Article Contents
| Clin Exp Pediatr > Volume 63(12); 2020 |
|
This article has been retracted. See "Coronavirus disease 2019 in a 2-month-old male infant: a case report from Iran" via https://doi.org/10.3345/cep.2020.00941.r1.
The global spread of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has posed a threat to the public health worldwide [1]. Emerging in Wuhan, China in December 2019, this pandemic has become a major public health and economic burden on the world. SARS-CoV-2 was the seventh coronavirus identified with human infection capacity [2]. It has been stated that the spike (S) protein of 2019-nCoV had a high affinity to angiotensin-converting enzyme (ACE-2), which mediates the mechanism of the virus entrance to the target cell [3]. As the viral mutations occur during transmission, investigation of related clinical features of SARS-CoV-2 during its spread is interesting. Therefore, recording all clinical symptoms in patients with the coronavirus disease 2019 (COVID-19) is not without merit and can be a great help in timely diagnosis and treatment of the disease [4].
Children are the important part of any society. Characterizing clinical manifestations can not only contribute to the management of COVID-19 infection in children, but also provide accurate information for healthcare workers. It is well documented that children of all ages are susceptible to COVID-19, but they do not develop as much severe symptoms as adults [4]. The first pediatric case was reported on January 20, 2020, in a 10-year-old boy from Shenzhen, China and the youngest one was a 36-hour-old newborn [5,6]. The underlying cause of the lower incidence and milder symptoms of COVID-19 in children is associated with the premature ACE in children. Fever and dry cough are the most commonly reported clinical manifestations of COVID-19 among children [7].
The present study reported a 2-month-old male infant with fever and diarrhea, hospitalized in Hazrat-e Fatemeh Masoumeh Hospital, Qom city, Iran. The clinical features including symptoms, hospitalizations time, and medications were recorded and analyzed.
In 19 April 2020, a 2-month-old male infant was taken to the hospital with fever (as the sublingual temperature more than 38°C) and diarrhea. He has these symptoms for 3 days (16 April, 2020). His past medical history was unremarkable, and he ignored using any drugs, medications, or hospitalization. Because his hospitalization occurred at the time of COVID-19 crisis, the infant was suspected to have the SARS-CoV-2. Therefore, the initial diagnosis for the infant was COVID-19 confirmed after the accurate evaluation with real-time reverse-transcriptase polymerase chain reaction or rRT-PCR (based on routine PCR testing and sequencing of PCR amplifiers using throat swabs) and computed tomography (CT) scan of the chest. Dyspnea, rhinorrhea, and increased frequency of diarrhea caused the infant's condition to worsen the second day after the initial diagnosis of COVID-19 pneumonia, which was why the patient was hospitalized in the intensive care unit. The chronology of events by the parents confirmed that the patient was not infected with COVID-19 due to the history of close contact with the parent. Given that the outbreak of COVID-19 began for the first time in the city of Qom, located in the center of Iran country, his parents reported that they resided at the high-risk areas of the Qom city. Interestingly, the child's parents who had to be close enough to take care of their child, regardless of the principles of protection and distance, have negative results for the SARS-Cov-2 (negative rRT-PCR, complete blood cell; erythrocyte sedimentation rate, C-reactive protein [CRP], and chest CT scan). They reported that their young child had no close contact with the suspected or confirmed COVID-19 in the close family. The parents of the child not presented any detailed data related to way of infection for the child.
Upon admission to the hospital, he looked ill, and his vital signs were as follows: blood pressure, 85/40 mmHg; pulse rate, 140 bpm; respiratory rate, 60 bpm; and oral temperature, 38°C. Laboratory assays were as follows: white blood cell, 15.1×103; red blood cell, 3.55×106/µL; platelets, 367×103/µL; hemoglobin, 10.7 g/dL; creatinine, 0.5 mg/dL; Na, 139 mEq/L; K, 5.4 mEq/L; Ca, 9.2 mg/dL. A summary of the clinical outcomes is given in Table 1. alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase values were in normal range. At day of hospitalization, echocardiography was performed that not revealed any abnormality as the patient was stable (mild tricuspid regurgitation, pressure gradient=17 mmHg, left ventricular ejection fraction=68%, good left ventricular function, normal pulmonary artery pressure). Total protein and CRP-1h levels were in normal range and were equal to 0.3 mg/dL (reference: quantities <10 considered as negative) and 4.2 g/dL (normal range; for child between 1 month to 6 months: 4.4 to 6.6 g/dL) (Table 1). In blood culture test, we did not find any bacteria growth after 72-hour incubation of the blood sample. After the initial diagnosis of COVID-19, the chest CT scan was performed as revealed bilateral lung involvement with pleural effusion and pneumothorax in right lobe (Fig. 1).
At the course of the disease, the SpO2 reduction was not seen for the patient. Due to the young age of the patient, we started medication with hydroxychloroquine (3–5 mg/kg/day twice daily) and lopinavir/ritonavir (3 mg/kg/dose, twice daily) after medical consultation with pediatricians in our department (similar treatment as algorithmic approach was proposed to management of children with severe 2019-nCoV pneumonia or those with underlying diseases) [8]. After 5 days of medication, the patient was discharged from the hospital with partial recovery (at the day of discharge, the patient still had mild diarrhea without fever). In the follow-up in which we performed a week after the patient's discharge from the hospital, the patient was completely recovered and all the initial symptoms were subsided. The follow-up for pneumonia was done by chest radiography and evaluation of the clinical symptoms. After the beginning of the treatment, we follow up the patient in duration of 4–6 weeks via chest radiography. After treatment, when the patient recovered, chest radiography was normal. Also, we did not find any evidence of crackle auscultation in the patient. After treatment and recovery, the patient did not show any clinical symptoms associated with pneumonia, including fever, dyspnea, and cough. For pneumothorax, the follow-up was performed within 12–24 hours, as decreasing of auscultation was not seen. Also, chest radiography was clear for the patient. After 1-week treatment, we do not find any clinical symptom related to pneumothorax in the patient such as dyspnea.
Approval was obtained from the ethics committee of Qom University of Medical Sciences. The procedures used in this study adhere to the tenets of the Declaration of Helsinki (Nu: IR.MUQ.REC.1399.056).
COVID-19, as a novel infectious disease, causing respiratory illness and death in humans. Children account a very small percentage of patients with the COVID-19 and often present mild symptoms that in adults. However, they can develop severe disease in some cases [7]. We must notice to this point that the mild symptoms in children do not mean that their disease should be ignored, because the infected children may spread the disease in the community [9,10]. Therefore, accurate and complete awareness of all clinical symptoms of children at the age of COVID-19 can help the clinicians control the disease [4].
It has been shown that infection mechanism of COVID-19 is mediated via ACE-2 receptor in both adults and children. To date, it has been proven that the prevalence and infection rate of COVID-19 in children is less common than in adults, and the mortality rate is also relatively low and the severity and type of symptoms are different in children as well [11]. Recently, the differences in the expression degree of ACE-2 receptor between children and adults, and also the dependence of the expression on the age of the individuals have been shown to be the cause of different outbreak and mortality rate between children and adults. Although, children are less likely to be affected by SARS-CoV-2, we must not forget that they can be the carrier of the disease to adults who are in close contact with them [7].
In this case report, we documented the youngest COVID-19 infant among patients hospitalized at Hazrat-e Fatemeh Masoumeh hospital, Qom, Iran. The patient's manifestations were fever and diarrhea, which were suspected to be COVID-19 infection. We conducted rRT-PCR and CT scan to confirm COVID-19 pneumonia in the patient's clinical evaluation. Radiology finding revealed bilateral lung involvement with pleural effusion and pneumothorax in right lobe. Given that the patient condition has been worst at the course of the illness, for better treatment and management, we make to hospitalize the infant. The interesting point for this patient was the high SpO2 value at the course of the disease. Despite the patient has lung involvement, SpO2 reduction was not observed as it was always near 97% (see similar finding for the youngest newborn with COVID-19 [6]. Lung involvement was treated by using hydroxychloroquine and lopinavir/ritonavir at suitable dose without any side effects. The patient's response to treatment was acceptable. The patient was discharged from the hospital, and after a week recovered in a good general condition.
Unlike adults, reported data about COVID-19 children is limited because children are rarely tested for the virus and they do not show any severe symptoms [4]. Therefore, we believe that sharing the finding of this case report may interesting to those involved in providing care for these patients all over the world. At the end, we concluded that we should be aware of the possibility of COVID-19 infection in children and newborns; however, they may have mild symptoms or without any symptoms. Also, it is true that the severity and prognosis of COVID-19 are likely to be different in children [5,7]. Unlike adults, COVID-19 is significantly affected by the timely diagnosis and treatment of infected children, which may play an important role in preventing COVID-19 community transmission it has been impressive especially in parents who are in close contact with the patient.
Acknowledgments
The authors would like to thank Sanam Ahmadpour for her contribution to the edit of the present manuscript.
Fig. 1.
Chest computed tomography scan (transverse plane) of the 2-month-old male infant revealed A) bilateral lung involvement with pleural effusion and B) pneumothorax in the right lobe.
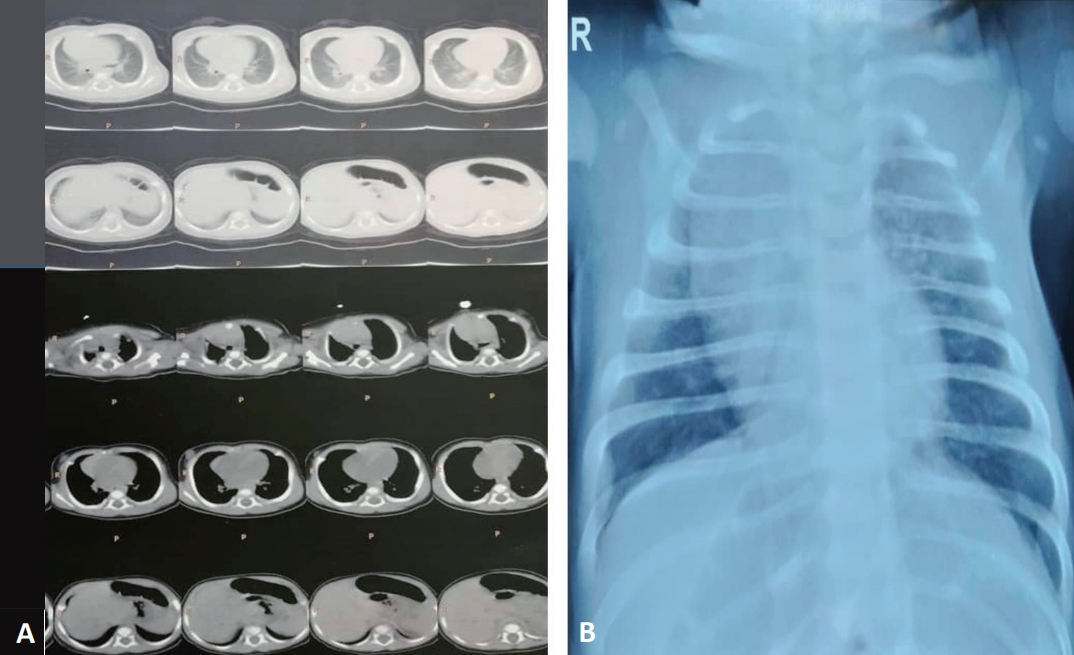
Table 1.
Serial laboratory results of a 2-month-old male infant with coronavirus disease 2019 on the day of hospitalization (April 19, 2020) versus the third day of hospitalization (April 22, 2020)
BS, blood sugar; SGOT, serum glutamic oxaloacetic transaminase; AST, aspartate aminotransferase; SGPT, serum glutamic-pyruvic transaminase; ALT, alanine aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; CRP, C-reactive protein; INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; HTC, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; ESR, erythrocyte sedimentation rate.
References
1. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15.



2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33.



3. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020;251:228–48.



4. She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol 2020;92:747–54.



5. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating personto-person transmission: a study of a family cluster. Lancet 2020;395:514–23.



6. Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, et al. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis 2020;71:853–7.



7. Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020;39:355–68.



8. Sankar J, Dhochak N, Kabra SK, Lodha R. COVID-19 in children: clinical approach and management. Indian J Pediatr 2020;87:433–42.