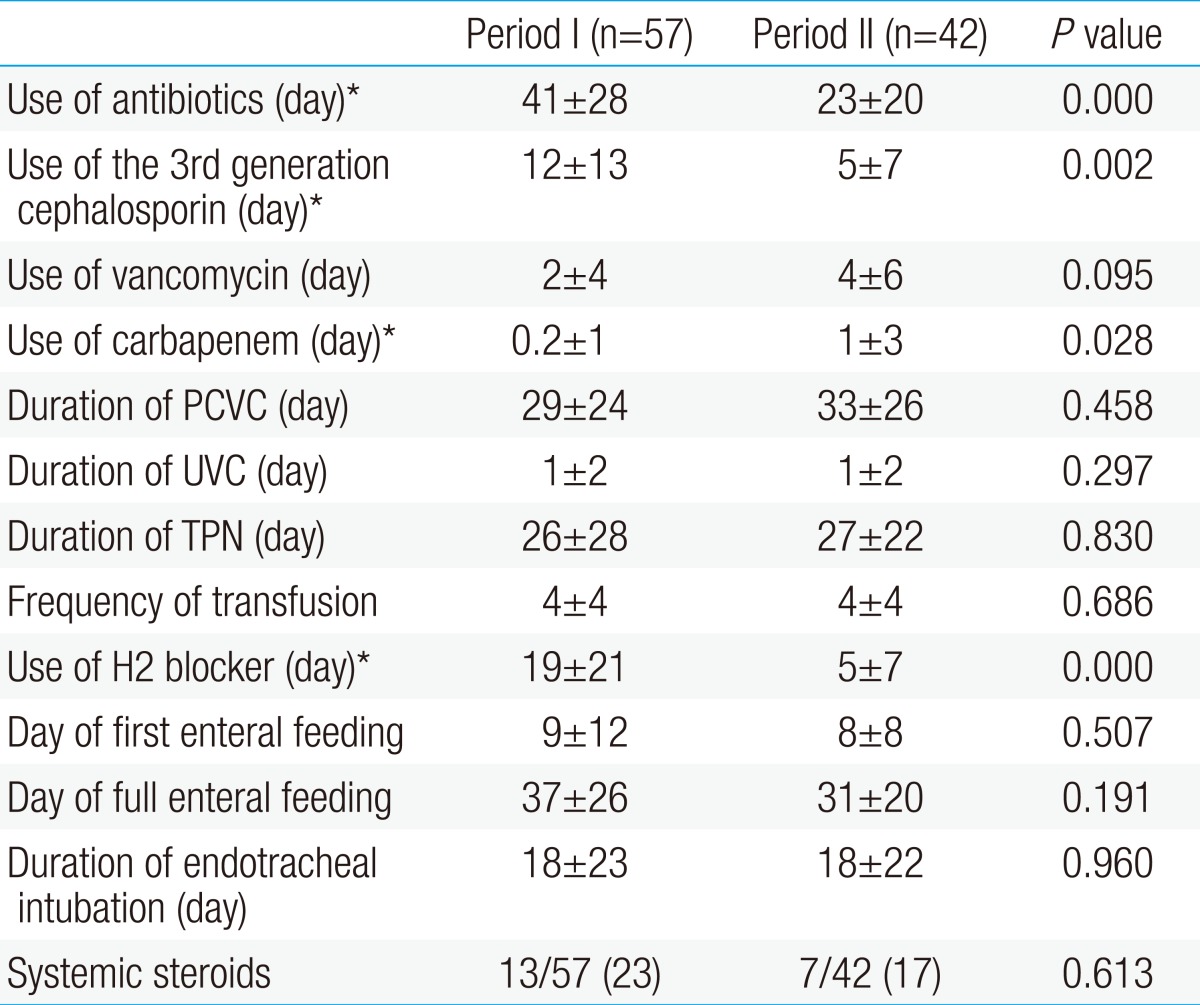
- 4. Comparisons of incidence of sepsis and associated factors between period I and period II
The use of the overall antibiotics (period I, 41±28 vs. period II, 23±20; P<0.001) and the 3rd generation cephalosporin (period I, 12±13 vs. period II, 5±7; P=0.002) decreased in period II when compared to period I. Although the use of vancomycin was not different between the two periods, the use of carbapenem increased in period II, compared to that of period I. The use of H2 blockers were decreased in period II compared to period I (period I, 19±21 vs. period II, 5±7; P<0.001). The duration of the central venous catheterization, total parenteral nutrition (TPN) and invasive mechanical ventilation were not significantly different between the two periods. Also, the use of systemic steroid and the day of the first enteral feeding and the full enteral feeding were not significantly different between the two periods (Table 4). Although the incidences of overall nosocomial, gram-positive cocci and gram-negative bacilli sepsis were not statistically different between the two periods, the incidence of invasive Candida infection exhibited a significant decrease in period II, in comparison to that of period I (period I, 11% [6/57] vs. period II, 0% [0/42]; P=0.037) (Table 5).- 5. Risk factor associated with invasive Candida infection
- 5. Risk factor associated with invasive Candida infection
In the univariate analysis, infants with invasive Candida infection (n=6) compared to infants without invasive Candida infection (n=93), showed lower birth weight, younger gestational age, longer use of overall antibiotics including 3rd generation cephalosporin, longer use of H2 blocker and longer duration of endotracheal intubation (P<0.05). Multiple logistic regression with identified significant risk factors in the univariate analysis showed that the gestational age (odds ratio [OR], 0.909; 95% confidence interval [CI], 0.829 to 0.996; P=0.042) and the use of 3rd generation cephalosporin (OR, 1.093; 95% CI, 1.009 to 1.183; P=0.029) were statistically significant.
- Discussion
- Discussion
The incidence of invasive fungal infection is known to be 1.6-3% in VLBW and 15-20% in ELBW infants4-6). Candida species are the most common fungal pathogens18) and comprise 70-90% of all invasive fungal infections in neonates19). Neonates themselves are known to be a risk factor for Candida infections, along with immune-compromised children, such as recipients of organ transplantations and children in the intensive care units20). In addition, lower birth weight, use of cephalosporin, difference in gender and lack of enteral feeding are associated with Candida infections in the neonatal period21). Particularly, VLBW infants are reported to have 25-35 times greater risk for invasive Candida infection when compared to the full term infants22). Recently, many studies have been reported on the use of fluconazole prophylaxis for the prevention of invasive fungal infection23-25) and fluconazole prophylaxis seems to inhibit the fungal colonization in the skin, gastrointestinal tract and respiratory tract26). However, routine prophylaxis with fluconazole is not recommended in all newborns27). Therefore, fluconazole prophylaxis should be used selectively in high risk preterm infants with additional risk factors, such as central venous catheterization and extended period of exposure to broad spectrum antibiotics28).For the prevention of invasive Candida infection in our NICU, 1) reduction in the use of antibiotics, especially the 3rd generation cephalosporin, 2) reduction in the use of H2 blockers, 3) reduction in the duration of central venous catheterization by encouraging enteral feeding and invasive mechanical ventilation by early removal of endotracheal tube were done. Routine fluconazole prophylaxis was not applied. Significant decrease in the use of the 3rd generation cephalosporin and H2 blockers were achieved. To reduce the use of 3rd generation cephalosporins, its administration was limited to infants with meningitis or with proven blood cultures only. Follow-up blood cultures and laboratory tests including C-reactive protein, complete blood count and differential count were done on 3rd and 7th day of administration of antibiotics for the early detection of improvement of infant's status. Results were interpreted for making decision for early discontinuation of antibiotics. However, compared to that of period I, the duration of central venous catheteriation and endotracheal intubation was not reduced in period II. In addition, compared to period I, day of the first enteral feeding and full enteral feeding was not achieved any earlier in period II. There were also no difference between the two groups on the duration of TPN, frequency of transfusions and use of systemic steroids. These results might be due to the improved survival rate of ELBW infants in period II, although, there was no statistical significance.Authors observed a decreased incidence of NEC in period II, compared to that of period I, and it is thought to be due to reduced use of antibiotics. Antibiotic use in the neonatal population has been previously described as a potential contributing factor in the development of NEC. Multivariate analyses aimed to exclude confounding factors, such as gestational age, birth weight and sepsis have shown similar persistent results29,30). Such antibiotic administration may result in suppression or eradication of protective anaerobic bacteria which normally colonize the gastrointestinal tract and thereby result in overgrowth of potentially pathogenic enteric aerobic gram-negative rods31,32). Combined with the impairment in the intestinal barrier, colonization of pathogenic bacteria superior to that of the normal flora may predispose preterm infants to NEC33).Despite the fact that new management policies of our unit did not include fluconazole prophylaxis, the new policies nevertheless succeeded in reducing the use of H2 blockers, and overall use of antibiotics, especially the 3rd generation cephalosporin, and thereby successfully decreased the invasive Candida infection in our unit. As shown in the table, out of total 75 VLBW infants in period I, 6 had invasive Candida infection (10.5%, 6/57) and all of them were treated with liposomal amphotericin B (5 mg/kg). First line antifungal agent commonly used in our hospital is amphotericin B or fluconazole. However, we have chosen liposomal amphotericin B because among the 6 infants with invasive Candida infection, 2 had initial renal insufficiency and 4 showed oliguria progressing to renal insufficiency34).There were 6 (20%) invasive Candida infection out of 30 VLBW infants in 2008, and 4 out of 34 (11.7%) in 2009, showing a consistent invasive Candida infection every year. These incidence rates are similar compared to the previously reported incidence rates in the literature. Invasive Candida infection were not in the form of outbreaks, but rather in sporadic manner with many various strains of Candida, including Candida parapsilosis, Candida albicans and Candida guilliermondii. However, after policy modification and application, there was not one case of invasive Candida infection in period II.In the univariate analysis comparing the infants with and without invasive Candida infection, lower birth weight, younger gestational age, longer use of overall antibiotics, including 3rd generation cephalosporin, longer use of H2 blocker and longer duration of invasive mechanical ventilation were statistically significant risk factors. However, after multiple logistic regression, gestational age and use of 3rd generation cephalosporin were the only significant risk factors.This study, therefore, shows that strict establishment and refreshment of clinical policies in the NICU can control invasive Candida infection in VLBW infants with critical conditions and additional risk factors. However, sepsis of gram-positive cocci has often occurred in our NICU in both periods I and II. More careful control in the insertion and the maintenance of central venous catheter may be needed to control such infections in our NICU.Most of the published data are retrospective comparative analysis of infants with or without invasive fungal infection. Manzoni et al.6) has showed that independent risk factors for invasive fungal infection are colonization of the central venous catheter and colonization in multiple sites, and Saiman et al.5) reported that gestational age <32 weeks, 5-minute Apgar <5, shock, disseminated intravascular coagulopathy, prior use of Intralipid, parenteral nutrition, central venous catheters, H2 blockers, intubation or length of stay >7 days before candidemia were all factors related to invasive fungal infection. Kaufman35) showed that a decrease in the use of the 3rd and 4th generation cephalosporin and carbapenem class broad-spectrum antibiotics, H2 blocker and proton pump inhibitors, and steroids, such as dexamethasone, could lower the incidence of invasive fungal infection. Early trophic feeding, typically within 3 days of birth, and breast feeding could also contribute to the prevention of invasive fungal infection35) .A study that changed the variety of parameters and management policies, along with investigating its efficacy and outcome, somewhat similar to our study, was done by Hwang et al.36). In that study, the restriction of antibiotic therapy, less use of invasive procedures, such as umbilical vein catheterization and endotracheal intubation, establishment of guidelines for hand-washing, infant handling, and central intravascular line management significantly decreased the nosocomial infection in ELBW infants. Only the duration of antibiotics treatment was an independent risk factor for nosocomial infection.The duration and follow-up of our study is somewhat short and the number of subjects is limited. Also, this study was done retrospectively. For a more objective result, there may be a need for a prospective study with a large number of subjects. Nonetheless, it is proven that decrease in the use of the 3rd generation cephalosporin in a single unit effectively lowers the incidence of invasive Candida infection. It is also a significant finding that with just preventive policies and without routine use of prophylactic fluconazole, invasive Candida infection can be reduced to less than reported rates. To maintain such low incidence rates, efforts must be put into decreasing the duration of ventilator care and central venous catheter insertion period, and to start early feeding, along with decreasing the use of the 3rd generation cephalosporin and H2 blockers.In conclusion, new management policies in our NICU resulted in a reduction of the overall use of antibiotics, especially the 3rd generation cephalosporin, and H2 blockers. Furthermore, reduction in the use of 3rd generation cephalosporin had a significant effect on decreasing the incidence of invasive Candida infection in our NICU.
- References
- 1. Perez-Gonzalez LF, Ruiz-Gonzalez JM, Noyola DE. Nosocomial bacteremia in children: a 15-year experience at a general hospital in Mexico. Infect Control Hosp Epidemiol 2007;28:418–422.
[Article] [PubMed]2. Bartels DB, Schwab F, Geffers C, Poets CF, Gastmeier P. Nosocomial infection in small for gestational age newborns with birth weight <1500 g: a multicentre analysis. Arch Dis Child Fetal Neonatal Ed 2007;92:F449–F453.
[Article] [PubMed] [PMC]3. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109:34–39.
[Article] [PubMed]4. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1): 285–291.
[Article] [PubMed]5. Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J 2000;19:319–324.
[Article] [PubMed]6. Manzoni P, Jacqz-Aigrain E, Rizzollo S, Franco C, Stronati M, Mostert M, et al. Antifungal prophylaxis in neonates. Early Hum Dev 2011;87(Suppl 1): S59–S60.
[Article] [PubMed]7. Manzoni P, Farina D, Leonessa M, d'Oulx EA, Galletto P, Mostert M, et al. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics 2006;118:2359–2364.
[Article] [PubMed]8. de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet 2000;355:973–978.
[Article] [PubMed]9. Kaiser JR, Cassat JE, Lewno MJ. Should antibiotics be discontinued at 48 hours for negative late-onset sepsis evaluations in the neonatal intensive care unit? J Perinatol 2002;22:445–447.
[Article] [PubMed]10. Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988-1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics 2000;106:1387–1390.
[Article] [PubMed]11. Patel SJ, Oshodi A, Prasad P, Delamora P, Larson E, Zaoutis T, et al. Antibiotic use in neonatal intensive care units and adherence with Centers for Disease Control and Prevention 12 Step Campaign to Prevent Antimicrobial Resistance. Pediatr Infect Dis J 2009;28:1047–1051.
[Article] [PubMed] [PMC]12. Brett S. Science review: the use of proton pump inhibitors for gastric acid suppression in critical illness. Crit Care 2005;9:45–50.
[PubMed]13. Gastmeier P, Geffers C, Schwab F, Fitzner J, Obladen M, Ruden H. Development of a surveillance system for nosocomial infections: the component for neonatal intensive care units in Germany. J Hosp Infect 2004;57:126–131.
[Article] [PubMed]14. Rubin LG, Sanchez PJ, Siegel J, Levine G, Saiman L, Jarvis WR, et al. Evaluation and treatment of neonates with suspected late-onset sepsis: a survey of neonatologists' practices. Pediatrics 2002;110:e42
[Article] [PubMed]15. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82:527–532.
[Article] [PubMed]16. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201.
[Article] [PubMed] [PMC]17. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–534.
[Article] [PubMed]18. Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Arch Dis Child Fetal Neonatal Ed 2006;91:F188–F192.
[Article] [PubMed]19. Filioti I, Iosifidis E, Roilides E. Therapeutic strategies for invasive fungal infections in neonatal and pediatric patients. Expert Opin Pharmacother 2008;9:3179–3196.
[Article] [PubMed]20. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39:309–317.
[Article] [PubMed]21. Lopez Sastre JB, Coto Cotallo GD, Fernandez Colomer B. Grupo de Hospitales Castrillo. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol 2003;20:153–163.
[Article] [PubMed]22. Long SS, Stevenson DK. Reducing Candida infections during neonatal intensive care: management choices, infection control, and fluconazole prophylaxis. J Pediatr 2005;147:135–141.
[Article] [PubMed]23. Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med 2001;345:1660–1666.
[Article] [PubMed]24. Aziz M, Patel AL, Losavio J, Iyengar A, Berven M, Schloemer N, et al. Efficacy of fluconazole prophylaxis for prevention of invasive fungal infection in extremely low birth weight infants. Pediatr Infect Dis J 2010;29:352–356.
[Article] [PubMed]25. Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics 2008;121:703–710.
[Article] [PubMed]26. Wade KC, Benjamin DK Jr, Kaufman DA, Ward RM, Smith PB, Jayaraman B, et al. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J 2009;28:717–723.
[Article] [PubMed] [PMC]27. Allen U, Richardson S, McGuire W, Bow E, Robinson J, Rotstein C, et al. Pediatric antifungal therapy. Part II: neonatal infections. Minerva Pediatr 2010;62:71–78.28. Reed BN, Caudle KE, Rogers PD. Fluconazole prophylaxis in high-risk neonates. Ann Pharmacother 2010;44:178–184.
[Article] [PubMed]29. Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 2011;159:392–397.
[Article] [PubMed] [PMC]30. Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009;123:58–66.
[Article] [PubMed] [PMC]31. Bonnemaison E, Lanotte P, Cantagrel S, Thionois S, Quentin R, Chamboux C, et al. Comparison of fecal flora following administration of two antibiotic protocols for suspected maternofetal infection. Biol Neonate 2003;84:304–310.
[Article] [PubMed]32. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 2009;3:944–954.
[Article] [PubMed] [PMC]33. Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 2001;15:1398–1403.
[Article] [PubMed]34. Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis 2000;30:662–678.
[Article] [PubMed]
Fig. 1
Selection and exclusion of study patients. ELBW, extremely low birth weight; Bwt, birth weight.
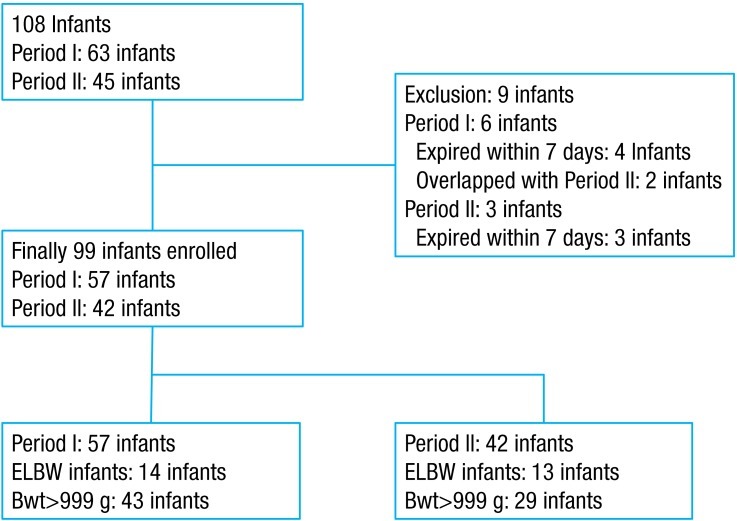
Table 1
Summary of cases diagnosed with sepsis in period I and II
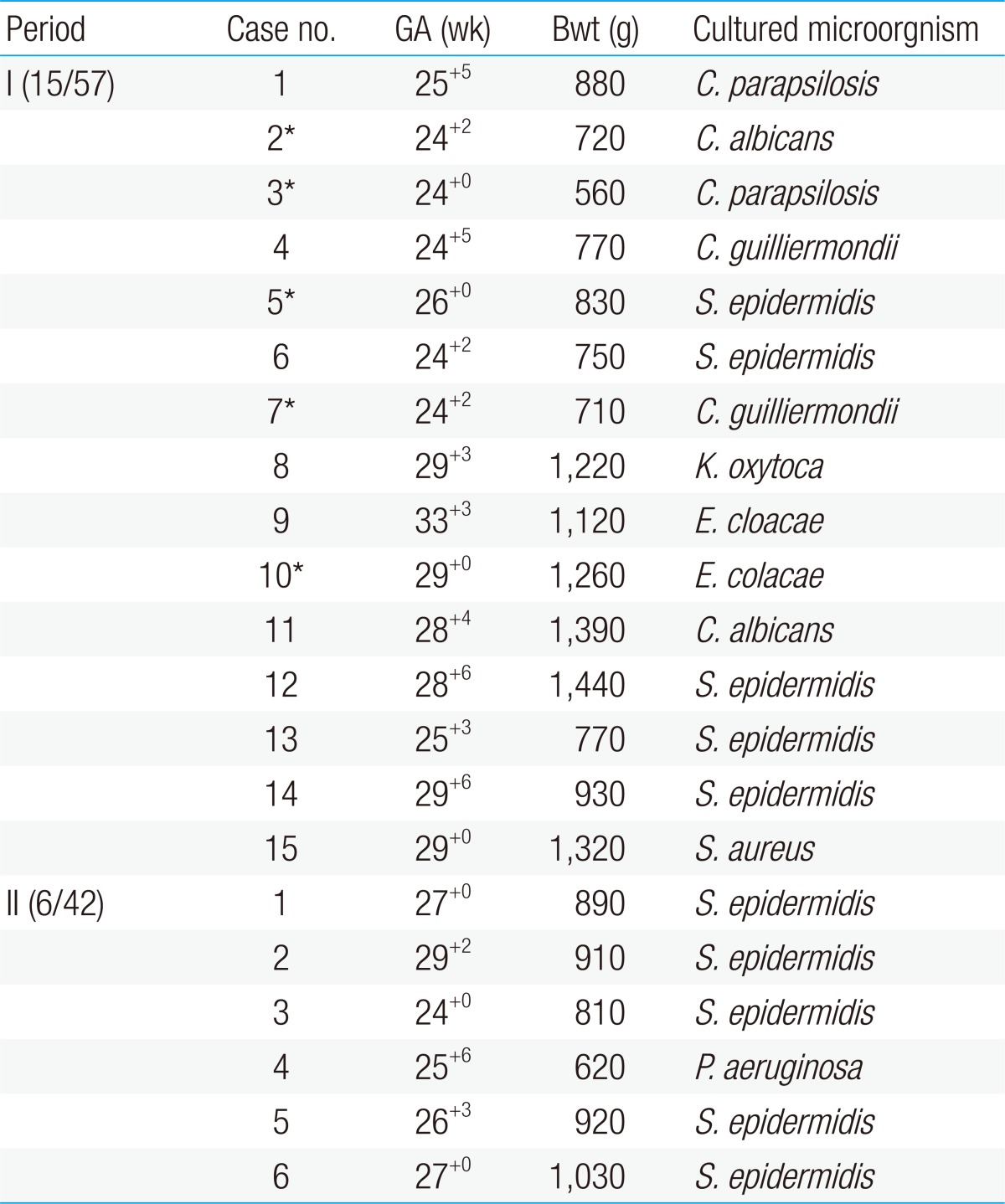
GA, gestational age; Bwt, birth weight; C. parapsilosis, Candida parapsilosis; C. albicans, Candida albicans; C. guilliermondii, Candida guilliermondii; S. epidermidis, Staphylococcus epidermidis; K. oxytoca, Klebsiella oxytoca; E. cloacae, Enterobacter cloacae; S. aureus, Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa.
*Expired case.

 About
About Browse articles
Browse articles For contributors
For contributors