Protocolized sedation may reduce ventilation and sedation requirements in the pediatric intensive care unit: a systematic review and meta-analysis
Article information
Abstract
This study aimed to evaluate the effectiveness and safety of protocolized sedation in mechanically ventilated pediatric intensive care unit (PICU) patients. A comprehensive search was conducted in MEDLINE, CENTRAL, Embase, Web of Science, and Scopus from inception to October 18, 2023. Randomized controlled trials (RCTs) and observational studies that compared protocol-directed sedation management with conventional sedation regimens in pediatric patients who required invasive mechanical ventilation (IMV) for >24 hours were included. Twenty-six studies (15,214 participants) were included. We found a statistically significant reduction in IMV duration (median difference [MD]=-13.88 hours; 95% confidence interval [CI], -25.46 to -2.29; P=0.022), PICU length of stay (MD=-0.64 days; 95% CI, -1.26 to -0.02; P=0.045). We found significant reductions in the duration (MD=-1.28 days; 95% CI, -2.26 to -0.31; P=0.016) and peak dose (MD=-0.05 mg/kg/hr 95% CI, -0.11 to 0.002; P=0.044) of benzodiazepines. A significant increase was found in the odds of unplanned extubation (odds ratio, 1.13; 95% CI, 1.02 to 1.26; P=0.029). We found no significant results regarding the other outcomes. Our results suggest that protocolized sedation may reduce ventilation requirements and PICU length of stay; however, these findings were not confirmed by RCTs. Moreover, we observed a trend toward a reduction in sedative exposure and an increased odds of unplanned extubation.
Key message
Protocolized sedation may reduce ventilation requirements, pediatric intensive care unit length of stay, and sedative exposure. However, it may increase the likelihood of unplanned extubation, highlighting the importance of incorporating preventive measures to mitigate this risk.
Introduction
In the pediatric intensive care unit (PICU), some form of analgosedation is often unavoidable to tolerate invasive mechanical ventilation (IMV) and other invasive procedures required during treatment. However, inadequate sedation can have detrimental effects. Undersedation may lead to life-threatening adverse effects such as endotracheal tube [1] and catheter removal in addition to possible long-term psychological consequences caused by pain, stress, and nxiety [2,3]. Similarly, oversedation and the use of certain sedative types may lead to adverse psychological outcomes such as delirium [4], withdrawal symptoms [5], and posttraumatic stress disorder [6]. Furthermore, oversedation reduces spontaneous respiratory activity, leading to longer PICU stays with all the resulting complications [3]. Therefore, optimizing sedation is of crucial importance for short- and long-term consequences.
Protocolized sedation has been proposed to optimize sedation, in which sedation is managed according to a predefined algorithm based on regular patient assessment. A meta-analysis of studies, mainly in adults, demonstrated some clinical improvements. However, it is essential to study children and adults separately since the pediatric population poses additional challenges. Moreover, the relatively low PICU mortality rate has led to a shift in focus toward post-PICU outcomes [7]. Thus, improving sedation practices in this regard might lead to quality improvement [7]. In 2022, 2 practice guidelines for PICU sedation recommended protocolized sedation for all patients requiring IMV coupled with regular patient assessments to maintain the designated sedation depth. However, due to low quality of evidence, neither guideline provides strong recommendations [3,8]. Therefore, here we aimed to synthesize the literature on the use of protocolized sedation in mechanically ventilated children.
Methods
This systematic review and meta-analysis was conducted based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (version 6.3.0) [9]. This study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10] (Supplementary Table 1). The review protocol was registered at the PROSPERO (International Prospective Register of Systematic Reviews) (no. CRD42021284277).
1. Information sources
A systematic literature search was conducted in MEDLINE (via PubMed), Cochrane Library (CENTRAL), Embase, Web of Science, and Scopus from inception to October 9, 2021, followed by an updated search on October 18, 2023. A query was applied to all fields, and no filters, language, or other restrictions were imposed. For a detailed description of the search keys, please refer to Supplementary Table 2.
2. Study selection process
The publications were screened using Endnote X20 and Clarivate [11]. Duplicates were removed using automated tools, followed by manual duplicate removal and eligibility screening by title, abstract and full text based on predefined eligibility criteria. If multiple studies with the same outcomes were identified using the same patient database, only that with the largest sample size was included; other studies were included only for outcomes that were originally not reported.
The selection process was performed separately by 3 pairs of reviewers (AS, ARS; AS, RE; or AS, VU) who independently screened each reference. Disagreements were resolved by a third reviewer (KH). Cohen's kappa coefficient (κ) was calculated after each step of the selection process to measure inter-rater reliability [12].
3. Eligibility criteria
The analysis included studies that reported on patients <18 years admitted to the PICU who received any form of analgosedation for >24 hours during IMV via an endotracheal or tracheostomy tube. Clinical trials including preterm infants were also excluded. Eligible studies compared protocolized and conventional sedation. We defined protocol-directed sedation management as a form of sedation in which predefined algorithms are followed to change the dose or type or to add or stop sedatives and/or major analgesic agents. This strategy involves regular patient assessment of sedation and comfort levels in most cases using an assessment tool, checklist, or questionnaire by any healthcare staff member. We included protocol-directed sedation weaning and daily sedation interruption strategies using predefined algorithms. Conventional sedation is determined by local practices that do not apply prearranged strategies or standardized patient assessments.
Our primary study outcome was IMV duration, while the secondary outcomes were length of stay (LOS) in the PICU and hospital, the incidence of adverse events associated with sedation and ventilation, exposure to sedatives and opioids, long-term psychological and cognitive recovery, and mortality. The definitions and measurements of the outcomes are summarized in Supplementary Table 3. Eligible studies reported at least one outcome.
Parallel and cluster randomized controlled trials (RCTs) and prospective and retrospective nonrandomized studies of intervention (NRSIs) were included. Case series, case reports, editorials, commentaries, qualitative studies, and literature reviews were excluded. Articles available only in abstract form or meeting reports were also excluded. No filters were applied, and no restrictions were imposed on the methodological quality or publication date or language.
4. Data extraction
Two independent authors (ARS and AS) extracted the data using a standardized data collection form in an Excel spreadsheet (Office 365; Microsoft, Redmond, WA, USA). The first author, publication year, study design, clinical settings, baseline characteristics of the study population, and results for all reported outcomes of interest were extracted. Disagreements were resolved by a third independent reviewer (KH). If data were insufficient, the original authors were contacted.
5. Risk of bias assessment and quality of evidence
The risk of bias was assessed using the Revised Cochrane Risk of Bias Tool for RCTs (RoB 2.0) [13] and the ROBINS-I (Risk Of Bias in Non-randomized Studies of Interventions) tool [14] by 2 independent authors; an independent third investigator resolved disagreements. The quality of the evidence from the included studies was evaluated according to the recommendations of the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) workgroup [15]. The tables used to assess the quality of evidence were prepared using the GRADEPro Guideline Development Tool [16].
6. Statistical analysis/data synthesis
Statistical analyses were performed using R v4.2.1 [17]. Meta [18] and metamedian [19] packages were used for calculations and plots. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to express binary outcomes. To calculate the OR, the total number of patients in each group and those with the events of interest were extracted from each study. Raw data from the selected studies were pooled using a random-effects model with the Mantel-Haenszel method. For the pooled results, the exact Mantel-Haenszel method (without continuity correction) was used to handle zero cell counts [20].
The median difference (MD) with 95% CI was calculated as the effect size for continuous outcomes. The values extracted to estimate the MD and its variance were the sample size, median, lower and upper quartiles, and minimum and maximum values in the 2 groups, when available. Some studies have reported the mean and standard deviation for both groups; in these cases, the outcome distribution was assumed to be normal to estimate the median and its variance. The sampling variance of the medians was estimated using the QE method [21] and a random-effect model was used to summarize the MD. The Hartung-Knapp adjustment [22,23] was applied to avoid false-positive findings. We used the restricted maximum likelihood method to estimate τ2.
Statistical heterogeneity across trials was assessed using the Cochran Q test and I2 values [24]. Forest plots were used to graphically summarize the results. Where applicable, we reported the prediction interval (i.e., expected range of effects in future studies) following the recommendations of IntHout et al. [25].
Results
1. Study search and selection process
A total of 11,331 records were identified. Twenty-six independent clinical trials [26-51] were included in the meta-analysis during the final selection process, with a total number of 15,214 participants.
The studies included RCTs and NRSIs. Three NRSIs [52-54] provided additional outcomes of the population of one RCT [27] and were included only in the qualitative synthesis. The PRISMA flow diagram in Fig. 1 summarizes the selection process.
2. Basic study characteristics
The baseline characteristics and designs of the eligible studies are shown in Supplementary Table 4. The clinical settings and sedation protocols with the sedative and major analgesic medications used are summarized in Supplementary Table 5. The eligible studies included medical, surgical, cardiac, and mixed PICU populations. Eighteen studies [26-34,36-38,45,46,48-51] investigated the effects of protocolized sedation, one pilot RCT [42] investigated the effects of daily sedation interruption, and 7 trials [35,39-41,43,44,47] studied the effects of protocolized weaning from sedation.
3. Primary outcomes
1) Duration of ventilation
A pooled analysis of 20 studies [26-31,33,35,37-39,41-43,46-50] involving 14,085 patients showed a statistically significant difference when comparing protocolized sedation versus usual care (MD=-13.88 hours; 95% CI, -25.46 to -2.29; P=0.022). We found moderate interstudy heterogeneity (I2=0.5, τ2=176.99, P=0.006). A subgroup analysis found dissimilarities among different study designs (χ2=8.15, degrees of freedom=2, P=0.017). A forest plot (Fig. 2) of prospective cohort studies suggested a significant decrease in the protocolized sedation arm only.
4. Secondary outcomes
1) PICU and in-hospital LOS
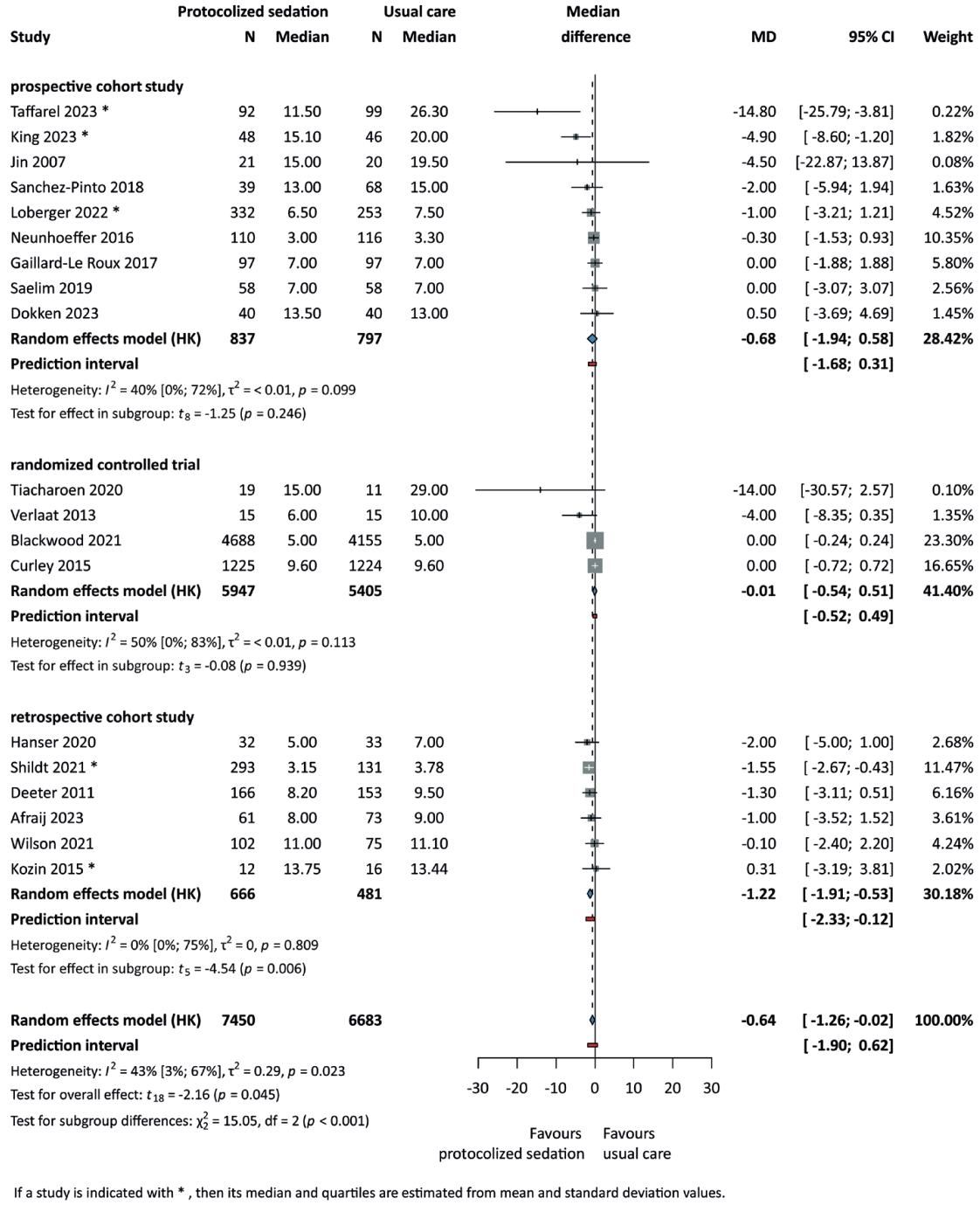
A statistically significant reduction in PICU LOS was found in the protocolized sedation arm (n=14,133; MD=-0.64 days; 95% CI, -1.26 to -0.02; P=0.045) [26,27,29-31,33,35,37-39,41-43,45-47,49-51], whereas no statistically significant difference was found in in-hospital LOS (n=11,930; MD=-1.83 days; 95% CI, -4.41 to 0.75; P=0.15) [26,27,35,38-40,44-48,50,51]. Notably, the significant difference in PICU LOS was driven by retrospective cohort studies (forest plots; Fig. 3, Supplementary Fig. 1).
2) Sedative and opioid exposure
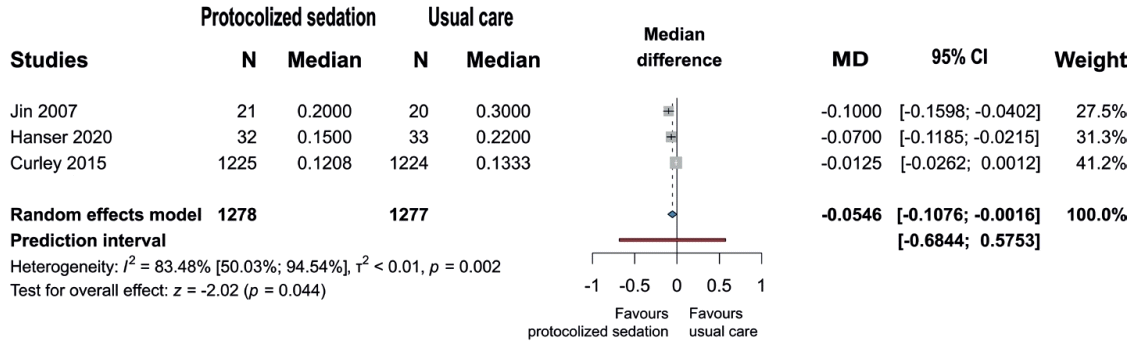
A statistically significant reduction was found in the duration of intravenous benzodiazepine use in the protocolized sedation arm (n=1,160; MD=-1.28 days; 95% CI, -2.26 to -0.31; P=0.016) [28-30,39,43-47,48,51] (forest plot, Fig. 4). However, no statistically significant differences were observed in the duration of intravenous sedative use (n=468; MD=-0.98 days; 95% CI, -3.1 to 1.14; P=.238) [29,33,35,46] as well as the duration of intravenous opioid analgesia (n=3,561; MD=-0.26 days; 95% CI, -1.62 to 1.1; P=0.678) [27-30,43,44,47,51] (Supplementary Figs. 2 and 3).

Forest plot of studies showing median differences in intravenous benzodiazepine exposure duration. Data pooling revealed a significant reduction in the protocolized sedation versus usual care group. MD, mean difference; CI, confidence interval.
A significantly lower peak benzodiazepine dose was observed in the protocolized sedation arm (n=2,555; MD=-0.05 mg/kg/hr; 95% CI, -0.11 to 0.002; P=0.044) [27,31,33] (forest plot, Fig. 5); however, no significant difference was noted in peak opioid dose (n=2,662; MD=-40.68 μg/kg/hr; 95% CI, -101.27 to 19.92; P=0.122) [27,31,33,39] cumulative opioid dose (n=3,063; MD=-5,549.73 μg/kg; 95% CI, -12,309.11 to 1,209.65; P=0.093) [27,31,33,37,39,43], cumulative benzodiazepine dose (n=3,176; MD=-1,719.73 mcg/kg; 95% CI -4,549.14 to 1,109.67; P=0.202) [27,31,33,37,39,43,44,46], and daily benzodiazepine dose (n=2,985; MD=-0.23 mg/kg/day; 95% CI, -0.58 to 0.13; P=0.207) [27,30,37,38] (Supplementary Figs. 4–7).
5. Adverse events and safety
No significant difference was found in PICU mortality (OR, 1.06; 95% CI, 0.65–1.73; P=0.734), the incidence of withdrawal syndrome (OR, 0.9; 95% CI, 0.46–1.75; P=0.708), or extubation failure rate (OR, 1.02; 95% CI, 0.78–1.35; P=0.851); however, we found a statistically significant increase in the odds of unplanned extubation (OR, 1.13; 95% CI, 1.02–1.26; P=0.029) (Supplementary Figs. 8–12).
6. Risk of bias assessment
A detailed description of the risk of bias assessment is presented in Supplementary Tables 6–8. A serious risk of bias indicates significant problems, such as major confounding or inaccurate measurements, that likely compromise the results, whereas a moderate risk of bias indicates minor confounding or measurement issues that are unlikely to substantially affect the results. For 2 NRSIs [30,35], the risk of bias was serious. Twenty NRSIs [28,29,31-40,43-45,47-51] had a moderate risk of bias due to confounding factors. One study [30] had a serious risk of bias due to baseline confounders, as a substantial number of patients were excluded due to a lack of data. Seven studies [30,31,44-46,48,50] had a moderate risk of bias due to missing data. One study [35] had a serious risk of bias since it did not provide sufficient information about missing data. Two pilot RCTs [41,42] suggested some concern in their selection of reported results, as neither the study protocol nor the statistical analysis plan were available before publication. Two cluster RCTs [26,27] suggested some concerns about the randomization process because information on allocation concealment was missing in the cluster randomization process. The risk of bias was generally low in all other domains.
7. Publication bias and heterogeneity
Egger test was available for only the OR analysis.
8. Certainty of evidence
The quality of evidence for IMV duration was low. Evidence quality was generally low for continuous outcomes and moderate to high for binary outcomes. The certainty of evidence for the 7 outcomes is summarized in Supplementary Table 9.
Discussion
Our meta-analysis revealed compelling findings regarding protocolized sedation, with statistically significant reductions in both IMV duration and PICU LOS. However, these benefits were primarily evident in NRSIs rather than in RCTs. Importantly, significant reductions in intravenous benzodiazepine duration [27,31,33] and benzodiazepine peak dose [27,31,33] were observed when sedation protocols were followed. Moreover, there was a trend toward lower cumulative and mean daily benzodiazepine doses, lower cumulative and peak opioid doses, and a reduced duration of intravenous sedation in the protocolized group. Moreover, 2 eligible studies reported significantly reduced dexmedetomidine [28] and clonidine [31] exposure in the protocolized group. Importantly, reports of sedative exposure differed among eligible studies; therefore, a limited number of patients could be pooled for sedation-related outcomes. In terms of adverse events, our analysis revealed a higher likelihood of unplanned extubation in the protocolized sedation arm. No other significant intergroup differences were observed. Consequently, we consider it feasible to safely implement protocolized sedation strategies, particularly in combination with bundles aiming to mitigate the risk of unplanned extubation.
Our findings of clinical outcomes differ somewhat from those of the previous meta-analyses focusing primarily on adults [8,9]. Aitken et al. [55] showed a significant reduction in-hospital LOS when using protocolized sedation in 4 RCTs, 3 in an adult population. Clear evidence was lacking of a reduction in ventilation days or PICU LOS, although the included studies showed a high degree of heterogeneity. Another meta-analysis comparing protocolized with nonprotocolized weaning strategies exclusively in adults showed a reduced duration of mechanical ventilation, weaning duration, and PICU LOS [56]. Similar to our results, both studies confirmed the safety of these strategies, whereas the overall sedative dosage was not investigated since it was not reported. The observed difference may be due to the specific characteristics of the pediatric population, as it is more difficult, if not impossible, to explain to these patients why treatment is necessary. Furthermore, different age groups have different situational awareness and abilities to express discomfort in addition to differences in pharmacokinetics and pharmacodynamics [57].
Reduced sedative exposure was a remarkable finding in our meta-analysis. Less sedative and opioid use inevitably leads to fewer adverse events. In addition, exposure to midazolam was significantly associated with the posttraumatic stress response at 1 month postdischarge [6], implying that sedation may be associated with long-term psychological outcomes. Thus, we also aimed to review the long-term effects of sedation; however, these data were available only for studies involving the same sample. Watson et al. [52] and Olszewski et al. [53] reported no statistically significant differences in the decline in cognitive function, health-related quality of life, or incidence of posttraumatic stress disorder at 6 months post-PICU discharge in the protocolized sedation arm. This finding was confirmed in a subset of patients with bronchiolitis [54].
These results provide high-quality data showing that minimizing sedation versus conventional sedation management has no harmful long-term impact. In addition, both groups reported a significant decline in cognitive function and quality of life from baseline levels at 6 months post-PICU discharge. A recent meta-analysis reported that children discharged from the PICU had a high burden of unfavorable psychological symptoms in several cognitive and affective domains after 3 months to 4 years of follow-up [58]; since some of these symptoms could be related to sedation practices, they warrant further study. The substantial statistical heterogeneity observed in our main outcomes was not disparate from meta-analyses in adults [8,9] and may be a consequence of variation among the included studies. Subgroup analyses comparing different study designs and sedation protocols did not reduce statistical heterogeneity, suggesting that differences in clinical settings, PICU populations, and sedation protocol implementation contributed to the reported heterogeneous results (Supplementary Tables 4 and 5). In addition, the eligible studies were heterogeneous in terms of sedation assessment tools [59,60], delirium [61], withdrawal syndrome [62], and sedatives used (Supplementary Table 5). These factors, which contributed to a moderate to high degree of observed statistical heterogeneity, were examined in the certainty assessment of evidence shown in Supplementary Table 9 and should be considered in the applicability and generalizability of our results.
The balance between reduced PICU LOS and IMV duration and the increased risk of unplanned extubation is a critical consideration in the implementation of protocolized sedation. Unplanned extubation is associated with significant risks including the potential for reintubation, respiratory failure, and cardiac arrest. However, the studies included in our meta-analysis reported no serious consequences. The RCT by Blackwood et al. [26], which contributed the most patients to the pooled analysis of unplanned extubation, reported no increase in reintubation rates, suggesting that patients were adequately prepared for extubation by the time the unplanned extubation occurred. Furthermore, no increased risk of other adverse events was observed, suggesting that an increase in unplanned extubation does not compromise patient safety in this context. Despite these findings, further research is needed to establish optimal sedation targets at different stages of illness and develop effective interventions for preventing unplanned extubations, such as improved taping methods and adjustments in nurse-to-patient ratios. The implementation of comprehensive care bundles, including protocolized sedation practices, may help mitigate the risks associated with unplanned extubations while maintaining the benefits of a reduced PICU LOS and IMV duration.
Apart from its strengths, this study has several limitations. Due to the insufficient number of eligible RCTs, data from cluster and pilot RCTs, as well as NRSIs were pooled. To overcome this limitation, we performed subgroup analyses, which showed significant differences among the different study designs in terms of IMV duration and PICU LOS. Furthermore, 10 studies [28,29,32,35,36,39-43] reported our main outcomes as secondary; consequently, they may have been statistically underpowered. Finally, we observed a moderate to high or unclear risk of bias for certain domains of several outcomes (Supplementary Tables 6–8), mainly due to baseline confounders and lack of reporting missing data. Hence, the quality of evidence for our main outcomes was rated as low (Supplementary Table 9). However, our meta-analysis is the most extensive review of the efficacy of protocolized sedation in PICUs. By encompassing a diverse patient cohort, our study offers a comprehensive assessment of the efficacy of protocolized sedation across various pediatric populations requiring critical care. Furthermore, our findings demonstrate the potential of protocolized sedation to significantly reduce sedative exposure.
The clinical efficacy of protocolized sedation remains debatable. Cohort studies demonstrated improvements after implementation, suggesting that increased focus on sedation may be beneficial. Despite this uncertainty, there is notable promise that protocolized sedation significantly reduces sedative exposure, thereby potentially minimizing sedative-related adverse effects. However, recognizing the potential risk of unplanned extubation associated with protocolized sedation is crucial. This underscores the need to incorporate prevention bundles into implementation strategies to mitigate such risks.
Further research should focus on the effects of protocolized sedation on standardized measurements of sedative exposure and include long-term follow-ups to evaluate the impact of sedation minimization strategies on psychological recovery, neurocognitive development, and quality of life. Additionally, investigating the distinct effects of various sedatives and sedation protocols could provide valuable insights into key associations. As the measurements of sedative and opioid exposure lacked standardization and were reported as different secondary outcomes, most studies were statistically underpowered for these outcomes, which may have reduced the impact of the implemented intervention. Therefore, future studies should include cumulative, peak, and average daily sedation doses in addition to duration.
Future clinical trials should consider methodological strategies to minimize the risk of bias, focus on individual randomization to adequately balance patients with different baseline characteristics among study cohorts, improve follow-up strategies, and address the implications of missing data.
In conclusion, protocolized sedation reduced IMV duration, PICU LOS, and hospital LOS based on a low to moderate quality of evidence. However, given that these results were not confirmed by RCTs, the superiority of protocolized sedation has not been established. Moreover, we observed a distinct trend of reduced sedative and opioid exposure; however, as the studies reported different measurements of sedation exposure, data pooling was severely limited. In addition, a significant difference was found only in unplanned extubation in terms of sedation-associated adverse events, implying that protocol-directed sedation management strategies can be safely implemented with certain precautions in various clinical settings.
Supplementary material
Supplementary Tables 1–9 and Supplementary Figs. 1–12 are available at https://doi.org/10.3345/cep.2024.01711.
PRISMA 2020 checklist
Detailed search strategy
Outcome definitions
Summary of baseline characteristics of included studies
Summary of clinical settings and implemented sedation protocols of the included studies
Risk of bias assessment at study and at domain level for cluster randomized controlled trials
Risk of bias assessment at study and at domain level for pilot randomized controlled trials
Risk of bias assessment at study and at domain level for nonrandomized studies
Certainty of evidence
Forest plot of studies representing the median differences (MD) of in-hospital length of stay. Pooled data showed no significant difference between the 2 study groups. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of the duration of intravenous sedation. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of the duration of intravenous opioid medications. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of peak dose of opioid medications. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of the cumulative dose of opioid medications. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of the cumulative dose of benzodiazepine medications. CI, confidence interval.
Forest plot of studies representing the median differences (MD) of the daily dose of benzodiazepine medications. SD, standard deviation; CI, confidence interval.
Forest plot of studies representing pooled odds ratios (OR) of intensive care unit mortality. CI, confidence interval.
Forest plot of studies representing pooled odds ratios (OR) of iatrogenic withdrawal syndrome. CI, confidence interval.
Forest plot of studies representing pooled odds ratios (OR) of unplanned extubations. CI, confidence interval.
Forest plot of studies representing pooled odds ratios (OR) of unplanned extubations/100 ventilator days. CI, confidence interval.
Forest plot of studies representing pooled odds ratios (OR) of extubation failure. CI, confidence interval.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
Klára Horváth is supported by the János Bolyai Research Scholarship awarded by the Hungarian Academy of Sciences, the National Research, Development and Innovation Office, Hungary. The authors thank Tamás Terebessy for his valuable comments on the manuscript. The authors are most grateful to Péter Mátrai, László Szabó, Mikolt Bakony and Andrea Harnos for their help with the statistical analysis.
Author Contribution
Conceptualization: AS, KH, ZM; Data curation: AS; Investigation: AS, ARS, VU, RE, MAE, KH, ZM; Methodology: AS, MAE, KH; Roles/Writing – original draft: AS, KH; Writing – review & editing: KH, MAE, ZM, PH; Formal analysis: AF; Funding acquisition: PH; Project administration: MAE; Supervision: KH, ZM