Inferior vena cava to aorta ratio in dehydrated pediatric patients: a systematic review and meta-analysis
Article information
Abstract
Background
Dehydration due to acute diarrhea is among the leading causes of mortality. However, advancements in management and technology do not help clinicians differentiate dehydration degrees. Ultrasound using the inferior vena cava to aorta (IVC/ Ao) ratio is a promising noninvasive technique to identify significant pediatric dehydration.
Puspose
Therefore, this systematic review and meta-analysis aimed to examine the diagnostic parameters of the IVC/Ao ratio for predicting clinically significant dehydration in pediatric patients.
Methods
We searched the MEDLINE, PubMed, Cochrane Library, ScienceDirect, and Google Scholar databases for studies of pediatric patients (≤18 years old) who presented with signs and symptoms of dehydration due to acute diarrhea, gastroenteritis, or vomiting. The inclusion criteria were cross-sectional, case-control, cohort, and randomized controlled trial study design and publication in any language. We then conducted a meta-analysis using the midas and metandi commands from Stata software.
Results
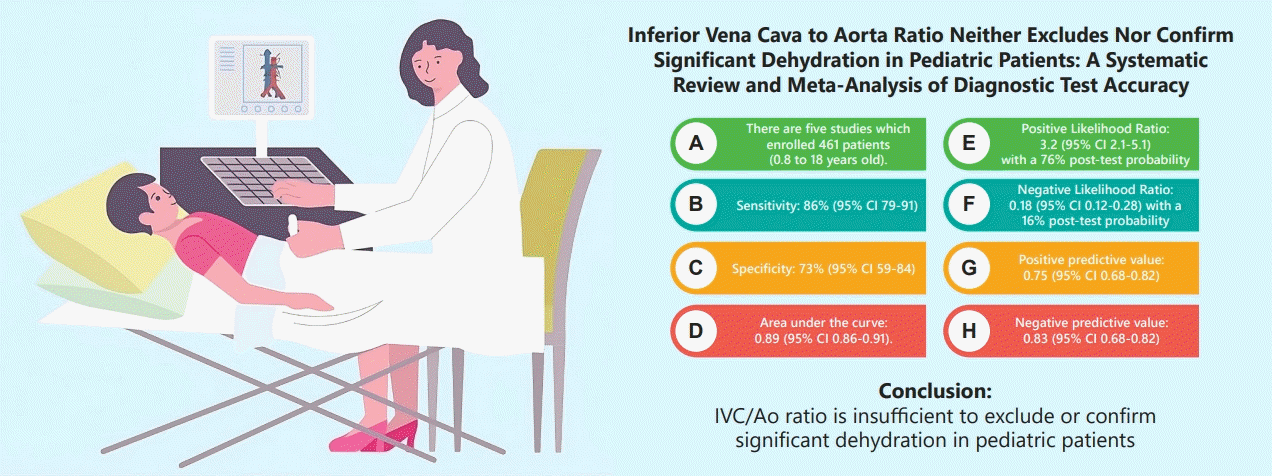
Five studies of 461 patients were included. The combined sensitivity was 86% (95% confidence interval [CI], 79%–91%), while the specificity was 73% (95% CI, 59%–84%). The area under the curve was 0.89 (95% CI, 0.86–0.91). The positive likelihood ratio (LR+) was 3.2 (95% CI, 2.1–5.1) with a 76% posttest probability, while the negative likelihood ratio (LR-) was 0.18 (95% CI, 0.12–0.28) with a 16% posttest probability. The combined negative predictive value was 0.83 (95% CI, 0.75–0.91), while the positive predictive value was 0.75 (95% CI, 0.68–0.82).
Conclusion
The IVC/Ao ratio was insufficient to exclude or confirm significant dehydration in pediatric patients. More studies are needed, especially multicenter, adequately powered diagnostic research, to will help establish the usefulness of the IVC/Ao ratio
Key message
Question: The inferior vena cava to aorta (IVC/Ao) ratio measured via ultrasound has been touted as a promising noninvasive technique to assess clinically significant dehydration in pediatric patients.
Finding: Our meta-analysis found that IVC/Ao ratio had a positive likelihood ratio of 3.2 (95% confidence interval [CI], 2.1–5.1) and negative likelihood ratio of 0.18 (95% CI, 0.12–0.28).
Meaning: Hence, IVC/Ao ratio is insufficient to exclude or confirm significant dehydration in pediatric patients.
Graphical abstract
Introduction
Dehydration is a physiological disturbance that affects salt and water loss, and the most common cause of pediatric dehydration is infectious diarrhea caused by viral or bacterial pathogens [1]. According to the World Health Organization (WHO), among the 1.7 billion diarrhea cases annually, around 525,000 children under 5 years of age are killed, making diarrhea the second most common cause of death in this population [2]. One study in Japan reported that the incidence of hospitalization in children <5 years of age due to rotavirus gastroenteritis is 13 per 1,000 person-years, while the cumulative incidence by 5 years of age is 6.6% [3]. In Indonesia, the burden of rotavirus diarrhea is around 60%, and children with rotavirus diarrhea are more likely to develop vomiting and dehydration [4].
Dehydration is a common concern for parents and clinicians because prompt and adequate rehydration therapy prevents mortality [5,6]. However, to prescribe sufficient rehydration therapy that does not under- or overtreat dehydration, the clinician must correctly determine its severity [7]. Under- or untreated dehydration results in acidosis, electrolyte disturbances, and end-organ damage, such as renal insufficiency, lethargy, and cardiovascular instability. Similarly, overtreatment of dehydration prolongs the length of stay and may result in unnecessary morbidity [7]. Despite advancements in knowledge and technology, classifying the degrees of dehydration in pediatric patients remains difficult [8].
Current methods for detecting significant dehydration, usually defined as a fluid deficit of ≥5% of the patient’s body weight, rely heavily on history taking, physical findings, complementary laboratory findings, and physician gestalt. Different guidelines vary significantly in determining pediatric dehydration criteria, cutoffs, and diagnosis [9]. One systematic review found that a combination of abnormal capillary refill time, abnormal skin turgor, and abnormal respiratory pattern are the most valuable signs for predicting 5% pediatric dehydration. The same systematic review also reported that history taking and laboratory tests are only moderately useful. However, these clinical signs possess poor sensitivity, limiting their utility [7]. A prolonged capillary refill time, touted as the best single sign to predict 5% pediatric dehydration [10], also suffers from low sensitivity [11].
Several clinical scoring systems, such as The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development score [12], modified Vesikari score [13], Community Diarrhea score [14], Clinical Dehydration Scale [15], Gorelick scale [16], and WHO scale [17], produce conflicting results due to their development in a specific population using specific definitions, settings, methodologies, and diarrhea etiologies [18-20]. Moreover, laboratory panels such as electrolyte, bicarbonate, blood urea nitrogen, serum anion gap, and urine specific gravity have no straightforward clinical utility for diagnosing pediatric dehydration [7,21-24].
Point-of-care ultrasonography has garnered significant attention, and an article even calls it a diagnostic tool beyond a stethoscope [25]. Because of its dynamic nature, high portability, and shallow learning curve [25-27], ultrasonography is increasingly accepted and used in many fields [28]. The first study to assess pediatric dehydration using ultrasonography was conducted by Kosiak et al. [29], who introduced the inferior vena cava to the aorta (IVC/Ao) diameter index.
However, since its first publication in 2007, the IVC/Ao ratio has not been widely used in pediatric dehydration settings. One explanation is the insufficient evidence to support its use. Studies are sparse, single-center, and underpowered and use different definitions and IVC/Ao ratio cutoffs. Only one meta-analysis assessed the IVC/Ao ratio in pediatric dehydration, and it included only one study, citing that current evidence does not support the routine use of ultrasound to determine dehydration severity [30]. In our opinion, an analysis of one study is insufficient to make a definitive statement about IVC/Ao ratio use in pediatric dehydration.
Therefore, this systematic review and meta-analysis aimed to examine the diagnostic parameters of the IVC/Ao ratio for predicting clinically significant dehydration in pediatric patients.
Methods
1. Eligibility criteria
The authors followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses) of Diagnostic Test Accuracy guidelines [31]. The study protocol was registered with the International PROSPERO (Prospective Register of Systematic Reviews) database (no. CRD42022324734).
The study population included all pediatric patients (≤18 years old) who presented with signs and symptoms of dehydration due to acute diarrhea, gastroenteritis, or vomiting. The primary aim of this study was to examine the sensitivity, specificity, and posttest probability of the IVC/Ao diameter ratio (the index test) measured by ultrasonography for detecting significant dehydration. The percent weight change from presentation to discharge was the benchmark for identifying substantial dehydration [20]. We excluded studies that did not use the same reference standard or assess dehydration or the IVC/Ao diameter ratio. Hence, our research question could be formulated as “In pediatric patients who present with dehydration due to acute diarrhea, gastroenteritis, or vomiting, how accurate is the IVC/Ao diameter ratio for ruling in and ruling out significant dehydration versus the percent weight change from presentation to discharge?”
Any cross-sectional, case-control, cohort, or randomized controlled trial published in any language met the inclusion criteria. The grey literature, such as conference abstracts, theses, and dissertations, was also searched. Case reports, case series, reviews, and animal studies were excluded. Studies with fewer than 50 samples were excluded. The reference lists of reviews were manually searched to ensure that all relevant studies were included.
2. Search strategy and study selection
The literature search began and ended on 7th April 2022. We searched five academic databases: MEDLINE, Cochrane Library, PubMed, ScienceDirect, and Google Scholar. The keywords used were related to the diagnostic tool (“diagnostic imaging,” “ultrasonography,” “inferior vena cava,” “aorta,” and “inferior vena cava to aorta”), population (“pediatric” and “adolescent”), and condition (“diarrhea,” “vomiting,” “gastroenteritis,” and “dehydration”).
Supplementary Table 1 lists the Medical Subject Heading terms for each database. We purposefully did not include any keywords mentioning “sensitivity," “specificity,” or any diagnostic-related terms to avoid missing potential studies [32]. All records were entered into the Rayyan program, which manually screened them and automatically identified duplicates [33].
Using this program, the authors might choose to group pertinent studies together. Two authors (GSO and JW) performed the first search and imported all information into Rayyan software. The initial searches were cross-checked by a different author (MI). Three authors independently reviewed each study that was identified. Conflicts were settled through group discussions and professional judgments (MW). When studies from the same dataset had overlapping time points, we selected data that provided the most information.
3. Data extraction and quality assessment
Two authors (GSO and NKH) independently extracted the data, whereas the others (JW and MI) verified their accuracy. We gathered pertinent data, including study identity (author and publication year), study characteristics (location, study design, participant age, and study duration), and information regarding ultrasonography (machines used, sensitivity and specificity, and IVC/Ao ratio cutoff).
We used the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool to assess the risk of bias. There were no formal cutoff scores in QUADAS-2, and the risk of bias was presented visually [32]. Four reviewers evaluated the scale independently (GSO, JW, MI, and NKH), and any disagreements were resolved internally and through an expert decision (MW) until consensus was reached. We contacted the associated authors by email in cases of missing or incomplete data.
4. Data synthesis
Before proceeding with the meta-analysis, model diagnostics were checked using a graphical depiction of residual-based goodness of fit, bivariate normality, influence, and outlier detection analysis. Any outlier studies were double-checked using a sensitivity analysis. Bivariate box plots were used to describe the degree of interdependence. We calculated the individual and pooled sensitivity and specificity using the bivariate model of sensitivity and specificity [34]. A summary receiver operating characteristic was used to display and illustrate the trade-off between sensitivity and specificity using the hierarchical summary receiver operating characteristic model [35]. An area under the curve was measured, and a value of 0.9–1 indicated excellent, 0.8–0.9 indicated very good, 0.7–0.8 indicated good, 0.6–0.7 indicated sufficient, and 0.5–0.6 indicated poor diagnostic accuracy [36]. The I2 index was used to measure heterogeneity; a value of 0% implied no observable heterogeneity, while a value of >50% was considered high heterogeneity [37]. A linear regression test of funnel plot asymmetry was used to evaluate publication bias, and a slope coefficient indicated significant asymmetry at values of P<0.1.
We calculated the posttest probability using a Fagan plot based on Bayes’ theorem and the likelihood ratio scattergram. As the prevalence of significant dehydration in pediatric patients varied, we chose a pre-test probability of 50% [38-40]. An arbitrary cutoff point for the likelihood ratio to have marked changes was >+10 for positive likelihood ratios (LR+) and <-0.1 for negative likelihood ratios (LR-) [41]. Additionally, the predictive values and probability modifying plots are displayed, more informative positive results producing curves that incline toward the (0,1) location and more informative negative results producing curves that incline toward the (1,0) line [42]. The “midas” and “metandi” commands of Stata ver. 17.0 (StataCorp, College Station, TX, USA) were used to conduct the analysis [43].
Results
A total of 11,546 articles were retrieved: of them, 279 were immediately removed and 11,267 unique articles were screened. A total of 444 articles were excluded after the title and abstract screening, and five were ultimately included in this meta-analysis (Supplementary Fig. 1).
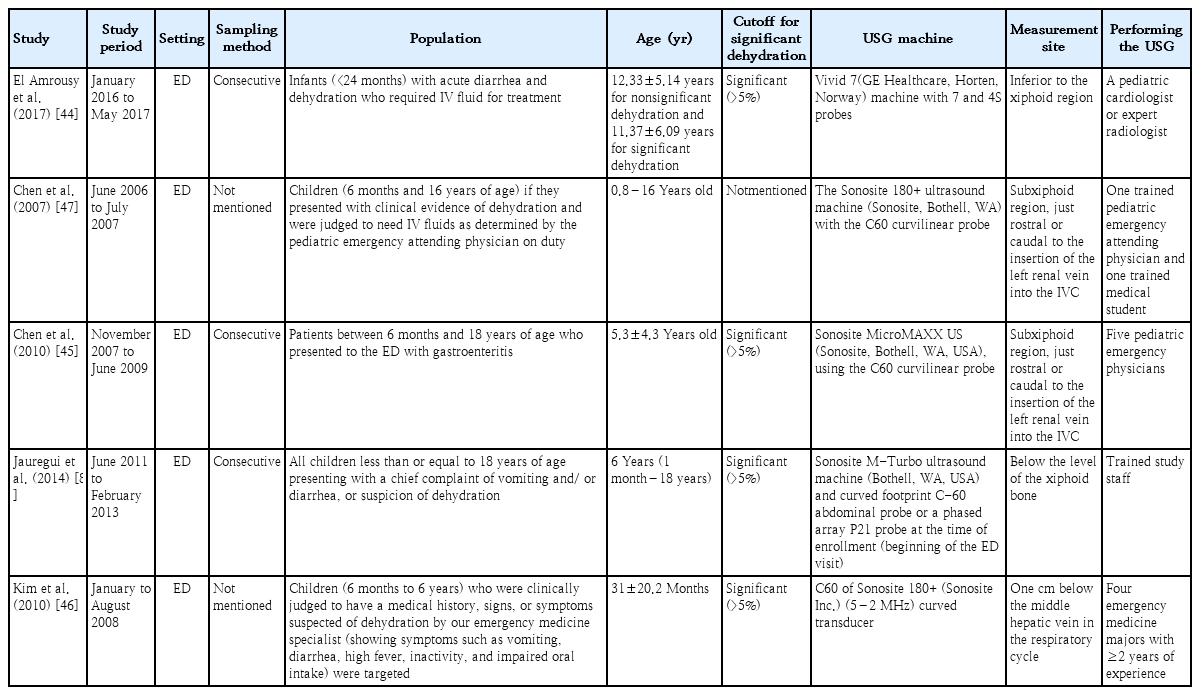
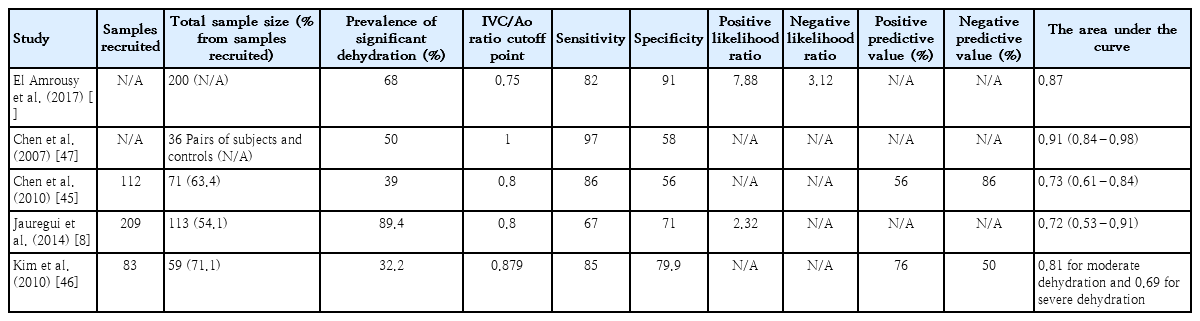
Additional manual citation searches did not yield additional studies. The characteristics of each study are shown in Table 1, and the descriptive diagnostic test parameters are listed in Table 2. The notable exclusions are presented in Supplementary Table 2. All studies were prospective cohort studies conducted in the emergency department. Across all five studies, there were 461 patients aged 0.8–18 years. Four studies [8,44-46] applied the same cutoff for significant dehydration (>5%), while the other [47] did not specify a cutoff. All studies used the transverse plane to measure IVC and Ao diameters. El Amrousy et al. [44] had the highest enrollment with 200 patients (43.4%). The prevalence of significant dehydration ranged from 32.2%8) to 89.4% [8]. In terms of the risk of bias, patient selection suffers from the highest risk of bias, followed by the index test, flow, and timing. The reference standard had the lowest bias risk. All studies had a low risk of concern in terms of their applicability. Overall, only one study [44] had a low risk of bias, while the other studies had some risk of bias (Supplementary Fig. 2, Supplementary Table 3).
Supplementary Fig. 3 shows the model diagnostics, whereas no studies were outliers with a moderate goodness of fit. However, the bivariate normality assumption was not fulfilled, and three studies [8,44,47] seemed more influential than others with a Cook’s distance of >0.5. The bivariate box plot (Supplementary Fig. 4) showed skewness of the test performance measures toward a higher specificity with lower sensitivity, providing indirect evidence of some threshold variability.
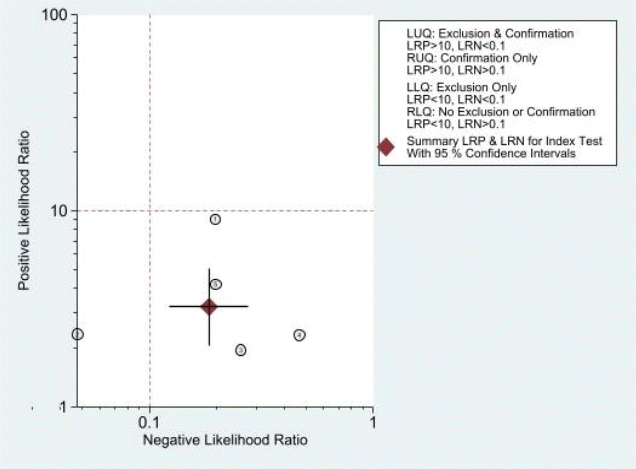
The combined sensitivity was 86% (95% CI, 79%–91%), while the specificity was 73% (95% CI, 59%–84%). Supplementary Fig. 5 shows the combined summary receiver operating characteristic curve with an area under the curve of 0.89 (95% CI, 0.86–0.91), indicating that the IVC/Ao ratio has very good diagnostic accuracy for detecting significant dehydration in pediatric patients. Supplementary Fig. 6 presents the studyspecific sensitivity and specificity, indicating threshold variability as sensitivity increased and specificity decreased and vice versa. The study of Jauregui et al. [8] had the highest sensitivity at 97% (95% CI, 85%–100%), while the study of Kim et al. [46] had the highest specificity at 91% (95% CI, 81%–97%). The I2 value was 83% (95% CI, 63%–100%) (P=0.002), indicating significant substantial heterogeneity. Supplementary Fig. 7 displays Fagan’s nomogram showing that the LR+ was 3.2 (95% CI, 2.1–5.1) with a 76% posttest probability while the LR- was 0.18 (95% CI, 0.12–0.28) with a 16% posttest probability. The likelihood ratio scattergram placed the summary point of likelihood ratios in the right lower quadrant, indicating that the IVC/Ao ratio could not be used to exclude or confirm significant dehydration in pediatric patients (Supplementary Fig. 8). The probability modifying plot tended toward the (1,0) line, indicating a more informative negative result. The combined negative predictive value was 0.83 (95% CI, 0.68–0.82), while the positive predictive value was 0.75 (95% CI, 0.68–0.82) (Fig. 1).
Probability modifying plot. LR, Likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
The linear regression test for funnel plot asymmetry yielded a P value of 0.16, indicating insignificant asymmetry. Thus, there was a low chance of publication bias (Supplementary Fig. 9). A sensitivity analysis revealed no significantly altered results when individual studies were omitted (Supplementary Table 3). According to the meta-regression analysis, probability methodology and having a 5% cutoff for significant dehydration contributed to heterogeneity with values of P<0.01 and P=0.1, respectively (Supplementary Table 4).
Discussion
The IVC/Ao diameter ratio achieved a good sensitivity of 86% and moderate specificity of 73% in this meta-analysis. While these numbers may seem encouraging, sensitivity and specificity alone do not provide a basis for informed decisions following positive and negative screening test results due to false positives and negatives, respectively. Therefore, sensitivity and specificity alone do not rule out significant dehydration in pediatric patients [48]. However, one methodological study recommended displaying a side-by-side forest plot of sensitivity and specificity to assess threshold variability. The forest plot indicates that sensitivity increases with decreasing specificity, indicating threshold variability. This result is also supported by a bivariate boxplot analysis [49]. A variability threshold is a type of variation in which an arbitrary choice of the threshold value may lead to overoptimistic measures of test performance [50]. Only one study [44] prespecified its IVC/Ao ratio cutoff, while the others conducted a series of tests to determine the cutoff, resulting in threshold variability.
The likelihood of a test result in patients with the disease divided by the chance of the test results in patients without the condition is known as LR, another diagnostic test indicator that can be examined. The more the LR+ is >1 or <1 for LR-, the more or less likely the disease, respectively [51]. Therefore, the IVC/Ao ratio has a slight increase and moderate decrease in the likelihood of detecting significant dehydration with an LR+ of 3.2 and LR- of 0.18, respectively [52]. However, prevalence affects all of the above parameters, affecting diagnostic test accuracy [53,54]. A posttest probability depends on prevalence, a clinically more useful diagnostic test accuracy parameter. In this meta-analysis, an IVC/Ao ratio below the suggested cutoff increased the probability of significant dehydration by 26% (from 50% to 76%), while an IVC/Ao ratio above the suggested cutoff decreased the probability by 34% (from 50% to 16%).
The likelihood scattergram indicated that the IVC/Ao ratio cannot be used to exclude or confirm significant dehydration, meaning that the IVC/Ao ratio may only be good in test research and not diagnostic research [55]. All the studies included in this meta-analysis were test research, which has limited applicability to clinical practice. Koeller et al. [56] performed diagnostic research in which they compared The Dehydration: Assessing Kids Accurately (DHAKA) score model with the DHAKA-US and found that ultrasonography has no added benefit. However, it is necessary to point out that they measured the Ao/IVC ratio and not the other way around. We believe that ultrasonography, especially the IVC/Ao ratio, belongs to the “add-on diagnostic test” category in the emergency department for detecting significant dehydration after history taking, clinical signs and symptoms, and basic laboratory work-ups [57]. However, the difficulty obtaining an accurate reading from crying children [58], high interobserver variability [59], and unspecified cutoff and test characteristics [30] complicate the use of ultrasonography in this setting.
Regarding overtreatment, a meta-analysis of different clinical scales to measure severe dehydration in children with acute gastroenteritis found insufficient evidence to warrant routine ultrasonography usage [30]. However, only 1 study in this meta-analysis analyzed ultrasonography, while the other four did not. These results strengthen the findings of a previous meta-analysis that the IVC/Ao ratio may not be clinically valuable for detecting significant dehydration in pediatric patients.
There are several explanations for the shortcomings of ultrasonography in identifying significant dehydration in pediatric patients. First, the best view, mode, and site of measurement were not established. Studies were underpowered, targeted different populations, or had different test characteristics to determine those three characteristics [60,61]. Second, IVC diameter changes with age, and the corresponding normal value for age has not been established in multicenter large-cohort studies of pediatric patients [62]. Pediatric patients with significant dehydration present with inconsolable crying, which affects respiratory variability.
A significant inspiratory effort will introduce more false positives into the results, while shallow breathing during crying will introduce false negatives [63]. Conflicting results have been reported regarding fluid responsiveness detected by ultrasonography in spontaneously breathing patients [64,65]. Finally, the optimal IVC/Ao cutoff ratio has not been determined, contributing to different results using different cutoffs [8,44-47].
The findings of our meta-analysis have some limitations. We found only five studies despite our thorough and systematic search. Meta-analyses of diagnostic test accuracy are notorious for missing relevant studies due to the use of nonspecific keywords [32]. However, we conducted a manual citation and study search to ensure that relevant studies were not missed. Different studies also employed different IVC/Ao ratio cutoffs, contributing to threshold variability that affects diagnostic test parameters. In our meta-analysis, the I2 index was high, which indicated heterogeneity. Combining different studies will introduce bias and variations in our meta-analysis, which may also introduce heterogeneity. Clinical variations, such as in the experience and expertise of ultrasonography operators, varying IVC/Ao ratios, and sampling methodology, may introduce some unexplained heterogeneity. However, the included studies were all conducted in a resource-rich setting with ultrasonography performed in the emergency department. In addition, almost all studies used >5% as the cutoff for significant dehydration.
Despite this heterogeneity, our results remain valid among these populations. The utility of the IVC/Ao ratio for identifying substantial dehydration in pediatric patients with acute diarrhea, gastroenteritis, or vomiting has never been adequately evaluated. We believe that this is the first meta-analysis to systematically examine this research question. Despite the small number of studies, Deeks’ test revealed no publication bias. Despite its low power, use of this test is suggested for diagnostic test accuracy meta-analyses [66]. Most importantly, this meta-analysis found that the IVC/Ao ratio is not valuable for excluding or confirming significant dehydration in pediatric patients. Although it may be too premature to conclude this finding, especially with a small number of studies and patients examined herein, it indeed guides researchers to shift their studies from test research to multicenter adequately powered diagnostic research to help establish the usefulness of the IVC/Ao ratio [55].
Conclusion
The IVC/Ao ratio had a positive posttest probability of 76% and negative posttest probability of 16%. This means that the IVC/Ao ratio is insufficient to exclude or confirm significant dehydration in pediatric patients. More studies are needed, especially multicenter adequately powered diagnostic research, to help establish the usefulness of the IVC/Ao ratio. More research is needed to determine the most suitable mode, view, and location to detect IVC in pediatric patients, normal IVC diameter for age, and the optimal IVC/Ao ratio cutoff. Further studies must identify whether this ratio is useful as a replacement, triage, or add-on test in clinical settings [57].
Supplementary materials
Supplementary Tables 1-4 and Figs. 1-9 can be found via https://doi.org/10.3345/cep.2022.01445.
Medical subject heading (MeSH) terms used in each database
Notable exclusions
Quality assessment of diagnostic accuracy studies tool result of each study
Meta-regression results
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart for selection of included studies.
Quality assessment of diagnostic accuracy studies graphical representation of risk of bias.
QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies.
Model diagnostics of each study.
Bivariate boxplot of each study.
Summary receiver operating curve with confidence and prediction regions. Sens, Sensitiviy; Spec, Specificity; SROC, Summary Receiver Operating Characteristic; AUC, Area Under the Curve
Study-specific sensitivity and specificity. CI, confidence interval.
Fagan’s nomogram of IVC/Ao ratio in detecting significant dehydration in pediatric patients. Prob, probability; LR, Likelihood ratio; Pos: Positive; Neg, negative.
Likelihood ratio scattergram. LUQ, Left upper quadrant; LRP, Likelihood ratio positive; LRN, Likelihood ratio negative; RUQ, Right upper quadrant; LLQ, Left lower quadrant; RLQ, Right lower quadrant.
Linear regression test of funnel plot asymmetry.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization: GSO, MW; Data curation: GSO, MI, JW, NKH; Formal analysis: GSO; Funding acquisition: None; Methodology: GSO, MI, JW, NKH, MW; Project administration: MI, JW, NKH; Visualization: GSO; Writing - original draft: GSO, MI, JW, NKH, MW; Writing - review & editing: GSO, MI, JW, NKH, MW